ABSTRACT
Background
Fluid management during thoracic anaesthesia is a challenge for the anaesthesiologists. The “safe zone” between volume overload (pulmonary oedema risk) and hypovolemia (renal failure risk) remains to be narrow and hard to determine.
Purpose
The aim of the present study was to assess goal-directed fluid therapy using cardiometry versus restrictive fluid therapy during one-lung ventilation in patients planned for having thoracic surgery.
Methods
After receiving the Ethics Committee approval and taking an informed written consent, a prospective randomized study was conducted. Fifty adult patients of both genders were scheduled for thoracic surgery with one lung ventilation.
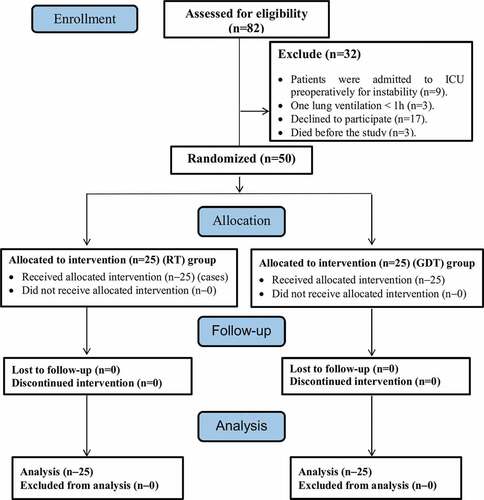
Patients were randomly categorized using closed envelope technique into two equal groups (25 patients each). Group I: a number of 25 patients underwent thoracic surgery and were managed with goal directed fluid therapy using intraoperative cardiometry. Group II: a number of 25 patients underwent thoracic surgery and were managed with intraoperative restrictive fluid therapy.
Results
There was a significantly increased serum lactate level, serum creatinine with decreased urine output in restrictive therapy group (RT) by the end of the surgery, immediately postoperative and 6 h postoperative (p value of <0.001). The restrictive hypoxic index values were much higher than the goal directed group values, yet, both were of normal range and no patient suffered of lung injury. A significant increase in serum neutrophil gelatinase-associated lipocalin (Ngal) in the restrictive therapy group compared to the goal directed therapy group. (p value of <0.001).
Conclusion
From these results, we concluded that patients undergoing thoracic surgery are preferably to be maintained over goal directed therapy protocol using electrical cardiometry. Fluid restriction is better to be individualized and aided by new technologies based on beat-to-beat variations.
1. Introduction
Fluid therapy is an important and life-saving part of perioperative management. The therapeutic goal of fluid therapy is to improve preload leading to optimized stroke volume and cardiac output [Citation1].
Thoracic surgeries are from the major surgeries that have a mortality risk of 1% to 7% caused by how much of lung tissue compromized. This risk is also augmented by surgical trauma caused by the removal of lung tissue, single lung ventilation and the worsening in pulmonary vascular resistance adds another risk [Citation2].
The excess in fluid administration and pulmonary oedema after thoracic surgery were reported. So, the fluid (amount and type) given around thoracic surgery (during and after) has been a challenge [Citation2].
One lung ventilation (OLV) poses a huge challenge to the anaesthesiologist. OLV is not a must for thoracic procedures, yet, lung injury induced by one-lung ventilation is increasingly becoming a concern. PEEP and low tidal volume ventilation are recommended as important lung protective measures together with low oxygen concentration [Citation2].
Perioperative fluid therapy has a direct effect on surgical outcome. Prescriptions should be individualized to the needs of the patient as regards fasting, anaesthesia and surgery [Citation3].
The goal-directed therapy (GDT) uses cardiac output and its parameters as a guide for fluid administration in order to improve tissue perfusion and oxygenation by increasing oxygen delivery. It was used in surgical patients to get normal or supernormal values of cardiac output and oxygen delivery and avoid the oxygen debt due to the perioperative rise in oxygen consumption. Several meta-analysis, clinical trials, and reviews showed its effectiveness, so, many societies published many guidelines that proposed its use in high-risk surgical patients [Citation4].
With the new advances in haemodynamic monitoring devices, the utilization of cardiovascular measurements as stroke volume variations (SVV) or pulse pressure variations (PVV) has been recommended along with applying algorithms for maximizing cardiac output (COP) and oxygen delivery [Citation4].
2. Patient and methods
After receiving approval from the Ethics Committee of the Faculty of Medicine and an informed written consent from patients, a prospective randomized study was performed. Fifty patients of both genders, according to sample size calculation, adult age (18–60) years, physical status American Society of Anaesthesiologists (ASA) I and II who were scheduled for thoracic surgery using one lung ventilation, admitted to Alexandria Main University Hospital. All the operations were conducted by the same surgical team.
Patients were randomly categorized using closed envelope technique into 2 equal groups (25 patients each as calculated by the biostatistical department in the high institute of public health, Alexandria University): goal-directed fluid therapy group using cardiometry and restrictive fluid therapy (RT) group. Patients with an ASA grade III and more, obesity class II (body mass index ≥35 kg/m2), pregnancy, frequent ventricular extra-systoles and haemodynamic instability, one-lung ventilation less than 1 h together with hepatic, renal or cardiac patients were excluded ().
The primary outcome was to evaluate and study the effects of goal-directed fluid therapy versus restrictive fluid therapy on lung, kidney and systemic perfusion during one-lung ventilation in thoracic surgery. The secondary outcome is assessing the effects of both fluid therapies on haemodynamic response.
Standard general anaesthesia was induced by intravenous fentanyl (2 µg/kg), propofol (2.5 mg/kg) and rocuronium (0.6 mg/kg) to facilitate endotracheal intubation. A left-sided double-lumen tube was inserted and adjusted using a fiberoptic bronchoscope. Anaesthesia will be maintained with isoflurane (1 Mac). Incremental doses of rocuronium will be used for further relaxation.
In goal-directed fluid therapy group using cardiometry, the non-invasive cardiac output monitoring device (ICON, Osypka Medical, Berlin, Germany) will be attached to the patient using four electrodes. Two electrodes will be on the left side of the neck, the two other electrodes on the left side of the chest opposite to the xiphoid process at the mid-axillary line.
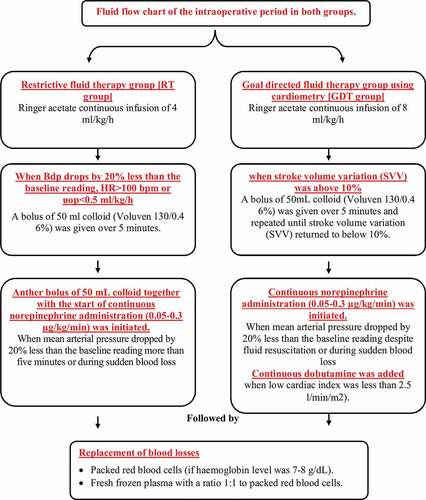
Fluid management in both groups was maintained as demonstrated in the flow chart in . Haemodynamic parameters were monitored continuously including heart rate (Beats/min), invasive measurement of mean arterial blood pressure (in mmHg) and oxygen saturation (SpO2%) along with end tidal carbon dioxide (mmHg). All previous parameters were continuously monitored and recorded before starting anaesthesia except for end tidal carbon dioxide was measured immediately after intubation and every 30 min until the end of surgery.
Intraoperative laboratory investigations for assessment of tissue perfusion: as in haemoglobin level (g/dl), serum lactate (mmol/l), central venous oxygen saturation (ScVO2) (%) and hypoxic index (HI). All were recorded after induction as a baseline record, 15 min after one-lung ventilation and 15 min after termination.
Intraoperative fluids and infusions as in total amount of blood losses, total amount of packed red blood cells received (ml), total amount of fresh frozen plasma received, number of patients who received noradrenaline, number of patients who received dobutamine in the goal-directed therapy group, amount of crystalloid boluses, amount of colloid boluses, urine output (ml/h) and diuretics given if any.
Intraoperative data in goal-directed fluid therapy group were stroke volume variation (SVV), cardiac index (CI) and thoracic fluid content (TFC). SVV was controlled at 10% and continuously monitored. Any increase above 10% was recorded and colloid infusion was started. Another recording of the SVV was recorded at the end of colloid infusion. Monitoring was stopped when spontaneous ventilation is resumed.
CI was controlled at a minimum of 2.5 l/min/m2 and continuously monitored. Any decrease was recorded and dobutamine infusion was started. Monitoring was stopped when spontaneous ventilation is resumed.
TFC recording started 15 min after the initiation of OLV, then another recording was done 15 min after the termination of OLV.
Postoperative data were recorded as lung perfusion parameters (HI and arterial blood gases), kidney perfusion measurements (urine output (UOP), serum creatinine (sCr), creatinine clearance and serum neutrophil gelatinase-associated lipocalin (Ngal)) and systemic perfusion measurements (serum lactate, haemoglobin level, central venous oxygen saturation and hematocrit level). All were measured in both groups first at the intensive care admission as 0 hour and then 6 h, 12 h and 24 h in the postoperative period except for Ngal was recorded 6 h after surgery.
3. Results
Data were entered to the computer and was processed by IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp). Qualitative data were presented as numbers and percent. The Kolmogorov–Smirnov test was utilized to verify the normality of distribution. Quantitative data were presented as range (minimum and maximum), mean, standard deviation and median. Significance of the obtained results was judged at the 5% level. The used tests were Chi-square test for categorical variables comparing between different groups, Fisher’s Exact or Monte Carlo correction for correction for chi-square (when >20% of the cells have expected count less than 5), Student t-test used for normally distributed quantitative variables among the two studied groups and Mann–Whitney test for abnormally distributed quantitative variables among the two studied groups.
The two studied groups showed no significant difference in age, gender and body mass index. Also, there were no significant difference regarding the type of operation, duration of surgery and OLV together with haemodynamic parameters, haemoglobin and heamatocrit.
A significant decrease in urine output in RT group in the third hour of intraoperative period, immediate and 6 hours postoperatively was reported (p value of <0.001) ().
Table 1. Intraoperative and postoperative urine output (ml/h)
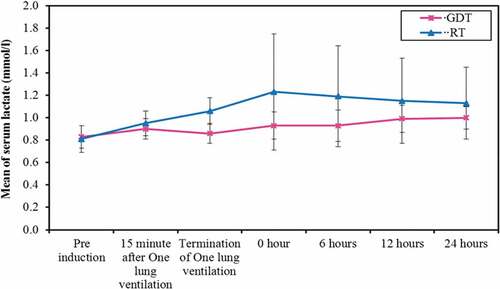
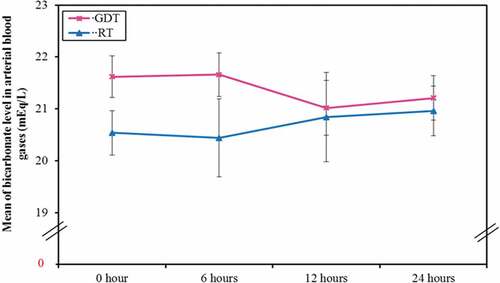
A significant increased serum lactate level (), creatinine () and creatinine clearance levels with decreased bicarbonate level () in the RT group at the end of the surgery, immediate and 6 hours postoperatively was plotted. However, it returned to be of no statistical significant difference among the two groups at 24 hours postoperatively.
Figure 4. Comparison between the two studied groups according to postoperative serum creatinine (mg/dL)
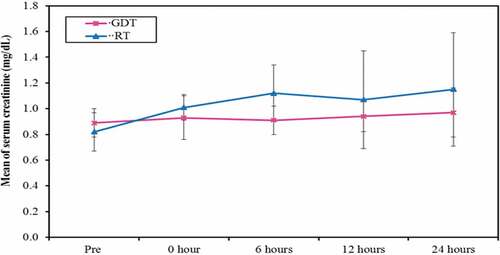
Figure 5. Comparison between the two studied groups according to bicarbonate level in arterial blood gases (mEq/L)
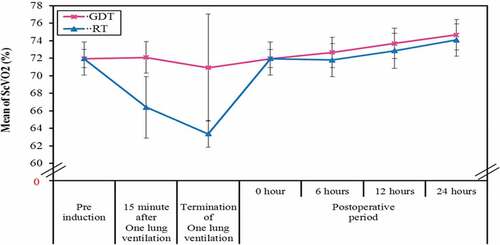
A statistically significant decrease was in ScVO2 in RT group at 15 minute after OLV and 15 minutes after the termination of OLV (p value of <0.001) ().
Figure 6. Comparison between the two studied groups according to central venous oxygen saturation (ScVO2) (%)
The HI was of no statistically significant difference the whole intraoperative values and the post-operative period. The restrictive HI values were much higher than the GDT group values, yet, both were higher than 300 and both groups were of normal range and no patient suffered of lung injury throughout the surgery or in the whole 24 hours of the post-operative period.
No significant difference in the total amount of blood losses, total amount of packed red blood cells (PRBCs) and fresh frozen plasma (FFP) received was reported. A statistically significant was increase in the amount of crystalloid infusion received in GDT group when compared to RT group. (p value of <0.001) While the amount of colloid boluses received were insignificant between both groups till the end of the study ().
Table 2. Intraoperative blood loss, fluids and blood products transfused in both groups
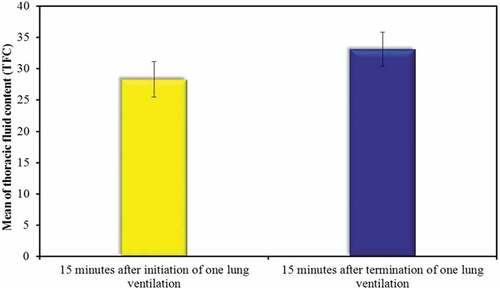
There was a significant statistic increase in the TFC in the GDT group in the interval of 15 minutes after termination of OLV than 15 minutes after the start of OLV ().
Figure 7. Comparison between the thoracic fluid content (TFC) (k.ohm−1) in the goal-directed therapy group (GDT) at different times of one lung ventilation
Acute kidney injury (AKI) occurred in four patients (26%) of the RT group while no patients experienced the same complication in the GDT group. Yet, there were no significant difference among both groups.
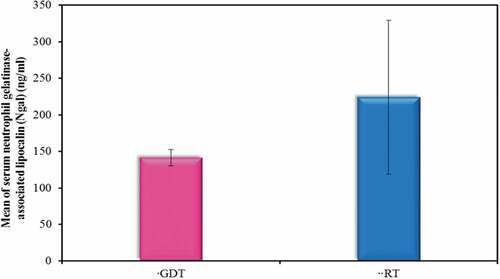
Moreover, there was a statistically significant increase in Ngal in the RT therapy group compared to the GDT group (p value of <0.001) ().
4. Discussion
Thoracic surgery has always a increased rate of postoperative morbidity. Many of which can be decreased by being operated at large volume centers where there is greater experience [Citation5].
Respiratory complications are of the most recurring postoperative complications in thoracic surgery. During the past twenty years, many management strategies have been used to decrease mortality and morbidity [Citation6].
In spite of the clinical impact of fluids in thoracic surgery, no randomized controlled trial has been done to compare the efficacy and safety of restrictive and goal-directed methods.
The conflict of different approaches (restrictive or liberal) in intraoperative fluids has not been decided in thoracic surgery [Citation1].
However, a restrictive perioperative fluid protocol may compromise renal perfusion, causing AKI. AKI incidence following pulmonary resection surgery in two studies was 6.8% and 5.9%, respectively [Citation7,Citation8]. This causes long hospital stay, more cardiopulmonary complications and higher mortality [Citation8,Citation9].
The haemodynamic parameters were of no significant difference among the studied groups. However, the RT group had more hypotensive readings during the intraoperative period.
These results matched Casado et al. [Citation10] study. They studied the different perioperative fluid management protocols in patients undergoing oesophagectomy.
Ahn et al. [Citation11] agreed to the same results. Matot et al. [Citation12], as well, reported no significant differences in the haemodynamic parameters.
No significant difference among the preoperative values of haemoglobin level in the whole intraoperative and the postoperative 24 hours values was reported.
Arslantas et al. [Citation13], Bjerregaard et al. [Citation14], Casado et al. [Citation10], Matot et al. [Citation12], Ahn et al. [Citation11] and Della Rocca et al. [Citation15] used restrictive fluid therapy protocols and agreed to have no significance regarding the haemoglobin level changes as in the present study.
Also, Sahutoglu et al. [Citation16], Kaufmann et al. [Citation17], Zhang et al. [Citation18], Veelo et al. [Citation19] and Xu et al. [Citation20] (as GDT studies) agreed with the present study results.
In the postoperative period, the restrictive therapy group showed a statistically significant increase in the heamatocrit level immediately after the surgery and after 6 hours in the postoperative period. Meanwhile, it returned to be of no statistical significant difference between the 12 and the 24 h interval in the post-operative period.
The haemo-concentrated values in the early 6 hours of the post-operative period mainly in the RT group was attributed to some dehydration and resolved as soon as oral hydration was resumed at the postoperative period.
In contrast to the present study, Matot et al. [Citation12] stated that there was a decrease in haematocrit level by the end of postoperative day one. The study did not mention the haematocrit values during the day which may have been of high value. They attributed the decrease by the end of postoperative day one to the early oral hydration as a part of fast track strategy.
A significant increased serum lactate level in restrictive therapy group at the end of surgery, immediately and 6 hours postoperatively was plotted.
After all, all values of serum lactate in both groups remained within the normal range. This may be due to the prompt use of colloid boluses and the administration of noradrenaline infusion.
The significant increase in the serum lactate in the restrictive group may reflect a degree of dehydration and hypoperfusion that may have occurred during the hypotensive episodes. These changes may have been masked by the innate activation of the sympathetic nervous system and the administration of noradrenaline infusion.
Della Rocca et al. [Citation21], Matot et al. [Citation12] and Arslanstas et al. [Citation13] agreed. While, Haas et al. [Citation22] disagreed concerning serum lactate level in the postoperative period which was significantly increased in the whole first postoperative day. These increased values may be present because it was conducted on oesophagectomy surgery which is a more aggressive, bloody, extensive and prolonged surgery.
The ScvO2 significantly decreased in the RT therapy group at 15 min after OLV and 15 min after the termination of OLV. Yet, they were of no significant difference during the whole postoperative 24 h.
These results may be due to the restricted fluid administration which compromised the cardiac output yet was masked by the sympathetic nervous system activation and the administration of noradrenaline proposed by the present study restrictive protocol.
The restrictive study of Bjerregaard et al. [Citation14] agreed. Yet, these values were improved and returned to normal values by the end of the surgery and continued to be of no significant change throughout the first postoperative day.
Concerning the GDT protocol, Haas et al. [Citation22] disagreed and showed further continued decrease in the central venous oxygen saturation in the postoperative day one. This may be attributed to aggressive nature of oesphagectomy as a surgery.
The HI was of no statistically significant difference between them during the in all intraoperative values and the post-operative period. The restrictive hypoxic index values were much higher than the GDT values. Yet, no patient suffered of lung injury. This may be due to more restrictive crystalloid administration (4 ml/kg/h) when compared to GDT where the basal crystalloid infusion was 8 ml/kg/h in the whole intraoperative period.
As a restrictive approach, Assaad et al [Citation9] used a fluid protocol of 1.5 ml/kg/h. They showed normal hypoxic index all over the intra and postoperative periods. Also, Sahutoglu et al. [Citation16], Zhang et al.. [Citation18] and Xu et al. [Citation20] agreed with the results of the present study.
In contrast to the present study, Haas et al. [Citation22], concluded that the PaO2/FiO2 ratio decreased significantly but remained more than 300 mmHg except during OLV. It may be due to longer duration of one-lung ventilation and more aggressive surgery with major fluid shifts and blood loss and its replacement.
The total amount of blood loss, total amount of PRBCs and FFP showed no statistically significant difference may be due to the conductance of the present study by the same surgical team and no extreme variation in the type of surgeries performed in the study.
Comparing the amount of crystalloids, the increased volume of crystalloids in the GDT group maybe due to the use of continuous crystalloid infusion of 8 ml/kg/h compared to 4 ml/kg/h in the restrictive group.
Licker et al. [Citation23,Citation24] quantified an infusion rate of 2.9–5.8 ml/kg/h for safe fluid restriction during thoracic surgery. Chau et al. [Citation5] suggested that the crystalloids use should be decreased to 2 L throughout the operation and less than 3 L within the first 24 h.
Sahutoglu et al. [Citation16] reported that the use of crystalloids was significantly higher in the SVV group. This is because they used crystalloids as a basal infusion and rescue boluses throughout the whole intraoperative period.
However, Veelo et al. [Citation19] and Xu et al. [Citation20] used less crystalloid infusions. This may be due to the lack of use of basal infusion fluids and the use of colloids as rescue boluses.
The amount of colloid boluses wasn’t significant. This may be attributed to the higher baseline crystalloid infusion in the GDT group that may have decreased the need for colloid rescue boluses. Meanwhile, the noradrenaline infusion has decreased the colloid administration in the RT group.
While Veelo et al. [Citation19], Zhang et al. [Citation18] and Xu et al. [Citation20] disagreed and reported significant increase in the colloid administration. That may be due to the lack of use of basal infusion fluids and the use of colloids as the only form of rescue boluses.
The number of the patients that received noradrenaline infusion and the total amount of noradrenaline infused were significantly increased in the RT group.
These results may be due to less basal crystalloid infusion used in the RT group that made noradrenaline to become a cornerstone pillar in supporting the circulation.
Electrical cardiometry was not used in thoracic surgery in the previous literature and no data were available using cardiometry in that type of surgery. Yet, data of other GDT studies using other devices were used to evaluate the results of the present study.
The entire GDT studies showed superior role of the goal-directed protocol over the control group in thoracic surgeries.
When TFC was measured, the increase may be caused by increased oncotic pressure of the subglycocalyceal space caused by the potential disruption of the glycocalyx endothelial layer. This subglycocalyceal oncotic pressure increases during one lung ventilation. Also, the inflammatory mediators’ release, especially tumor necrosis factor alpha, may aggravate the glycocalyceal fragility.
TFC does not reflect the absolute fluid levels; but, dynamic values of TFC can point to a directional shifts in thoracic fluids, either an increase or a decrease, especially in surgeries with volume shifts, as in thoracic surgery [Citation25].
A statistically significant decrease was in UOP (ml/h) in RT group in the third intraoperative hour, immediate and 6 hours postoperatively.
Sahutoglu et al. [Citation16], Kaufmann et al. [Citation17], Zhang et al. [Citation18], Veelo et al. [Citation19] and Xu et al. [Citation20] showed comparable results to the present study. However, Matot et al. [Citation12] found that intraoperative UOP together with postoperative renal parameters was not changed by fluid therapy infusions in the range of 2 to 8 ml/kg/h.
A significant decrease in the bicarbonate level and an increase in sCr and creatinine clearance in the immediate and 6 hour postoperative period in the restrictive therapy were reported.
According to Ahn et al. [Citation11], there was an increase in the postoperative sCr which subjected some of the patients to AKI. However, this increase was not of a statistical significant value and the number of AKI patients turned to be of no statistical significant value.
Moreover, there was a statistically significant increase in Ngal in the RT group compared to the GDT group.
These changes have resolved due to early oral hydration and insignificant intraoperative fluid shifts and blood loss by the end of the first postoperative day.
Makris [Citation26] and Haase et al. [Citation27] found that Ngal level was more accurate than sCr in AKI prediction when measured at the same time; however, Lassnigg et al. [Citation28] reported that the sCr criteria in RIFLE classification for the definition of AKI was considered to be valuable.
5. Conclusion
Fluid restriction is better to be individualized and aided by new technologies based on beat to beat variations.
Cardiometry can be used as a dynamic fluid responsive tool in thoracic surgery. Moreover, TFC derived from electrical cardiometry can help preventing pulmonary complications post thoracic surgeries.
RT protocols can prevent pulmonary complications yet can put the patient at risk of hypoperfusion especially kidney injuries.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Assaad S, Popescu W, Perrino A. Fluid management in thoracic surgery. Curr Opin Anaesthesiol. 2013;26(1):31–39.
- Sato Y. Management of Fluid and nutrition after lung resection to prevent complications. Kyobu Geka. 2017;70(8):692–695.
- Harris B, Schopflin C, Khaghani C, et al. Perioperative intravenous fluid prescribing: a multi-centre audit. Perioper Med (Lond). 2015;4:15.
- Arora RC. To give or not to give? Are we ready to use noninvasive monitors to guide fluid management in cardiothoracic surgical patients? J Thorac Cardiovasc Surg. 2014;148(6):3146–3147.
- Chau EH, Slinger P. Perioperative fluid management for pulmonary resection surgery and ooesophagectomy.ضظ Semin Cardiothorac Vasc Anesth. 2014;18(1):36–44.
- Vallet B, Blanloeil Y, Cholley B, et al. Guidelines for perioperative haemodynamic optimization. Ann Fr Anesth Reanim. 2013;32(10):e151–158.
- Ishikawa S, Griesdale DE, Lohser J. Acute kidney injury after lung resection surgery: incidence and perioperative risk factors. Anesth Analg. 2012;114(6):1256–1262.
- Licker M, Cartier V, Robert J, et al. Risk factors of acute kidney injury according to RIFLE criteria after lung cancer surgery. Ann Thorac Surg. 2011;91(3):844–850.
- Assaad S, Kyriakides T, Tellides G, et al. Extravascular lung water and tissue perfusion biomarkers after lung resection surgery under a normovolemic fluid protocol. J Cardiothorac Vasc Anesth. 2015;29(4):977–983.
- Casado D, López F, Martí R. Perioperative fluid management and major respiratory complications in patients undergoing oesophagectomy. Dis Esophagus. 2010;23(7):523–528.
- Ahn HJ, Kim JA, Lee AR, et al. The risk of acute kidney injury from fluid restriction and hydroxyethyl starch in thoracic surgery. Anesth Analg. 2016;122(1):186–193.
- Matot I, Dery E, Bulgov Y, et al. Fluid management during video-assisted thoracoscopic surgery for lung resection: a randomized, controlled trial of effects on urinary output and postoperative renal function. J Thorac Cardiovasc Surg. 2013;146(2):461–466.
- Arslantas MK, Kara HV, Tuncer BB, et al. Effect of the amount of intraoperative fluid administration on postoperative pulmonary complications following anatomic lung resections. J Thorac Cardiovasc Surg. 2015;149(1):314–320.
- Bjerregaard LS, Møller-Sørensen H, Hansen KL, et al. Using clinical parameters to guide fluid therapy in high-risk thoracic surgery. A retrospective, observational study. BMC Anesthesiol. 2015;15:91.
- Della Rocca G, Langiano N, Baroselli A, et al. Survey of thoracic anesthetic practice in Italy. J Cardiothorac Vasc Anesth. 2013;27(6):1321–1329.
- Sahutoglu C, Turksal E, Kocabas S, et al. Influence of stroke volume variation on fluid treatment and postoperative complications in thoracic surgery. Ther Clin Risk Manag. 2018;14:575–581.
- Kaufmann KB, Stein L, Bogatyreva L, et al. Oesophageal Doppler guided goal-directed haemodynamic therapy in thoracic surgery - a single centre randomized parallel-arm trial. Br J Anaesth. 2017;118(6):852–861.
- Zhang J, Chen CQ, Lei XZ, et al. Goal-directed fluid optimization based on stroke volume variation and cardiac index during one-lung ventilation in patients undergoing thoracoscopy lobectomy operations: a pilot study. Clinics (Sao Paulo). 2013;68(7):1065–1070.
- Veelo DP, van Berge Henegouwen MI, Ouwehand KS, et al. Effect of goal-directed therapy on outcome after oesophageal surgery: a quality improvement study. PLoS One. 2017;12(3):e0172806.
- Xu H, Shu SH, Wang D, et al. Goal-directed fluid restriction using stroke volume variation and cardiac index during one-lung ventilation: a randomized controlled trial. J Thorac Dis. 2017;9(9):2992–3004.
- Della Rocca G, Vetrugno L, Tripi G, et al. Liberal or restricted fluid administration: are we ready for a proposal of a restricted intraoperative approach? BMC Anesthesiol. 2014;14:62.
- Haas S, Eichhorn V, Hasbach T, et al. Goal-directed fluid therapy using stroke volume variation does not result in pulmonary fluid overload in thoracic surgery requiring one-lung ventilation. Crit Care Res Pract. 2012;2012:687018.
- Licker M, Fauconnet P, Villiger Y, et al. Acute lung injury and outcomes after thoracic surgery. Curr Opin Anaesthesiol. 2009;22(1):61–67.
- Licker MJ, Widikker I, Robert J, et al. Operative mortality and respiratory complications after lung resection for cancer: impact of chronic obstructive pulmonary disease and time trends. Ann Thorac Surg. 2006;81(5):1830–1837.
- Narula J, Kiran U, Malhotra Kapoor P, et al. Assessment of changes in haemodynamics and intrathoracic fluid using electrical cardiometry during autologous blood harvest. J Cardiothorac Vasc Anesth. 2017;31(1):84–89.
- Makris K, Markou N, Evodia E, et al. Urinary neutrophil gelatinase-associated lipocalin (NGAL) as an early marker of acute kidney injury in critically ill multiple trauma patients. Clin Chem Lab Med. 2009;47(1):79–82.
- Haase-Fielitz A, Bellomo R, Devarajan P, et al. Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery–a prospective cohort study. Crit Care Med. 2009;37(2):553–560.
- Lassnigg A, Schmid ER, Hiesmayr M, et al. Impact of minimal increases in serum creatinine on outcome in patients after cardiothoracic surgery: do we have to revise current definitions of acute renal failure? Crit Care Med. 2008;36(4):1129–1137.