ABSTRACT
Background and Aims
Laparoscopic cholecystectomy (LC) is favored by less postoperative pain compared with the open approach; nevertheless, pain is still a frequent complaint. Dexmedetomidine is a centrally acting α2 agonist that has sedative, sympatholytic, and analgesic properties. We aimed primarily to study the effect of different doses of dexmedetomidine on the quality of anesthesia in the patients undergoing LC. The secondary aims were to detect the implications of these different doses on the postoperative outcome (postoperative pain, nausea, and vomiting.
Methods
Sixty patients of the American Society of Anesthesiologists (ASA) physical grades I and II scheduled for elective LC were randomly divided into three equal groups. Group Dex 0.2, Group Dex 0.4, and Group Dex 0.6 (patients received dexmedetomidine infusion at 0.2 mcg/kg/h, 0.4 mcg/kg/h, and 0.6 mcg/kg/h, respectively) 15 min before induction and throughout the surgical procedure. Hemodynamic parameters, spontaneous respiratory recovery time, extubation time, incidence of cough, postoperative pain, and postoperative nausea and vomiting (PONV) were recorded.
Results
Significantly attenuated hemodynamic stress response was observed in Dex 0.4 and Dex 0.6 groups. The incidence of cough, PONV, was significantly less and postoperative analgesic requirements were fewer in Dex 0.6 group compared to the other two groups. Nevertheless, the time of spontaneous respiratory recovery and extubation in Dex 0.6 group was insignificantly longer compared to the other two groups.
Conclusion
Intravenous infusion of 0.6 μg/kg/h dexmedetomidine before induction can attenuate hemodynamic stress response, reduce cough incidence, PONV, and postoperative analgesic requirements in patients undergoing LC without significant prolongation of spontaneous respiratory recovery time.
1. Introduction
While the first laparoscopic cholecystectomy (LC) procedure was performed by Professor Erich Mühe in Germany on 12th of September 1985, [Citation1] LC is currently the most commonly performed abdominal surgical procedures. [Citation2]
LC is favored by less postoperative pain compared with the open approach; nevertheless, pain is still the main postoperative complaint that sometimes could lengthen hospital stay. [Citation3] Incidence of moderate postoperative abdominal and shoulder pain is reported to occur in about 60% of the patients following LC, while 10% experience a severe degree of pain. [Citation4] Opioids are commonly used in postoperative pain relief after LC. However, opioids have many side effects including respiratory depression, nausea and vomiting, ileus, and urinary retention, which may overshadow the analgesic advantages, especially after abdominal surgery. [Citation5]
Creation of pneumoperitoneum is a principal step of any laparoscopic procedure; this is usually achieved through carbon dioxide insufflation into the peritoneal cavity at a rate of 4–6 l/min to attain a pressure of 10–15 mmHg. [Citation6] Consequently, the increase of intra-abdominal pressure (IAP) could lead to hemodynamic instability and the incidence of postoperative nausea and vomiting (PONV). [Citation7]
Dexmedetomidine (Dex) is a highly selective centrally acting alpha-2-adrenoceptor agonist, which has a sedative, anxiolytic, perioperative sympatholytic, and analgesic sparing effects. [Citation8] Dexmedetomidine is approved at the end of 1999 by the Food and Drug Administration (FDA) as a potential nonopioid sedative agent with analgesic properties, which attenuates hemodynamic responses to intubation, pneumoperitoneum, and reduces opioid-related adverse events. [Citation9]
The primary aim of this study is to assess the effect of different doses of dexmedetomidine on quality of anesthesia in patients undergoing LC. The secondary aim is to detect the effect of these different doses on postoperative outcome (postoperative pain, nausea, and vomiting).
2. Materials and methods
After the approval of institutional ethical committee. A randomized, prospective, controlled double-blinded clinical study enrolled 60 adult patients scheduled for elective LC. Patients were 18–60 years and their body mass index (BMI) <30 kg/m2. ASA physical classification status was I–II. Patients suffered from PONV, motion sickness, gastroparesis, bradycardia, atrioventricular block, severe cardiac dysfunction, diabetes, hypertension, coronary heart disease, liver and kidney function seriously damaged, chronic pain, upper respiratory tract infection, asthma, smoking, and allergic to dexmedetomidine were excluded from the study.
A written informed consent was obtained from the participating patients in this study. The sample size was approved to be sufficient by the Department of Statistics, Medical Research Institute, Alexandria University, Egypt. [Citation10]
The preoperative assessment was accomplished by detailed history, clinical examination, and routine laboratory investigations. All patients were fasting according to the American Society of Anesthesiology guidelines. [Citation11]
The patients were randomly distributed by closed envelope technique into three equal groups, Group Dex 0.2 (patients received dexmedetomidine infusion 0.2 mcg/kg/h), Group Dex 0.4 (patients received dexmedetomidine infusion 0.4 mcg/kg/h), and Group Dex 0.6 (patients received dexmedetomidine infusion 0.6 mcg/kg/h).
2.1. Anesthetic technique
According to the group, dexmedetomidine infusion was prepared in a different room by mixing 1 ml of Dex containing 100 μg of the drug with 50 ml of normal saline, so 2 μg/kg final concentration resulted, then Dex infusion was given via a B Braun Infusomat Space infusion pump. According to the weight of the patient, the pump was set to deliver the targeted infusion rate, then concealed using colored label. Thus, the assessor and the patient were unaware of the group.
On arrival to the operation theater, a multichannel monitor was attached to the patient HR, systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse oximetry (SpO2), end-tidal carbon dioxide (ETCO2), and bispectral index (BIS) were recorded.
Two wide bore intravenous cannulas were inserted one for the intravenous fluids, and another line was secured for the infusion pump. Fifteen minutes after initiating Dex infusion, preoxygenation was done for 3 min. Induction of general anesthesia was attained by intravenous midazolam 0.03 mg/kg, propofol 1.5–2 mg/kg, fentanyl 1–2 mcg/kg, and rocuronium 0.6 mg/kg. Tracheal intubation was carried out and mechanically controlled ventilation initiated with the tidal volume was 8 ml/kg, the respiratory rate was 14 breath/min and the inhalation/ exhalation ratio was 1:2. Respiratory parameters modified to maintain ETCO2 at 35–45 mmHg and SpO2 ≥ 98%.
Intraoperative maintenance of anesthesia done with sevoflurane and incremental doses of 0.2 mg/kg rocuronium to keep BIS values at 40–60. During surgery, all patients were positioned in the reverse Trendelenburg with left lateral inclination 15°, and abdominal pressure maintained at 12 mmHg.
At the end of surgery dexmedetomidine infusion and anesthetic agents were stopped, then extubation was carried out after reversal of muscle relaxation with sugammadex 2 mg/kg, and the patient transferred to the postanesthesia care unit (PACU). Nonsteroidal anti-inflammatory drugs (100 mg/day ketoprofen) and paracetamol (3 g/day) were administered intravenously to achieve multimodal postoperative analgesia. [Citation12]
2.2. Surgical technique
LC was carried on using the original technique described Reddick & Olsen, this technique entails positioning of the patient and surgical team in specific order, closed technique for creation of pneumoperitoneum, insertion of four trocars, wide display of calot’s triangle, and infundibular dissection first technique. [Citation13]
Demographic information (age, BMI, sex), hemodynamic measurements including HR, SBP, DBP, and peripheral oxygen saturation (SPO2) were logged at the time of arrival at the operating theater (baseline value), immediate after intubation, with start of surgical manipulation, starting insufflation, every 15 min intraoperative, immediate after extubation, and every 6 h postoperatively.
According to American College of Cardiology/American Heart Association (ACC/AHA) perioperative hypotension defined as reduction of the blood pressure more than 20% of the base value, that continues for longer than 15 min treated with immediate use of 6–10 mg of intravenous ephedrine, while hypertension defines as an upsurge of the blood pressure more than 20% of the base value, that lasts more than 15 min managed with nitroglycerin intravenous infusion 5 μg/min administered initially, then titrated in 5 μg/min increments [Citation14], whereas bradycardia defined as drop of the HR less than 50 beat per minute (bpm) treated with 0.5 mg of intravenous atropine conversely, tachycardia defined as increase of the HR more than 110 bpm managed primarily with correction of triggering factors as hypovolemia and inadequate depth of anesthesia, if tachycardia persisted 10 mg of intravenous esmolol required [Citation15].
Operative time was recorded from skin incision to dressing, spontaneous respiratory recovery time noted down from discontinuation of inhalational anesthetic agent to spontaneous respiratory effort regain, and extubation time measured from discontinuation of inhalational anesthetic agent till endotracheal tube removal.
On recovery incidence and severity of cough were assessed and recorded in grades: (grade 0: no cough; grade 1: mild, single cough; grade 2: moderate, frequent cough, lasting time <5 s, no effect on extubation; grade 3: severe, continuous cough, lasting time ≥5 s, affecting extubation).
Postoperative pain was assessed using visual analog scale [Citation16], when the VAS ≥4 so 0.5 μg/kg intravenous fentanyl was given and time to first rescue analgesic requirement was documented with total amount of analgesic drug required during first 24 h postoperatively. Postoperative nausea and vomiting (PONV) were evaluated with a 4 points scale (1 = absent, 2 = nausea, 3 = retching, and 4 = vomiting) and treated with 4 mg of intravenous ondansetron administration per time. Both postoperative pain and PONV were noted down at 20 min (t1), 2 h (t2), 6 h (t3), 12 h (t4), and 24 h (t5) postoperatively.
The results were tabulated, and statistical data were analyzed using IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp), Fisher’s exact test was used for qualitative variables comparison, while paired t-test used to compare normally distributed quantitative variables within the group against baseline values. ANOVA test was used to compare continuous variables between three groups; if ANOVA was significant, post‑hoc test was used to compare two groups and the results were expressed as mean ± standard deviation. p > 0.05 was considered insignificant, <0.05 as significant, and highly significant if <0.001.
3. Results
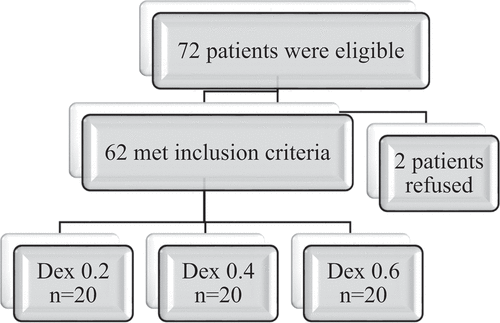
A total of 72 patients were screened for eligibility; 62 patients met the inclusion criteria and were approached to participate, and 2 of them refused participation in the study, .
There were statistically insignificant differences regarding demographic data and operation time among three groups. However, the time of spontaneous respiratory recovery and extubation in Dex 0.6 group was insignificantly longer compared to Dex 0.2 and Dex 0.4 groups (p value = 0.070 and 0.053, respectively) as shown in .
Table 1. Demographic data and clinical characteristics in three groups
At the time of arrival at the operating theater hemodynamic measurements including HR, SBP, and DBP (baseline values) had statistically insignificant differences between three groups (p value = 0.647, 0.948, and 0.977, respectively). HR immediately after intubation, at the start of surgical manipulation, and immediately after extubation showed significant increase in Dex 0.2 group compared to the other two groups (p value = 0.026, 0.028, and 0.003, respectively), . Similarly, a significant increase of SBP and DBP was noted in Dex 0.2 group, . Additionally, a statistically significant decrease of HR, SBP, and DBP were observed in Dex 0.2 group during insufflation compared to Dex 0.4 and Dex 0.6 group (p value = 0.045, 0.015, and 0.012, respectively). An insignificant hemodynamic differences were recorded either intraoperative during pneumoperitoneum or postoperatively between three groups.
Table 2. Heart rate changes in three groups at different times
Table 3. Systolic blood pressure changes in three groups at different times
Table 4. Diastolic blood pressure changes in three groups at different times
During emergence the incidence of cough was significantly lower in Dex 0.6 group compared to other groups (20% in Dex 0.6 vs. 65% and 55% in Dex 0.2 and Dex 0.4 groups, respectively, p value = 0.013). Nevertheless, no statistically significant difference was recorded regarding degree of severity of cough between the three groups, .
Table 5. The incidence of cough in four groups during emergence
As regards postoperative pain, Dex 0.6 group showed a better analgesic effect with significantly lower VAS compared to the other two groups at different time points. The time to first rescue analgesia was longer (245 min) in group Dex 0.6 compared to Dex 0.4 and Dex 0.2 groups (180 min and 150 min, respectively). Furthermore, the first 24 h postoperative total analgesic requirement was significantly lower in Dex 0.6 group in comparison to other groups (42.5 ± 17.3 mg in Dex 0.6 group vs. 122.2 ± 12.4 mg, 84.7 ± 14.1 mg in Dex 0.2 and Dex 0.4 groups, respectively, p value = 0.000), .
Table 6. Comparison of VAS at different time points in the three groups
The results of the present study showed statistically insignificant difference in the incidence of PONV among the three groups except at 12 h postoperatively. Group Dex 0.2 showed significant increase of PONV incidence compared to the other two groups with p value = 0.018, . In group Dex 0.2, the scale of PONV ranged between 2 and 4 points with a mean of 2.4 ± 1.3 points. On the other hand, it ranged between 2 and 3 points in group Dex 0.4 with a mean of 1.9 ± 1.05 points, while it ranged between 1 and 2 points in group Dex 0.6 with a mean of 1.6 ± 0.84 points. PONV score was significantly higher in group Dex 0.2 with p value of 0.007. There were no recorded adverse reactions among the three groups.
Table 7. The incidence of PONV in four groups at different time points
4. Discussion
More than 230 million people scheduled for surgical intervention every year worldwide. Intraoperative stress response lead to disruption of the hypothalamic–pituitary–adrenal (HPA) axis and relative glucocorticoid impairment which are considered a contributor to perioperative organ injury. [Citation17]
The present results showed that perioperative intravenous infusion of dexmedetomidine 0.4 μg/kg/h and 0.6 μg/kg/h attenuated hemodynamic stress response during intubation, with start of surgical manipulation, pneumoperitoneal insufflation and extubation in patients undergoing LC, Weerink et al. [Citation18] studied pharmacokinetics and pharmacodynamics of dexmedetomidine and concluded that dexmedetomidine could activate the medullary vasomotor center receptors and inhibit epinephrine and norepinephrine release to achieve intraoperative hemodynamic stability. Moreover, Niyogi et al. [Citation19] documented that intravenous 0.5 μg/kg/h DEX can attenuate the hemodynamic stress responses of laryngoscopy and intubation.
Postoperative pain caused by either inflammation or neural tissue damage is accompanied by increased complication rate and extended hospital stay. [Citation20] Multimodal analgesia implies the concept of different analgesics combination to improve analgesia, reduce opioids requirement (opioid sparing effect), and thereby reduce opioids side effects. [Citation21]
The present randomized controlled study found that patients received Dex 0.6 μg/kg/h experienced better analgesic effect with significantly lower VAS, longer time to first rescue analgesia and lower total analgesic requirement compared to other groups. A meta-analysis verified that dexmedetomidine could reduce inflammatory mediators and substance P induced by surgical trauma and thereby reduces postoperative analgesic requirements. [Citation22]
Kang et al. [Citation23] suggested that intraoperative dexmedetomidine infusion decreases incidence of emergence agitation (EA), rescue analgesia, and shivering in adults after lung surgery.
General anesthesia and endotracheal intubation increase postoperative incidence of cough which could be severe enough to cause wound dehiscence. During emergence, the present study found that the incidence of cough was significantly lower in Dex 0.6 group compared to other two groups.
Kim et al. [Citation24] documented that continuous infusion of 0.5 μg/kg dexmedetomidine could slightly reduce the incidence of cough; however, the incidence was still about 70%. Conversely, Aouad et al. [Citation25] agreed with the current study and concluded that increased doses of intravenous dexmedetomidine infusion on recovery could reduce the incidence of cough, while 0.5 μg/kg/h dexmedetomidine had insignificant inhibitory effect on cough.
Postoperative nausea and vomiting (PONV) are considered one of the most distressing symptoms that follow surgery under general anesthesia, with an incidence reaching as high as 30%. However, the incidence of PONV is higher after LC compared to other types of surgery and the peak of PONV incidence in patients with LC specially at 6 h and 12 h postoperative interval. [Citation26]
The results of the present study verified that incidence of PONV increased significantly after 6 h postoperative in group Dex 0.2 patients compared to other groups. Bakri et al. [Citation27] noted that intravenous infusion of dexmedetomidine 1 μg/kg preoperative could reduce the incidence of PONV in patients undergoing LC. Latest studies concluded that the incidence of PONV could be significantly reduced by perioperative dexmedetomidine 0.6 μg/kg/h infusion. [Citation28]
LC is a surgical procedure with a relatively short duration, the operative time is 45 min on the average in straightforward cases. Many studies documented that high DEX infusion rates associated with prolonged spontaneous respiratory recovery time and extubation time. [Citation29] The present results showed that the spontaneous respiratory recovery time and extubation time increased insignificantly in the Dex 0.6 group compared with other groups.
5. Conclusion
Intravenous infusion of 0.6 μg/kg/h dexmedetomidine before induction can attenuate hemodynamic stress response, reduce cough incidence, PONV, and postoperative analgesic requirements in patients undergoing LC without significant prolongation of spontaneous respiratory recovery time.
Declaration of patient consent
The authors confirm that they have obtained all applicable patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be described in the journal. The patients recognize that their names and initials will not be published, and due efforts will be made to obscure their identity, but secrecy cannot be guaranteed.
Ethics approval and consent to participate
The present study was permitted by the ethical committee of Faculty of Medicine, Alexandria University (IRB no. 00007555, FWA no. 00018699). A consent to participate was attained as well.
Consent for publication
Written informed consent was achieved from the parents for publication of this article and any accompanying tables/images. The copies of the written consents are accessible for review by the Editor of this journal upon request.
Availability of data and material
All data supporting the study are offered in the manuscript or obtainable upon request.
Funding
The authors did not receive any funding support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Reynolds W Jr. The first laparoscopic cholecystectomy. JSLS. 2001 Jan-Mar;5(1):89–94.
- Gaillard M, Tranchart H, Lainas P, et al. New minimally invasive approaches for cholecystectomy: review of the literature. World J Gastrointest Surg. 2015;7(10):243–248.
- Jabbour-Khoury SI, Dabbous AS, Gerges FJ, et al. Intraperitoneal and intravenous routes for pain relief in laparoscopic cholecystectomy. JSLS. 2005;9:316–321.
- Bisgaard T, Warltier D. Analgesic treatment after laparoscopic cholecystectomy: a critical assessment of the evidence. Anesthesiology. 2006;104(4):835–846.
- Mitra S, Khandelwal P, Roberts K, et al. Pain relief in cholecystectomy–a review of the current options. Pain Pract. 2012;12(6):485–496.
- Gharaibeh H. Anaesthetic management of laparoscopic surgery. East Mediterr Health J. 1998;4(1):185–188.
- O’Malley C, Cunningham AJ. Physiologic changes during laparoscopy. Anesthesiol Clin N Am. 2001;19(1):1–19.
- Andjelkovic L, Novak-Jankovic V, Pozar-Lukanovic N, et al. Influence of dexmedetomidine and lidocaine on perioperative opioid consumption in laparoscopic intestine resection: a randomized controlled clinical trial. J Int Med Res. 2018;46(12):5143–5154.
- Tufanogullari B, White PF, Peixoto MP, et al. Dexmedetomidine infusion during laparoscopic bariatric surgery: the effect on recovery outcome variables. Anesth Analg. 2008;106(6):1741–1748.
- Manne GR, Upadhyay MR, Swadia VN. Effects of low dose dexmedetomidine infusion on haemodynamic stress response, sedation and postoperative analgesia requirement in patients undergoing laparoscopic cholecystectomy. Indian J Anaesth. 2014;58(6):726–731.
- American Society of Anesthesiologist Committee., Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists task force on preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration. Anesthesiol. 2017;126:376–393.
- Gan TJ, Habib AS, Miller TE, et al. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin. 2014;30(1):149–160.
- Reddick EJ, Olsen D, Spaw A, et al. Saye W Safe performance of difficult laparoscopic cholecystectomies. Am J Surg. 1991 Mar;161(3):377–381.
- Eagle KA, Berger PB, Calkins H, et al. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee to update the 1996 guidelines on perioperative cardiovascular evaluation for noncardiac surgery). J Am Coll Cardiol. 2002;39:542–553.
- Dua N, Kumra VP. Management of perioperative arrhythmias. Indian J Anaesth. 2007;51:310–323.
- Duncan GH, Bushnell MC, Lavigne GJ. Comparison of verbal and visual analogue scales for measuring the intensity and unpleasantness of experimental pain. Pain. 1989;37(3):295–303.
- Miller T, Gibbison B, Russell GM. Hypothalamic–pituitary–adrenal function during health, major surgery, and critical illness. BJA Educ. 2017;17(1):16–21.
- Weerink MAS, Struys MMRF, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893–913.
- Niyogi S, Biswas A, Chakraborty I, et al. Attenuation of haemodynamic responses to laryngoscopy and endotracheal intubation with dexmedetomidine: a comparison between intravenous and intranasal route. Indian J Anaesth. 2019 Nov;63(11):915–923.
- Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. 2008;372(9633):139–144.
- Young A, Buvanendran A. Recent advances in multimodal analgesia. Anesthesiol Clin. 2012;30(1):91–100.
- Wang X, Liu N, Chen J, et al. Effect of intravenous dexmedetomidine during general anesthesia on acute postoperative pain in adults: a systematic review and meta-analysis of randomized controlled trials. Clin J Pain. 2018;34(12):1180–1191.
- Kang X, Tang X, Yu Y, et al. Intraoperative dexmedetomidine infusion is associated with reduced emergence agitation and improved recovery profiles after lung surgery: a retrospective cohort study. Drug Des Devel Ther. 2019;13:871–879.
- Kim JH, Ham SY, Kim DH, et al. Efficacy of single-dose Dexmedetomidine combined with low-dose Remifentanil infusion for cough suppression compared to high-dose Remifentanil infusion: a randomized, controlled, non-inferiority trial. Int J Med Sci. 2019;16(3):376–383.
- Aouad MT, Zeeni C, Al Nawwar R, et al. Dexmedetomidine for improved quality of emergence from general anesthesia: a dose-finding study. Anesth Analg. 2019;129(6):1504–1511.
- Acalovschi I. Postoperative nausea and vomiting. Curr Anaesth Crit Care. 2002;13(1):37–43.
- Bakri MH, Ismail EA, Ibrahim A. Comparison of dexmedetomidine and dexamethasone for prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy. Korean J Anesthesiol. 2015;68(3):254–260.
- Chilkoti GT, Karthik G, Rautela R. Evaluation of postoperative analgesic efficacy and perioperative hemodynamic changes with low dose intravenous dexmedetomidine infusion in patients undergoing laparoscopic cholecystectomy - a randomised, double-blinded, placebo-controlled trial. J Anaesthesiol Clin Pharmacol. 2020;36(1):72–77.
- Jones CR. Perioperative uses of dexmedetomidine. Int Anesthesiol Clin. 2013;51(2):81–96.