ABSTRACT
Background
To improve the outcome after shoulder arthroscopy, effective pain control is needed. We aimed to compare the efficacy of ultrasound (US) guided erector spinae plane block (ESPB) versus the intraarticular injection (IAI) of bupivacaine in managing postoperative pain in patients undergoing shoulder arthroscopy.
Methods
This prospective randomized, double-blind, parallel-controlled trial was conducted on sixty patients aged 18–60 years of either gender, BMI < 40 kg/m2, American Society of Anesthesiologists physical status I–II, posted for elective shoulder arthroscopy. Patients were randomly allocated into two equal groups. Group IA: received IAI using 20 ml of bupivacaine 0.25% done through the surgical port by the surgeon after closure of the shoulder capsule and sham ESBP. Group ES: received US guided ESPB at the T2 level using 20 ml bupivacaine 0.25% after the end of surgery and sham IAI.
Results
Postoperative visual analogue scale (VAS), heart rate and mean arterial blood pressure were significantly decreased at 30 min, 1 h, 2 h, 8 h, 12 h and 24 h in group ES compared to group IA. VAS increased at 4 h, 6 h in group ES compared to group IA. The time of first postoperative analgesic requirement was significantly increased in group ES compared to group IA. The total diclofenac consumption over 1st 24 hrs postoperatively was decreased in group ES compared to group IA.
Conclusion
US-guided ESPB controlled postoperative pain effectively in patients undergoing shoulder surgeries with superiority over IAI of bupivacaine.
1. Introduction
Shoulder arthroscopy is a common procedure done is orthopedics for many surgical indications as rotator cuff tears, stiffness and instability [Citation1]. This procedure has a well – documented postoperative pain. To improve the outcome after surgery, effective pain control is needed [Citation2].
There are many postoperative pain management modalities after shoulder arthroscopy including non-steroidal anti-inflammatory drugs, intraarticular injection (IAI), regional nerve blocks, patient controlled analgesia and cryotherapy [Citation3].
ggIAI of local anesthetics is a simple technique and preserves the motor function but carries a potential risk to chondrolysis [Citation4].
The erector spinae plane block (ESPB) is one of the emerging regional techniques for managing postoperative pain. ESPB has been used successfully in many surgeries such as mastectomy [Citation5], thoracotomies [Citation6], percutaneous nephrolithotomies [Citation7], lumbar fusions [Citation8], hernia repair [Citation9], cesarean delivery [Citation10] and even in total hip arthroplasty [Citation11]. However, there is paucity in the literature about the use of ESPB in shoulder surgeries [Citation12]. Also, no previous study, to our knowledge, compared ESPB with IAI of bupivacaine in shoulder arthroscopy.
Therefore, our aim was to compare the efficacy of ultrasound (US) guided ESPB versus the IAI of bupivacaine for managing acute postoperative pain in patients undergoing shoulder arthroscopy.
2. Materials and methods
2.1. Patient population and eligibility criteria
This prospective randomized double-blind parallel-controlled study was carried out after obtaining approval from the ethical committee (ID: 33,097/04/19), registration on clinicaltrals.gov (ID: NCT04483323) and written informed consent from all cases. We enrolled 60 patients aged 18–60 years of either gender, BMI < 40 kg/m2, American Society of Anesthesiologists (ASA) physical status I–II, posted for elective shoulder arthroscopy from August 2020 to March 2021. The design of trial and pain score [visual analogue scale (VAS)] were clarified for participant during the preoperative anesthesia visit.
Patients with opioids chronic use, local anesthetics allergy, coagulopathy, and the need for postoperative drain were excluded.
2.2. Randomization
Computer‐generated randomization numbers were used to randomly allocate patients into two equal groups. The sealed envelope was opened by another investigator (who had no other roles in the trial). Group IA: patients received IAI using 20 ml of bupivacaine 0.25% and sham ESBP. Group ES: patients received US guided ESPB using 20 ml bupivacaine 0.25% and sham IAI.
Both patients and outcome assesors were blinded. A dedicated anesthetist, who had no subsequent participation in the trial, prepared the study solutions and performed ESBP and IAI. Intraoperative and postoperative measurements were evaluated by another anesthetist who was blinded to assignment of groups.
2.3. Study design
After canula insertion, all patients were premedicated with intravenous (IV) midazolam 2 mg. For all case, the standard technique of general anesthesia was used. We used the standard monitoring (pulse oximetry, temperature probe, noninvasive blood pressure, 5- lead ECG, and capnography). Induction of general anesthesia was done by IV propofol 2–2.5 mg/kg and IV fentanyl 1ug/kg. After IV cisatracurium 0.15 mg/kg, endotracheal intubation was done. Maintenance of anesthesia was isoflurane (1–1.5%) with 50% oxygen. Incremental doses of IV cisatracurium 0.03 mg/Kg was given.
Insufficient analgesia was considered if there was heart rate (HR) or mean arterial blood pressure (MAP) increase > 20% than baseline values and IV fentanyl 1 ug/kg was administered.
In group IA (n = 30): IAI of 20 ml bupivacaine 0.25% was done through the surgical port by the surgeon after closure of the shoulder capsule. Sham ESBP (by 20 ml saline) was received.
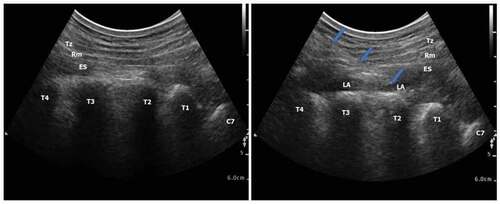
In group ES (n = 30): Sham IAI (by 20 ml saline) was done. After the end of surgery, the position of patient was in the lateral one. After disinfection of skin, the counting started from the spinous process of C7 and down. The level of T2 transverse process was identified. A 2–5 MH2 curved probe (Philips CX50 Extreme edition) was positioned transversely to visualize the lateral tip of T2 transverse Process. A longitudinal para sagittal orientation was obtained over the transverse process using a 22 – gauge 8 cm block needle (visioplex, Vygon, France) was inserted in plane to US beam in a caudal to cranial direction to place the needle tip between the posterior fascia of the erector spinae (ES) muscle and the T2 transverse process. The needle tip position was deep to the ES muscle was confirmed using hydrodissection with 0.5–1 ml of normal saline and visualization of the linear fluid spread deep to the ES muscle following which 20 ml of 0.25% bupivacaine was injected. ().
Figure 1. Ultrasound‐guided erector spinae plane block. (a) before local anesthetic injection (b) after local anesthetic injection. Tz: trapezius muscle, Rm: rhomboid major muscle, ES: erector spinae muscle, T: transverse processes of the thoracic vertebrae, C: transverse processes of the cervical vertebrae, LA: local anesthetic
At the end, all anesthetics were stopped. Extubation was performed when spontaneous breathing is adequate and following prompt reversal. Patients were transferred to the post-anesthesia care unit (PACU).
Postoperative pain (using VAS at admission), HR and MAP were measured at PACU, 30 min, 1 h, 2 hr. 4 hr, 6 hr, 8 hr, 12 hr, 18 hr, and 24 hrs postoperative. All patients in both groups were scheduled to receive paracetamol 1 gm/8 hrs. Rescue analgesia in the form of diclofenac sodium 75 mg intramuscularly (IM) was given if the VAS ≥ 40. Time to the 1st rescue analgesic request was recorded. Total amount of rescue analgesic (Diclofenac sodium) in 24 hrs. Recording of adverse events was done [e.g., nausea, vomiting, hypotension (MAP < 20% of baseline value) and bradycardia (HR < 60)].
The primary outcome was the total 24 hrs postoperative rescue analgesic consumption. Secondary outcomes were postoperative VAS along the study time as well as the time to first request of rescue analgesia.
2.4. Sample size calculation
G*Power 3.1.9.2 (Universitat Kiel, Germany) was used to calculate the sample size. We recruited 10 cases in each group and performed a pilot study. The mean difference of total 24 hrs postoperative rescue analgesic consumption was 37.5 and the common SD was 33.9. The following parameters were used: 1.10 effect size, 95% confidence limit and 95% power of the study, group ratio 1:1. Seven cases were added to each group to overcome dropout. Therefore, we recruited 25 patients in each group.
2.5. Statistical analysis
Statistical analysis was done by SPSS v25 (IBM Inc., Chicago, Il, USA). Normality of data was checked with Shaprio–Wilks test and histograms. Quantitative parametric variables were described as mean and SD and compared by unpaired student’s T test. Quantitative non-parametric variables were described as median and interquartile range (IQR) and compared between the two groups by Mann Whitney (U) test. Qualitative variables were described as frequency and percentage (%) and compared by the Chi – Square test or Fisher’s exact test when appropriate. A two tailed P value less than or equal 0.05 was adopted for the level of statistical significance.
3. Results
For enrollment, 79 patients were evaluated; 13 patients didn’t match the inclusion criteria and six patients refused to participate in the study. Randomization of 60 patients were done into two equal groups. All patients were followed up and statistically analyzed ().
Demographic data (age, sex, body mass index and ASA physical status) and duration of surgery were comparable among the two studied. ()
Table 1. Patient characteristics of both groups
Postoperative VAS was significantly decreased at 30 min, 1 h, 2 h, 8 h, 12 h and 24 h (P < 0.001, <0.001, 0.007, <0.001, <0.001 and 0.027 respectively) but increased at 4 h, 6 h (P < 0.001 and 0.039 respectively) in group ES compared to group IA. Postoperative VAS was insignificantly different between-group ES and group IA at PACU, 3 h and 18 h. ()
Table 2. Postoperative visual analog scale (VAS) in both groups
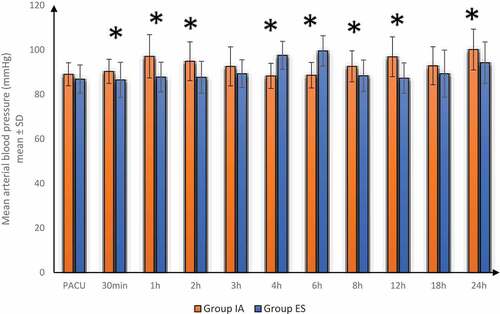
Postoperative HR and MAP were significantly lower in group ES at 30 min, 1 h, 2 h, 8 h, 12 h and 24 h but increased at 4 h, 6 h compared to group IA. Postoperative HR and MAP were insignificantly different between-group ES and group IA at PACU, 3 h and 18 h. ()
Figure 3. Postoperative heart rate in both groups
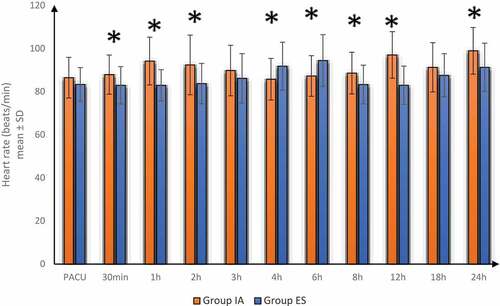
Figure 4. Postoperative mean arterial blood pressure in both groups
The time of first analgesic requirement was significantly prolonged in group ES compared to group IA [median (IQR): 5 (4–6 h) vs 2 (1–2.75hrs), P < 0.001]. The total diclofenac consumption over 1st 24 hrs postoperatively was lower in group ES compared to group IA [median (IQR): 150 (75–150 mg) vs 225 (168.75–225 mg), P < 0.001]. ()
Table 3. Time of first analgesic requirement and total diclofenac consumption at 1st 24 hours in both groups
As regards complications, postoperative nausea and vomiting occurred in two patients in group ES and four patients in group IA (P = 0.389), hypotension occurred in one patient in group ES and two patients in group IA (P > 0.99).
4. Discussion
ESPB has established itself as a useful technique for regional anesthesia in a variety of thoracic, abdominal, and lower limb operations. ESPB became popular because it is an easy technique with low incidence of severe adverse events.
ESPB has a role in effective multimodal analgesia regimens and Enhanced Recovery after Surgery (ERAS) [Citation13].
There are only case reports regarding ESPB in managing postoperative pain after shoulder surgery without obvious guidelines or randomized clinical trials. Several factors may affect the outcome of block; block related (such as volume and concentration of local anesthetics), surgery related (such as type of surgery), patient related (such as individual variations of the anatomy) [Citation14].
In our study, postoperative VAS, HR and MAP were significantly lower at 30 min, 1 h, 2 h, 8 h, 12 h and 24 h but were increased at 4h, 6 h in group ES compared to group IA and were insignificantly different between-group ES and group IA at PACU, 3 h and 18 h. The time of first postoperative analgesic requirement was significantly increased and the total diclofenac consumption over 1st 24hrs postoperatively was decreased in group ES compared to group IA. ES group had better analgesia; therefore, VAS, HR and MAP were significantly decreased at 30 min, 1 h, 2 h, 8 h, 12 h and 24h in group ES compared to group IA. However, VAS, HR and MAP increased at 4h, 6 h in group ES compared to group IA as group IA required analgesia earlier and was covered by the effect of analgesia at 4h and 6 h resulting in higher VAS as group ES started to need analgesia.
Forero et al. managed chronic shoulder pain in one case by performing ESPB at T2/T3 level. After the block, range of motion improved with good control of pain. The motor function was preserved, and the sensory block was in the cervico-thoracic dermatomes. The radiocontrast spread up to the level of C3 as shown by computed tomography imaging [Citation15].
In agreement with our results, Ciftci et al. [Citation12] in a randomized prospective double-blind study demonstrated that ESPB at the T2 level can offer effective analgesia after shoulder arthroscopy. As ESPB decreased postoperative fentanyl consumption, the need for rescue analgesia and the pain score compared to sham block.
Also, a case report done by Selvi et al. [Citation14] demonstrated that ESPB at T2 managed postoperative pain in shoulder surgeries effectively. Also. Papa et al. [Citation16] case report showed that ESPB can be used for treating upper extremity cancer pain with preserving the motor power.
Moreover, many case reports [Citation17–20] showed that high thoracic ESPB can be used in shoulder surgeries with phrenic nerve sparing.
In a study done on a cadaver, ESPB injection led to LA anterior penetration to the spinal nerves [Citation21]. Mechanism of action of ESPB may be explained by placement of LA near the costotransverse foramina where the origin of both the dorsal and ventral e spinal nerves rami [Citation22].
A recent metanalysis [Citation23] stated that US guided ESPB decreased the postoperative 24 h opioid consumption.
Additional studies including a large number of patients are required for generalization of these results. Also, further studies are needed for assessment of using bupivacaine in different concentrations and with different additives.
Further studies are needed to compare thoracic with cervical ESPB. A cadaveric study [Citation24] showed that US-guided ESPB at C6 and C7 stained the roots of the brachial plexus and dorsal rami. Also, a case report [Citation25] demonstrated that ESPB at C7 controlled post-shoulder disarticulation acute pain. Moreover, a case report for forequarter amputation [Citation26] showed that insertion of ESPB catheter threaded from the thoracic region to cervical region was an effective, method.
5. Conclusion
US-guided ESPB controlled postoperative pain effectively in patients undergoing shoulder surgeries with superiority over IAI.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Hill JR, McKnight B, Pannell WC, et al. Risk factors for 30-day readmission following shoulder arthroscopy. Arthroscopy. 2017 Jan;33(1):55–61.
- Desai N. Postoperative analgesia for shoulder surgery. Br J Hosp Med (London, England: 2005). 2017 Sep 2; 78(9):511–515.
- Hurley ET, Maye AB, Thompson K, et al. Pain control after shoulder arthroscopy: a systematic review of randomized controlled trials with a network meta-analysis. Am J Sports Med. 2020;15:363546520971757.
- Beaudet V, Williams SR, Tétreault P, et al. Perioperative interscalene block versus intra-articular injection of local anesthetics for postoperative analgesia in shoulder surgery. Reg Anesth Pain Med. 2008 Mar-Apr;33(2):119–120.
- Leong RW, Tan ESJ, Wong SN, et al. Efficacy of erector spinae plane block for analgesia in breast surgery: a systematic review and meta-analysis. Anaesthesia. 2021 Mar;76(3):404–413.
- Elsabeeny WY, Ibrahim MA, Shehab NN, et al. Serratus anterior plane block and erector spinae plane block versus thoracic epidural analgesia for perioperative thoracotomy pain control: a randomized controlled study. J Cardiothorac Vasc Anesth. 2021 Jan 4;35(10):2928–2936.
- Abd Ellatif SE, Abdelnaby SM. Ultrasound guided erector spinae plane block versus quadratus lumborum block for postoperative analgesia in patient undergoing open nephrectomy: a randomized controlled study.Egypt J Anaesth. 2021 [2021 January 01];37(1):123–134.
- Zhu L, Wang M, Wang X, et al. Changes of opioid consumption after lumbar fusion using ultrasound-guided lumbar erector spinae plane block: a randomized controlled trial. Pain Physician. 2021 Mar;24(2):E161–e168.
- Abu Elyazed MM, Mostafa SF, Abdelghany MS, et al. Ultrasound-guided erector spinae plane block in patients undergoing open epigastric hernia repair: a prospective randomized controlled study. Anesth Analg. 2019 Jul;129(1):235–240.
- Hamed MA, Yassin HM, Botros JM, et al. Analgesic efficacy of erector spinae plane block compared with intrathecal morphine after elective cesarean section: a prospective randomized controlled study. J Pain Res. 2020;13:597–604.
- Lennon MJ, Isaac S, Currigan D, et al. Erector spinae plane block combined with local infiltration analgesia for total hip arthroplasty: a randomized, placebo controlled, clinical trial. J Clin Anesth. 2021 May;69:110153.
- Ciftci B, Ekinci M, Gölboyu BE, et al. High thoracic erector spinae plane block for arthroscopic shoulder surgery: a randomized prospective double-blind study. Pain Med. 2021 Apr 20;22(4):776–783.
- Kot P, Rodriguez P, Granell M, et al. The erector spinae plane block: a narrative review. Korean J Anesthesiol. 2019 Jun;72(3):209–220.
- Selvi O, Tulgar S, Ozer Z. Case report presentation of ultrasound-guided erector spinae plane block in shoulder surgery: three patients and two different results. Cureus. 2018;10(11):150–165.
- Forero M, Rajarathinam M, Adhikary SD, et al. Erector spinae plane block for the management of chronic shoulder pain: a case report. Can J Anaesthesia. 2018 Mar;65(3):288–293.
- Papa P, Antunez-Maciel M, Asenjo JF. Cancer shoulder pain treated with a neurolytic erector spinae plane block. Can J Anaesthesia. 2020 Sep;67(9):1262–1263.
- Ma W, Sun L, Ngai L, et al. Motor-sparing high-thoracic erector spinae plane block for proximal humerus surgery and total shoulder arthroplasty surgery: clinical evidence for differential peripheral nerve block? Can J Anaesthesia. 2019 Oct;66(10):1274–1275.
- Tsui BCH, Ip VHY. Can electrical nerve stimulation guidance assist in cervical erector spinae plane block catheter placement for total shoulder arthroplasty?Can J Anesth. 2019 [2019 November 01];66(11):1417–1418.
- Nair A, Diwan S. Erector spinae block as a phrenic nerve sparing block for shoulder surgeries. Reg Anesth Pain Med. 2020; rapm-2019-101230.
- Tsui BCH, Sun LY, Ip VHY, et al. Diaphragm-sparing erector spinae plane block for shoulder surgery: emerging evidence. Reg Anesth Pain Med. 2021 Mar;46(3):287–288.
- Forero M, Rajarathinam M, Adhikary S, et al. Continuous erector spinae plane block for rescue analgesia in thoracotomy after epidural failure: a case report. A & A Case Rep. 2017 May 15;8(10):254–256.
- Hamilton DL, Manickam B. Erector spinae plane block for pain relief in rib fractures. Br J Anaesth. 2017 Mar 1;118(3):474–475.
- Huang J, Liu J-C. Ultrasound-guided erector spinae plane block for postoperative analgesia: a meta-analysis of randomized controlled trials.BMC Anesthesiol. 2020 [2020 April 14];20(1):83.
- Elsharkawy H, Ince I, Hamadnalla H, et al. Cervical erector spinae plane block: a cadaver study. Reg Anesth Pain Med. 2020 Jul;45(7):552–556.
- Hamadnalla H, Elsharkawy H, Shimada T, et al. Cervical erector spinae plane block catheter for shoulder disarticulation surgery. Can J Anaesthesia. 2019 Sep;66(9):1129–1131.
- Tsui BCH, Mohler D, Caruso TJ, et al. Cervical erector spinae plane block catheter using a thoracic approach: an alternative to brachial plexus blockade for forequarter amputation. Can J Anaesthesia. 2019 Jan;66(1):119–120.