ABSTRACT
Background
Cirrhotic patients are more vulnerable to sedation-related complications than the general population, and there is no consensus on sedation during endoscopic procedures for these patients, whose numbers are increasing globally. Our study compared the efficacy of sedation, hemodynamic, respiratory effects, the incidence of side effects, and patient and endoscopist satisfaction with dexmedetomidine-ketamine versus propofol-ketamine during upper gastrointestinal endoscopy (UGIE) in hepatic patients with Child-Pugh classification A & B.
Patients and Methods
Seventy adult hepatic patients with Child-Pugh classification class A and B scheduled for UGIE were randomly assigned to one of two groups:
The ketamine/dexmedetomidine (KD) group received an IV loading of 1 mg/kg ketamine and 1 µg/kg dexmedetomidine over 10 minutes, followed by 0.1 mg/kg/hr ketamine and 0.1 µg/kg/hr dexmedetomidine maintenance. Ketamine/propofol (KP) group received an IV loading of 1 mg/kg ketamine and 1 mg/kg propofol over 10 minutes, followed by 0.1 mg/kg/hr ketamine and 0.1 mg/kg/hr propofol. Heart rate (HR), respiratory rate (RR), mean arterial pressure (MAP), and peripheral oxygen saturation (SpO2) were measured every 5 minutes (min) until the procedure was completed. The time required to reach the target Ramsay Sedation Score (RSS) (3–4) which is called the induction time, the time required to recover (the recovery time), and the occurrence of side effects were all recorded. The mini-mental state examination (MMSE) at baseline and 2 hours after recovery were measured.
Results
Demographic data showed no significant differences between the two groups. HR changes in the KD group were significantly lower than in the KP group at T1 (after the loading dose), T2 (after the endoscopy was inserted), and at all time points until the procedure was completed. MAP values were lower in the KD group compared to the KP group, but this difference was not statistically significant. Induction and recovery times were longer in the group (KD) than in group (KP), with (8.00 ± 1.26 min in the group (KD) vs 3.00 ± 1.14 min in the group (KP) for induction and (19.00 ± 1.53 min in the group (KD) vs 9.00 ± 1.41 min in the group (KP) for recovery. The prevalence of oxygen desaturation was higher in the group (KP) than in group (KD) (“9” pts in the group (KP) vs “3” pts in the group(KD)) (KD). However, unwanted movements were statistically more common in group KD than in group KP (6 patients versus two patients, respectively). Baseline MMSE and MMSE at PACU values were comparable within and between the two groups. The levels of satisfaction among patients and endoscopists were comparable in both groups.
Conclusion
The dexmedetomidine/ketamine combination is as effective as the propofol/ketamine combination in terms of sedation efficacy, with more hemodynamic and respiratory stability, but it has longer induction and recovery times with comparable results in terms of ketamine bolus consumption, MMSE scores after recovery, and patient and endoscopist satisfaction.
1. Introduction
Upper gastrointestinal endoscopy (UGIE) is commonly used to assess and diagnose a variety of gastrointestinal disorders. Follow-up UGIE is performed routinely on hepatic patients to assess the presence and severity of esophageal and gastric varices, as well as to treat them[Citation1]. Sedation is required during UGIE to reduce anxiety and allow the endoscopist to perform the procedure efficiently. Sedative drugs include benzodiazepines, opioids, and sedative-hypnotics. The best sedation agent should have quick induction and recovery times with few side effects. There is no single agent which completely meets all of these requirements. As a result, different types of drugs are used in combination to provide the best sedation with the fewest side effects [Citation2]. Ketamine causes sedation, analgesia, and amnesia. It keeps airway reflexes and respiration going. The main drawbacks of its use are vomiting, excessive salivation, sympathomimetic effects, and psychotic emergent reactions [Citation3]. Propofol is a commonly used agent in UGIE due to its quick induction and recovery time. The main disadvantages of its use are dose-dependent respiratory and cardiovascular depression [Citation4]. The safety and efficacy of ketamine/propofol combination as a sedoanalgesic agent are doses and mixture ratio-dependent [Citation5]. Dexmedetomidine is an alpha-2 agonist with high selectivity. It has analgesic, sedative, and anxiolytic effects while causing far less respiratory depression than other sedatives [Citation6,Citation7]. Cirrhotic patients are more vulnerable to sedation-related complications than the general population, and there is no agreement on sedation during endoscopic procedures for these patients. It is critical to research safer sedation methods in cirrhotic patients because the global prevalence of chronic liver disease is rising due to chronic hepatitis C and nonalcoholic fatty liver disease. According to our knowledge, no study has reported the use of dexmedetomidine/ketamine combination in sedation for hepatic patients undergoing gastrointestinal endoscopy. We hypothesized that the combination of ketamine and dexmedetomidine would improve the sedative and analgesic effects of both drugs with less cardiovascular and respiratory depression, allowing the endoscopist to perform the procedure more effectively. The primary goal of this prospective randomized comparative study was to compare the sedation efficacy of ketamine/dexmedetomidine versus ketamine/propofol combinations on hepatic patients with Child-Pugh classification A and B undergoing UGIE, and the secondary goals were to compare the respiratory, hemodynamic, and adverse effects of each, as well as the patients’ and endoscopists’ experiences.
2. Patients and methods
This prospective randomized double-blind clinical trial included seventy patients who were randomly assigned to one of two groups of 35 patients each using computer-generated random numbers and numbered sealed envelopes in a double-blind fashion. After receiving approval from the Faculty of Medicine, Ain-Shams University’s Research Ethics Committee (number FMASU R 21/2021), registration in ClinicalTrial.gov (NCT 04906772), and informed written consent from all patients. We enrolled hepatic patients aged 18 to 60 years with Child-Pugh classification (class A and B), American Society of Anesthesiologists physical status II, III, who were scheduled for elective UGIE. Exclusion criteria included emergency UGIE, severe hepatic disorder (Child C), chronic neuropsychiatric disorder, history of neuro-psychiatric drug intake, severe cardiovascular diseases (impaired systolic function (EF<50%), severe stenotic valve lesion, and recurrent attacks of unstable angina), pregnancy, history of drug abuse, allergy to any of the drugs used in the study, and the patients who developed any surgical complications like bleeding and perforation. Patients were randomly assigned to one of two groups: the ketamine/dexmedetomidine (KD) group or the ketamine/propofol (KP) group. The patients’ preoperative evaluation included a history, physical examination, and laboratory tests (complete blood picture, liver, and renal function tests). All patients had a coagulation profile and an electrocardiogram. All the study drugs were prepared in 50 mL infusion syringes in the same way: The ketamine/dexmedetomidine syringe contained 200 mg ketamine and 200 µg dexmedetomidine (precedex; united pharmaceutical group company, USA) diluted in 50 ml of 0.9% normal saline. The ketamine/propofol syringe contained 200 mg ketamine and 200 mg propofol (1% Fresenius Kabi Austria Gmbh 20 ml) diluted in 50 ml of 0.9% normal saline. An anesthesiology technician who was not involved in the study fully wrapped all the study syringes in aluminum foil sheets to conceal their contents from the anesthesiologist who performed the procedure. An anesthesiologist who was neither involved nor interested in the study administered the study drugs. When the patient arrived in the operating room, ECG, pulse oximetry, and non-invasive blood pressure were all connected to him. A nasal prong was connected to a 2–3 liter/min oxygen flow rate. A 20 G peripheral cannula was inserted into the dorsum of the hand, and Lactated Ringer’s solution 6–8 ml/kg/hr was started. The patient received NO premedication. Participants in the KD group received an IV loading of 1 mg/kg ketamine and 1 µg/kg dexmedetomidine over 10 minutes, followed by 0.1 mg/kg/hr ketamine and 0.1 µg/kg/hr dexmedetomidine maintenance. Participants in the KP group received an IV loading of 1 mg/kg ketamine and 1 mg/kg propofol over 10 minutes, followed by 0.1 mg/kg/hr ketamine and 0.1 mg/kg/hr propofol. Throughout the procedure, we aimed to maintain a sedation level of ≥ 3 according to Ramsay Sedation Scale (RSS) [Citation8]. Additional 100 mg of ketamine prepared in a 10 mL syringe to be given as Supplementary IV shots at a dose of 0.25 mg/kg to patients in both groups if they moved, to be repeated after 3 minutes if no response. Heart rate (HR), respiratory rate (RR), mean arterial pressure (MAP), and peripheral oxygen saturation (SpO2) were measured in the induction room before the start of the loading dose of the studied agents and immediately after completion of the loading dose (10 minutes) in both groups, as well as immediately after the insertion of the UGIE, then every 5 minutes (min) until the end of the procedure and then every 15 minutes (min) for 1 hour after finishing the procedure. Time to reach target Ramsay sedation scores (RSS) ≥3 which is called induction time was recorded. Any hemodynamic instability was managed appropriately such as bradycardia (HR< 50 beats/min), tachycardia (HR > 20% of baseline values), and hypotension (MAP < 60 mmHg) by asking the endoscopist to stop the procedure till giving atropine 0.01 mg/kg to treat bradycardia, or an extra bolus dose of ketamine to increase the depth of sedation and analgesia to treat tachycardia, and if hypotension was encountered, it was treated by giving 250 ml of iv crystalloids bolus or giving iv ephedrine 6 mg to be repeated after 5 min if no improvement. Supplementary ketamine bolus consumption during the procedure was calculated and recorded. At the end of the procedure, we stopped the drug infusion and recorded the Recovery time (which is the time from the stoppage of the drug infusions till achieving a score of 6 according to the modified steward recovery score) [Citation9]. The occurrence of postoperative nausea and vomiting (PONV) was recorded and treated with 4 mg ondansetron. Mini-Mental State Examination (MMSE) [Citation10] was used to assess the baseline cognitive function and the postoperative cognitive function at PACU after 2 h and recorded. The incidence of Respiratory compromise in the form of bradypnea (RR < 10/min), hypoxia (SpO2 < 90%) were recorded and managed non-invasively by increasing oxygen flow to 6 − 10 L/ min, and chin-left or jaw-thrust maneuver with or without nasal airway insertion, if failed Bag-Mask ventilation was done to improve desaturation. Patients’ and the endoscopists’ satisfaction scores were recorded evaluating the overall score out of 4 (1 = excellent, 2 = good, 3 = fair, 4 = poor).
2.1. Sample size justification
The sample size was calculated using the STATA program, setting the type-1 error (α) at 0.05 and the power (1-β) at 0.8. the results from the previous study [Citation11] showed that the ketamine consumption among the dexmedetomidine/ketamine group was 1.35 ± 0.75 mg/kg/h while among the midazolam/ketamine group was 2.15 ± 1.43 mg/kg/h with a p-value less than 0.05. According to these values, a sample size of 35 cases per group is enough.
2.2. Statistical analysis
SPSS version 16.0 computer software was used to analyze the patients’ data (Chicago, IL, USA). Data were presented as means ± standard deviation. An unpaired Student’s t-test was used to compare numerical variables between the two study groups, and a paired Student’s t-test was used to compare numerical variables within the same group. The chi-square test was used to compare categorical variables between the two study groups. Mann–Whitney U test was used to analyze the sedation score and MMSE. Statistical significance was defined as P values less than 0.05.
3. Results
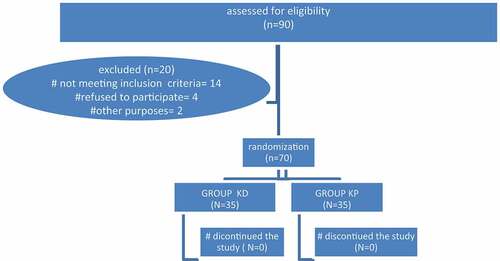
A total of 90 hepatic patients were evaluated and scheduled for UGIE. Twenty patients were excluded from the study due to either not meeting the inclusion criteria (14 patients), refusing to participate (4 patients), or other reasons such as displaying a picture of hepatic encephalopathy on the morning of the procedure (2 patients). Seventy patients were assigned in a random way to one of two groups, each of which had 35 patients. ().
Table 1. Demographic data of the two studied groups (data are presented as mean ± SD or number of patients)
In terms of heart rate changes in the two groups studied, there was a significant decrease in the KD group compared to the KP group at T1 (after the loading dose), T2 (after the endoscopy was inserted), and at all time points until the end of the procedure. When compared to the baseline in each group, there was a significant decrease in heart rate after the loading dose (T1) and a significant increase in heart rate after the endoscopy insertion (T2) in groups KD and KP. In terms of the remaining time points, there was a decrease in all heart rate values in the group KD compared to the group KP, but this difference was not statistically significant ().
Table 2. Changes in the heart rate (beat/min) in the two studied groups (data are presented as mean ± SD)
At all time points, MAP values were lower in the KD group compared to the KP group, but this difference was not statistically significant. However, when compared to the baseline values, there was a statistically significant decrease in MAP values after giving the loading dose (T1) and the increase in MAP after inserting the endoscopy (T2) was statistically lower than the baseline values within each group ().
Table 3. Changes in the MAP (mmHg) in the two studied groups (data are presented as mean ± SD)
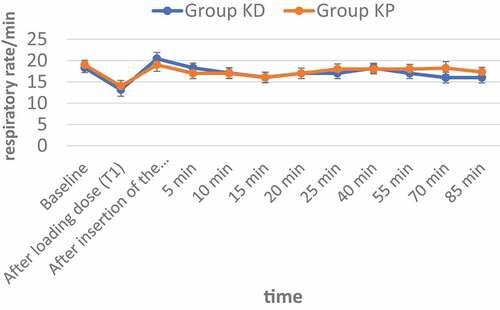
No statistical significance was found between the two groups regarding respiratory rate changes at all time points () and ().
Table 4. Changes in the respiratory rate in the two studied groups (data are presented as mean ± SD)
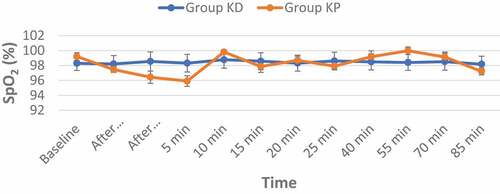
In terms of changes in oxygen saturation at all time points, there was no statistical difference between the two groups. Three cases (6%) in the group (KD) and nine cases (18%) in the group (KP) experienced desaturation of Oxygen (SpO2 < 90%) (p-value = 0.049), which was managed with chin-lift or jaw-thrust maneuver and oxygen flow increase to 6 L/min, without manual ventilation or artificial airway needed () and ().
Table 5. Changes in the Oxygen Saturation (SpO2) in the two studied groups (data are presented as mean ± SD)
In terms of induction and recovery times, group KP was statistically shorter than group KD ().
Table 6. Times of induction and recovery (data are presented as mean ± SD)
The ketamine bolus consumption was higher in group KD than group KP (52.29 ± 1.81 mg, 47.26 ± 3.25 mg respectively) but without a statistical significance between the two studied groups ().
Table 7. Ketamine bolus consumption in the two studied groups all over the study (data are presented as mean ± SD)
In terms of Ramsay sedation scores, there was no statistically significant difference between the two studied groups ().
Table 8. Ramsay sedation scores in the two studied groups (data are expressed as median (inter-quartile range)
There was no significant difference in baseline MMSE and MMSE at PACU values between the two groups studied, either within or between the two groups ().
Table 9. Evaluation of cognitive functions by mini-mental state examination (MMSE) (data are expressed as median (inter-quartile range))
in terms of the occurrence of side effects, both groups had comparable rates of bradycardia, tachycardia, hypotension, laryngeal spasm, nausea, vomiting, and postoperative cognitive dysfunction. The incidence of bradycardia in both groups was comparable (six patients in group (KD) versus four patients in group (KP), which was managed by stopping the procedure and administering 0.01 mg/kg atropine once). Only two patients in group KP experienced tachycardia, which was treated with an extra bolus dose of ketamine to increase the depth of sedation and analgesia. The incidence of hypotension was higher in group KP than in group KD, but the difference was not statistically significant (three patients in group (KD) versus five patients in group (KP), which was managed with a 250 mL ringer solution and a 6 mg ephedrine increment once). The prevalence of oxygen desaturation was statistically higher in group KP than in group KD (9 patients, and 3 patients respectively). All patients were managed by chin-lift or jaw-thrust maneuver and increasing oxygen flow to 6–10 L/min without the need for a nasal airway or manual ventilation. Unwanted movements were statistically more common in group KD than in group KP (6 patients, and two patients respectively). Three patients in group KD and two patients in group KP experienced nausea/vomiting in the recovery room and were given 4 mg ondansetron once. There were no patients who experienced laryngeal spasms or post-procedural psychotic symptoms ().
Table 10. Incidence of side effects in the two studied groups (data are presented as number of patients (%))
There were comparable rates of satisfaction scores between the two studied groups in terms of patient and endoscopist satisfaction ().
Table 11. Patients’ and endoscopists’ satisfaction (data are presented as number of patients (%))
4. Discussion
Our study showed that the use of ketamine/dexmedetomidine combination for sedation of hepatic patients during UGIE versus ketamine/propofol combination was characterized by lower heart rate values at T1 (end of loading dose) and T2 (insertion of endoscopy), comparable MAP values, respiratory profile, SpO2 changes, and ketamine extra bolus consumption. However, the KD group had longer induction and recovery times. In terms of side effects, the ketamine/dexmedetomidine combination had a lower incidence of oxygen desaturation giving the dexmedetomidine group a significant advantage in terms of respiratory safety and airway protection. but a higher incidence of unwanted movements during the procedure was reported in the dexmedetomidine group. Both combinations had comparable rates of satisfaction scores in terms of patient and endoscopist satisfaction.
Ketamine is an NMDA receptor antagonist that is commonly used as an anesthetic agent, particularly outside of the operating room. Tachycardia, hypertension, increased salivation, hallucinations, psychotic emergence, and delayed recovery are all common side effects of ketamine [Citation12,Citation13]. To reduce these side effects, ketamine and other sedative agents are combined. Propofol is an anesthetic agent that has a rapid onset of action, a satisfactory level of sedation, and a shorter recovery time when compared to other sedative agents [Citation14,Citation15]. Using propofol alone in GIE necessitates large doses, which may result in respiratory and hemodynamic instability [Citation16]. The combination of ketamine and propofol was frequently used to reduce side effects and shorten the duration of recovery in a variety of settings, including interventional radiology, gynecological, ophthalmological procedures, and coronary artery surgery in adults [Citation17–20]. Dexmedetomidine is an alpha-2 receptor agonist with eight times the potency of clonidine. It has anti-anxiety, sedative, and analgesic properties. In the United States, the Food and Drug Administration has approved dexmedetomidine for sedation in intensive care units. It has been used in conjunction with other sedatives during various procedures. Dexmedetomidine exerts its sedative effect by activating pathways that initiate endogenous non-rapid eye movement sleep. Its anxiolytic and sedative effects are caused by the stimulation of alpha-2 receptors in the pons locus ceruleus. Analgesic properties are used throughout the stimulation of alpha-2 adrenergic receptors in the dorsal horn of the spinal cord [Citation21]. Activation of postsynaptic alpha −2 receptors causes sympatholysis, which causes bradycardia and hypotension. Furthermore, stimulation of presynaptic alpha-2 receptors decreases noradrenaline release, resulting in an even greater drop in blood pressure. Dexmedetomidine should be avoided in patients with atrioventricular nodal block, severe hypovolemia, or cardiovascular instability because these hemodynamic changes return to baseline after fifteen minutes [Citation22]. Dexmedetomidine does not affect the gas exchange or ventilation and thus has no depressive effect on respiratory function [Citation23]. Because dexmedetomidine undergoes extensive first-pass metabolism, it has a low bioavailability. It has linear pharmacokinetics with an intravenous infusion dose of 0.2–0.7 µg/kg/hr. It has a rapid distribution volume of 118 L and an elimination half-life of 2 hours. Without displacing the majority of the protein-bound drugs, the protein-bound fraction of the given dose is 94%. Its half-life ranges from 4 minutes for a 10-minute infusion to 250 minutes for an 8-hour infusion. Dexmedetomidine is metabolized by glucuronidation and hydroxylation mediated by cytochrome P-450 to inactive metabolites that are excreted in urine (95%) and feces (4%). Due to lower rates of metabolism, the dose must be adjusted and reduced by up to 32% in patients with severe hepatic impairment and failure [Citation24]. The combination of dexmedetomidine and ketamine is used to balance the sympatho-inhibitory effects of dexmedetomidine with the cardio-stimulatory effects of ketamine, to provide effective sedation and analgesia without compromising ventilation, and to reduce the undesirable central effects of ketamine [Citation25].
All the findings in our study could be explained by the pharmacological effects of the drugs used, as previously mentioned. The longer induction time with dexmedetomidine was due to the slow initial infusion over 10 minutes to avoid the undesirable hemodynamic changes caused by faster infusion. Although dexmedetomidine has a short half-life (2–3 h) due to its rapid distribution and extensive metabolization by the liver, the recovery time was longer than that of propofol, which has a three times shorter half-life (30–60 min). Many studies have been conducted to evaluate the use of dexmedetomidine as a single agent for sedation of gastrointestinal endoscopic procedures, with better results in terms of hemodynamic stability, sedation efficacy, the incidence of side effects, and endoscopist satisfaction [Citation26–31]. However, studies conducted by [Citation32–34] revealed that using dexmedetomidine as a single agent for sedation of GIE procedures was ineffective in achieving the required sedation level except by using higher doses of rescue sedation doses, resulting in a significant decrease in hemodynamics with a longer time required for recovery and home discharge.
The findings of our study are consistent with the findings of many previous studies [Citation35–39] that compared the effects of dexmedetomidine in combination with other drugs to assess the efficacy of sedation, hemodynamic stability, recovery criteria, and satisfaction scores for patients scheduled for GIE procedures, which revealed that the combination dexmedetomidine group outperformed the other combinations in terms of sedation efficacy and safety, hemodynamic stability, recovery criteria, analgesia, and satisfaction scores. However, other studies conducted by Citation[40–44] revealed inefficacy of sedation scores and the need for higher doses of adjuvant drugs to achieve the target level of sedation with less hemodynamic stability and poor recovery criteria.
The limitations of our study are as follows: (1) it was conducted in only one center; (2) a small sample size, which may prevent the detection of clinical events that occur infrequently; (3) hepatic patients with Child-Pugh classification (class C) were not included in our study, which may limit the applicability of our findings to critically ill hepatic patients; (4) hepatic pediatric patients weren’t included in our study; and (5) The cost-benefit ratio should be considered because dexmedetomidine is a more expensive drug than propofol, which may limit its use unless it is indicated, particularly in patients with tense ascites who have a respiratory compromise. As a result, we recommend multi-centric studies with a larger number of patients, including those with Child-Pugh classification (class C), to validate the findings in future studies.
5. Conclusion
The combination of dexmedetomidine and ketamine used for sedation in hepatic patients scheduled for UGIE is as effective as the combination of propofol and ketamine as regards the sedation efficacy and it provides more respiratory and hemodynamic stability compared to the combination between propofol and ketamine with comparable results regarding the incidence of side effects, ketamine bolus consumption, MMSE scores after recovery, and the patient and endoscopist satisfaction but with longer induction and recovery times presenting itself as a good alternative for sedation for the hepatic patients scheduled for endoscopic procedures.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Waring JP, Baron TH, Hirota WK, et al. Guidelines for conscious sedation and monitoring during gastrointestinal endoscopy. Gastrointest Endosc. 2003;58(3):3.
- Tolia V, Peters JM, Gilger MA. Sedation for pediatric endoscopic procedures. J Pediatr Gastroenterol Nutr. 2000;30(5):477–485.
- Himmelseher S, Durieux ME, Weiskopf RB. Ketamine for perioperative pain management. Anesthesiology. 2005;102(1):211–220.
- Anderson JL, Pribble CG, Guenther E P ropofol for Procedural Sedation in Children in the Emergency Department. 2003;December. doi:10.1016/mem.2003.368
- Ketofol: a combination of ketamine and propofol. J Anesth Crit Care Open Access. 2014;1(5): doi:10.15406/jaccoa.2014.01.00031.
- Lee SK. Clinical use of dexmedetomidine in monitored anesthesia care. Korean J Anesthesiol. 2011;61(6):451.
- Afonso J, Dexmedetomidine: RF. Current role in anesthesia and intensive care. Brazilian J Anesthesiol. 2012;62(1):118–133.
- Ramsay MAE, Savege TM, Simpson BRJ, et al. 656 BRITISH MEDICAL JOURNAL Hospital Topics Controlled Sedation with Alphaxalone-Alphadolone.. 1974.
- Steward DJ. A simplified scoring system for the post-operative recovery room. Can Anaesth Soc J. 1975;22(1):111–113.
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198.
- Menshawi MA, Fahim HM. Midazolam–ketamine versus dexmedetomidine–ketamine combinations for anesthesia of pediatric patients undergoing cardiac catheterization. Ain-Shams J Anesthesiol. 2019;11(1):1–7.
- Okamoto N, Nakai T, Sakamoto K, et al. Rapid antidepressant effect of ketamine anesthesia during electroconvulsive therapy of treatment-resistant depression: comparing ketamine and propofol anesthesia. J ECT. 2010;26(3):223–227.
- Vallejo MC, Romeo RC, Davis DJ, et al. Propofol-ketamine versus propofol-fentanyl for outpatient laparoscopy: comparison of postoperative nausea, emesis, analgesia, and recovery. J Clin Anesth. 2002;14(6):426–431.
- McQuaid KR, Laine L. A systematic review and meta-analysis of randomized, controlled trials of moderate sedation for routine endoscopic procedures. Gastrointest Endosc. 2008;67(6):910–923.
- Wang D, Chen C, Chen J, et al. The use of propofol as a sedative agent in gastrointestinal endoscopy: a meta-analysis. PLoS One. 2013;8(1). DOI:10.1371/journal.pone.0053311
- Aydin Erden I, Gulsun Pamuk A, Akinci SB, et al. Comparison of propofol-fentanyl with propofol-fentanyl-ketamine combination in pediatric patients undergoing interventional radiology procedures. Paediatr Anaesth. 2009;19(5):500–506.
- Akin A, Guler G, Esmaoglu A, et al. A comparison of fentanyl-propofol with a ketamine-propofol combination for sedation during endometrial biopsy. J Clin Anesth. 2005;17(3):187–190.
- Frey K, Sukhani R, Pawlowski J, et al. Propofol versus propofol-ketamine sedation for retrobulbar nerve block: comparison of sedation quality, intraocular pressure changes, and recovery profiles. Anesth Analg. 1999;89(2):317–321.
- Botero CA, Smith CE, Holbrook C, et al. Total intravenous anesthesia with a propofol-ketamine combination during coronary artery surgery. J Cardiothorac Vasc Anesth. 2000;14(4):409–415.
- Lee DWH, Chan ACW, Sze TS, et al. Patient-controlled sedation versus intravenous sedation for colonoscopy in elderly patients: a prospective randomized controlled trial. Gastrointest Endosc. 2002;56(5):629–632.
- Bajwa S, Dexmedetomidine: KA. An adjuvant making large inroads into clinical practice. Ann Med Health Sci Res. 2013;3(4):475.
- Bloor BC, Ward DS, Belleville JP, et al. Effects of intravenous dexmedetomidine in humans: II. Hemodynamic changes. Anesthesiology. 1992;77(6):1134–1142.
- Venn RM, Hell J, Grounds RM. Respiratory effects of dexmedetomidine in the surgical patient requiring intensive care. Crit Care. 2000;4(5):302–308.
- De Wolf AM, Fragen RJ, Avram MJ, et al. The pharmacokinetics of dexmedetomidine in volunteers with severe renal impairment. Anesth Analg. 2001;93(5):1205–1209.
- Levanen J, Makela ML, Scheinin H. Dexmedetomidine premedication attenuates ketamine-induced cardiostimulatory effects and postanesthetic delirium. Anesthesiology. 1995;82(5):1117–1125.
- Samson S, George SK, Vinoth B, et al. Comparison of dexmedetomidine, midazolam, and propofol as an optimal sedative for upper gastrointestinal endoscopy: a randomized controlled trial. J Dig Endosc. 2014;5(2):051–057.
- Demiraran Y, Korkut E, Tamer A, et al. The comparison of dexmedetomidine and midazolam used for sedation of patients during upper endoscopy: a prospective, randomized study. Can J Gastroenterol. 2007;21(1):25–29.
- Sula H, Domi R, Ohri I, et al. Propofol versus dexmedetomidine for sedation in colonoscopy: a prospective, randomized study. Eur J Anaesthesiol. 2012;29:32.
- Kilic N, Sahin S, Aksu H, et al. Conscious Sedation for Endoscopic Retrograde Cholangiopancreatography: dexmedetomidine Versus Midazolam. Eurasian J Med. 2011;43(1):13–17.
- Eberl S, Preckel B, Bergman JJ, et al. Safety and effectiveness using dexmedetomidine versus propofol TCI sedation during oesophagus interventions: a randomized trial. BMC Gastroenterol. 2013;13(1):1.
- Takimoto K, Ueda T, Shimamoto F, et al. Sedation with dexmedetomidine hydrochloride during endoscopic submucosal dissection of gastric cancer. Dig Endosc. 2011;23(2):176–181.
- Jalowiecki P, Rudner R, Gonciarz M, et al. Sole use of dexmedetomidine has limited utility for conscious sedation during outpatient colonoscopy. Anesthesiology. 2005;103(2):269–273.
- Eldesuky A, Hassan HI. Dexmedetomidine versus ketofol for moderate sedation in Endoscopic Retrograde Cholangiopancreatography (ERCP) comparative study. Egypt J Anaesth. 2015;31(1):15–21.
- Muller S, Borowics SM, Fortis EAF, et al. Clinical efficacy of dexmedetomidine alone is less than propofol for conscious sedation during ERCP. Gastrointest Endosc. 2008;67(4):651–659.
- Techanivate A, Verawattaganon T, Saiyuenyong C, et al. A comparison of dexmedetomidine versus propofol on hypotension during colonoscopy under sedation. J Anesth Clin Res Techanivate. 2012;3:11.
- Dere K, Sucullu I, Budak ET, et al. A comparison of dexmedetomidine versus midazolam for sedation, pain and hemodynamic control, during colonoscopy under conscious sedation. Eur J Anaesthesiol. 2010;27(7):648–652.
- Sethi P, Mohammed S, Bhatia PK, et al. Dexmedetomidine versus midazolam for conscious sedation in endoscopic retrograde cholangiopancreatography: an open-label randomised controlled trial. Indian J Anaesth. 2014;58(1):18–24.
- Abdalla MW, El Shal SM, El Sombaty AI, et al. Propofol dexmedetomidine versus propofol ketamine for anesthesia of endoscopic retrograde cholangiopancreatography (ERCP) (A randomized comparative study). Egypt J Anaesth. 2015;31(2):97–105.
- Mukhopadhyay S, Niyogi M, Sarkar J, et al. The dexmedetomidine “augmented” sedato analgesic cocktail: an effective approach for sedation in prolonged endoscopic retrograde cholangio-pancreatography. J Anaesthesiol Clin Pharmacol. 2015;31(2):201.
- EL Shmaa NS. The efficacy of etomidate-fentanyl versus dexmedetomidine-ketamine for procedural sedation and analgesia during upper endoscopy and biopsy: a prospective, randomized study. J Anesth Clin Res. 2014;5(12). DOI:10.4172/2155-6148.1000480
- Koksal E, Ustun YB, Kaya C, et al. Use of remifentanil or dexmedetomidine with ketamine for upper gastrointestinal endoscopy. J Exp Clin Med. 2014;31(4):221–224.
- Ramkiran S, Iyer SS, Dharmavaram S, et al. BIS targeted propofol sparing effects of dexmedetomidine versus ketamine in outpatient ERCP: a prospective randomised controlled trial. J Clin Diagn Res. 2015;9(5):UC07–UC12.
- Mazanikov M, Udd M, Kylänpää L, et al. Dexmedetomidine impairs success of patient-controlled sedation in alcoholics during ERCP: a randomized, double-blind, placebo-controlled study. Surg Endosc. 2013;27(6):2163–2168.
- Nagaraj MC, Geetha CRRR. IS DEXMEDETOMIDINE A POOR SURROGATE TO PROPOFOL FOR. J Evol Med Dent Sci. 2013;2(42):8165–8175.