ABSTRACT
Background
The usage of opioid sparing analgesia in different surgeries leads to varied results. The aim of this study is to compare between the effects of continuous intraoperative infusion of dexmedetomidine versus lidocaine as opioid sparing analgesia in children undergoing intracranial surgeries.
Methods
It is a double-blind randomized clinical study in which, 64 children were randomly allocated into 2 equal groups; 32 children for each group. The first one was dexmedetomidine group which received continuous intraoperative dexmedetomidine iv infusion [1ug/kg dexmedetomidine over 15 minutes as a loading dose and 0.5ug/kg/h for maintenance]. The second one was lidocaine group which received continuous intraoperative lidocaine iv infusion [1.5mg/kg lidocaine over 15 minutes as a loading dose and 1.5mg/kg/h for maintenance]. The continuous intraoperative iv infusion of each tested drug was stopped 10 minutes before the end the surgical procedure.
Results
Intraoperative total fentanyl consumption was significantly reduced in dexmdetomidine compared to lidocaine group ([19.33±11.15 vs 43.10±21.15] ug, 95% CI, -45.8 to -1.7). Children in dexmedetomidine group were significantly more sedated compared to lidocaine group. The time to reach Wong–Baker Faces Pain Rating Scale (WBFPS) scores 4 or more was around 4 hours in dexmedetomidine and around 1hour in lidocaine group. The time to first call of naluphine with dexmedetomidine was significantly prolonged compared to lidocaine ([235.03±5.02 vs 55.87±6.28] min, 95% CI, 176.3-182) with significantly reduced total postoperative naluphine consumption in dexmedetomidine compared to lidocaine ([4.22±1.46 vs 7.96±2.8] mg, 95% CI,-4.9 to -2.6) . The duration of PACU stay was significantly prolonged in lidocaine compared to dexmedetomidine ([99.75±14.36 vs 114.28±10.56] min, 95% CI,-20.8 to -8.2).
Conclusion
Continuous Intraoperative intravenous infusion of dexmedetomidine was superior to lidocaine in opioid sparing analgesia during intracranial surgeries in children.
1. Introduction
Pain after major surgeries in children is a challenge for anesthetists because of difficulty in assessing pain and opioid-related side effects such as hypoventilation, nausea and vomiting, ileus, urinary retention and prolonged hospital stay [Citation1,Citation2].
Dexmedetomidine is considered a relatively new drug in pediatric anesthesia and has gained great interest due to its satisfactory sedative effect without respiratory depression [Citation3,Citation4]. Moreover, it produces less damage to developing brain than other anesthetics do. Dexmedetomidine has analgesic, neuroprotective, anti-inflammatory and sympatholytic effects [Citation5–7]. A meta-analysis showed the analgesic effect of intraoperative dexmedetomidine in neonates, infants and children with decreased postoperative opioid consumption [Citation8].
Systemic lidocaine has centrally and peripherally analgesic, anti-hyperalgesic and anti-inflammatory effects with reduced side-effects especially if used with appropriate dose in children [Citation9]. Recently, intravenous lidocaine infusion is considered a part of analgesic therapy regimen that decreases postoperative opioid requirements and enhances children convalescence after major surgeries [Citation10].
Dexmedetomidine and lidocaine have been used for pain relief and reduction of opioid consumption; but it is still uncertain which drug is more effective. So, this study aims to compare the effects of intraoperative infusion of dexmedetomidine versus lidocaine as opioid sparing analgesia for intracranial surgeries in children.
2. Materials and methods
The ethical approval of Zagazig University’s Institutional Review Board number of this study was (IRB #6318-23-8-2020) and registration number on clinicaltrials.gov was (NCT04535089 date: 1-9-2020). The study was carried out according to the guidelines and regulations of the Helsinki Declarations. The nature and complications of the study were explained in detail to the parents and informed written consent was obtained from parents before enrolment. The first patient had been enrolled on 1 October 2020.
This was a prospective randomized double-blind clinical study, conducted on 64 children from October 2020 to October 2021. The age of children included in this study ranged from 6 to 18 years of either sex; belonging to class I and II physical status according to American Society of Anesthesiologist (ASA) classification; body mass index (BMI) ≥5th and ≤ the 85th percentile for age; scheduled for elective intracranial surgeries lasting for less than 4 hduration under general anesthesia. Children with altered mental status, known history of allergy to study drugs, unsuitability for extubation, on beta blocker, alpha 2 agonist, pain killer, hepatic, respiratory, renal and cardiovascular diseases or uncontrolled neurological state were excluded from this study.
The parents and the outcome assessors (anaesthesiologists collecting the data) were blinded to the study drugs. The primary outcome was to determine the total dose of intraoperative fentanyl consumption in dexmedetomidine infusion compared to lidocaine infusion in children undergoing intracranial surgeries. The secondary outcomes were to assess postoperative level of sedation, pain intensity, time to first call of naluphine, total postoperative naluphine consumption, postoperative side effects and Post Anesthesia Care Unit (PACU) stay.
3. General anesthesia
All children were hospitalized and evaluated a day before the surgery. Full history with complete physical examination and routine investigations were done. All children were kept fasting 6 hfor solid and 2 h for clear fluids before the operation.
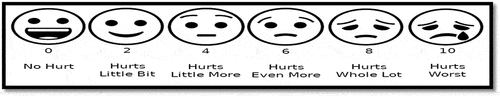
In the preparation room, the Wong–Baker Faces Pain Rating Scale (WBFPS) was explained to the child to choose the face that best expressed the intensity of pain postoperatively. WBFPS () contains a series of faces ranging from a happy face at 0, or “no hurt”, to a crying face at 10, which represents “hurts like the worst pain imaginable” [Citation11].
In the operating room, an intravenous (iv) line was inserted, warm iv fluid infusion was started and iv 0.05 mg/kg of midazolam was given. Full standard monitoring was initiated, including five leads electrocardiogram (ECG), pulse oximeter (SpO2), automated non-invasive blood pressure (NIBP), end tidal carbon dioxide partial pressure, temperature, and urine output. A 22-gauge radial artery cannula was inserted in the non-dominant hand after performing Allen’s test for continuous invasive monitoring of BP. Warm blankets were applied under children to avoid intraoperative hypothermia.
Then, children were randomly allocated into two groups: 32 children for each group via computer generated randomization table. The first one was dexmedetomidine group which received continuous intraoperative dexmedetomidine iv infusion [1ug/kg dexmedetomidine over 15 min as a loading dose and 0.5ug/kg/h for maintenance]. The second one was lidocaine group which received continuous intraoperative lidocaine iv infusion [1.5 mg/kg lidocaine over 15 min as a loading dose and 1.5 mg/kg/h for maintenance]. The continuous intraoperative iv infusion of each tested drug was stopped 10 min before the end the surgical procedure.
Pre-oxygenation with 100% O2 for 3 min was performed, then general anesthesia was inducted by iv injection of 2 mg/kg propofol and tracheal intubation was facilitated by iv injection of 0.5 mg/kg of atracurium. Maintenance of anesthesia with 1.5% sevoflurane in 50% O2 and 50% air, atracurium 0.1 mg/kg/h and fentanyl 0.5ug/ kg (when the heart rate and mean arterial blood pressure of patients increased > 20% from basal measurement after exclusion of other causes) and the total intraoperative fentanyl consumption in ug was recorded. Mechanical ventilation was adjusted to maintain the Etco2 (end tidal co2) at 30 to 35 mm Hg. All surgeries were operated by an experienced neurosurgeon who had 10 years training after MD.
Any intraoperative complications such as hypotension and bradycardia were recorded and managed. Intraoperative decrease of MABP and bradycardia less than 20% of the minimum range for age were treated by decreasing rate of infusion up to stopping infusion. Then, 0.2 mg/kg ephedrine and 0.02 mg/kg atropine were given, respectively, if hypotension and bradycardia were not responding.
After the end of the surgery, the inhalational anesthetic was turned off and the muscle relaxant was reversed by giving neostigmine 0.05 mg/kg and atropine 0.02 mg/kg. The child was extubated and transferred to PACU. Children who could not be extubated were transferred to Pediatric Intensive Care Unit (PICU) and excluded from the study.
In PACU, the intensity of pain was evaluated using The Wong–Baker Faces Pain Rating Scale (WBFPS) at 30 min, 1h, 2 h, 4 h, 6 h, 8 h, 10 h and 12 h postoperatively. The child was ready for discharge from PACU when attained an Aldrete score ≥9 [Citation12] and free from pain, nausea and vomiting. The duration of PACU stay from arrival till discharge was recorded.
Protocol for pain management was IV paracetamol 15 mg/kg every 6 h with a maximum daily dose 60 mg/kg not exceeding 2 grams.
Children with WBFPS score ≥ 4 were treated with naluphine 0.1 mg/kg as a rescue analgesic. Time to first call for rescue analgesic (naluphine) per minutes and the total postoperative amount of naluphine consumption per mg were recorded.
The children were monitored for vital signs (Mean arterial blood pressure, heart rate and SpO2). The level of sedation was also evaluated by 6-point Pediatric Sedation State Scale (PSSS) [Citation13] in the PACU.
Any side effects like: hypotension, bradycardia, nausea and vomiting, or any other complications such as lidocaine systemic toxicity that could be diagnosed by the following: tongue parathesia (early sign), circumoral numbness, drowsiness, lightheadedness, irritability, convulsions, hypotension and bradycardia were recorded and managed.
Sample size: Calculation of the sample size was based on estimation of the percent of children that required intraoperative fentanyl. A pilot study was carried out and found that (10%) of children in dexmedetomidine group needed intraoperative fentanyl compared to (40%) in lidocaine group at 0.05 α error and 0.2 β error. Sample size was estimated using OPEN EPI program. So, at power of 80% and 95% confidence interval, the sample size was 64 cases (32 in each group).
4. Statistical analysis
Data were collected and statistically analyzed using Version 26.0 SPSS Statistics for Windows, NY: IBM Corp, Armonk. Quantitative data were expressed as the mean ± SD and qualitative data were expressed as absolute frequencies (number) and relative frequencies (percentage). Comparison between normally distributed variables of the two groups was done using the T test. Fisher exact or Chi-square tests were used to calculate the percent of categorical variables. P-value was statistically significant if its value ≤ 0.05. Confidence interval (CI) 95% was estimated for quantitative data.
5. Results
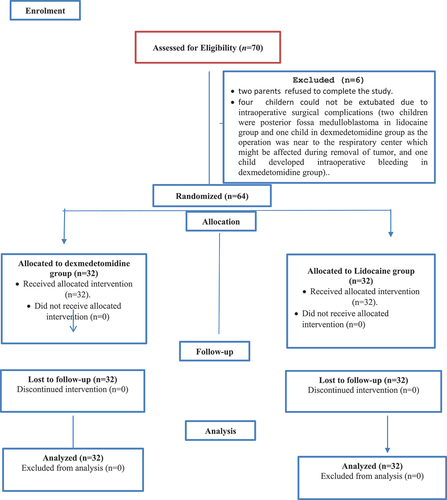
Seventy children were prepared for the study. However, two parents refused to complete the study and four children could not be extubated due to intraoperative surgical complications: Three children were posterior fossa medulloblastoma, two in lidocaine group and one in dexmedetomidine group as the operation was near to the respiratory center which could have been affected during removal of tumor, and one child developed intraoperative bleeding in dexmedetomidine group.
So, 64 children were randomly allocated into two groups (32 children for each group) (). Both groups were comparable regarding demographic data, type and duration of surgery ().
Table 1. Children’s demographic data, type and duration of surgery in the two studied groups
The intraoperative total dose of fentanyl consumption was significantly lower in dexmdetomidine group compared to lidocaine group ([19.33 ± 11.15 vs 43.10 ± 21.15] ug, mean difference = −23.8 [95% CI, −45.8 to −1.7], P = 0.03). The number of children who received first and second dose fentanyl were significantly lower in dexmedetomidine group [3(9.4%) and 1(3.1%)] compared to lidocaine group [(10(31.3%) and 8(25%)], respectively. The intraoperative complications were comparable between the two groups ().
Table 2. Intraoperative total fentanyl consumption, number (%) of children which received either one or two doses of fentanyl and the rate of various intra-operative complications in the two studied groups
In the PACU, the level of sedation in children of dexmedetomidine group were statistically significant more sedated compared to lidocaine group as 4(12.5%) children were state 1 and 22 (68.8%) children state 2 compared to 1(3.1%) and 7(21.9%) in lidocaine group, respectively. While children who had states 3 and 4 were more statistically significant in lidocaine group [18 (56.3%) and 6(18.8%)] compared to dexmedetomidine group [5(15.6%) and 1(3.1%)], respectively. Children with sedation score zero were not detected in both groups ().
Table 3. Distribution of children on the various sedation states, analgesic parameters, rates of various postoperative side effects and duration of PACU stay in the two studied groups
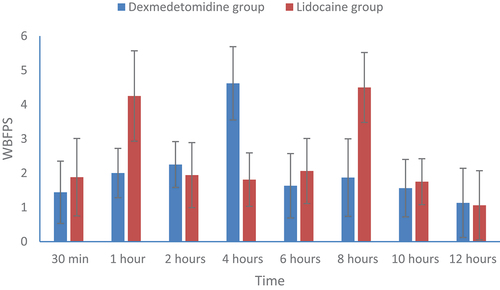
The postoperative WBFPS scores (mean ± SD) at various times of measurements were presented in . The time to reach WBFPS scores 4 or more was around 4 hours in dexmedetomidine and around 1 h in lidocaine group ().
Figure 3. WBFPS (Mean ± SD) at various times of measurement time in the two studied groups. WBFPS = Wong-Baker Faces Pain Rating score.
The mean time to first call of naluphine in dexmedetomidine group was significantly prolonged compared to lidocaine group ([235.03 ± 5.02 vs 55.87 ± 6.28] min, mean difference = 179.2[95% CI, 176.3 to –182], P = <0.001). The total postoperative naluphine consumption was significantly reduced in dexmedetomidine group compared to lidocaine group ([4.22 ± 1.46 vs 7.96 ± 2.8] mg, mean difference = −3.7[95% CI,-4.9 to −2.6], p < 0.001) ().
The rate of postoperative nausea and vomiting was statistically significant lower in dexmedetomidine group (3.1%) than in lidocaine group (25%) and the rate of other side effects (hypotension and bradycardia) were comparable in both groups ().
The mean duration of PACU stay was prolonged in lidocaine group (114.28 ± 10.56 min) compared to dexmedetomidine group (99.75 ± 14.36 min), mean difference = −14.5[95% CI,-20.8 to −8.2], p < 0.001) ().
6. Discussion
This study was designed to determine the intraoperative fentanyl consumption among children undergoing intracranial surgeries receiving continuous intraoperative dexmedetomidine iv infusion [1ug/kg dexmedetomidine over 15 min as a loading dose and 0.5ug/kg/h for maintenance] versus lidocaine [1.5 mg/kg lidocaine over 15 min as a loading dose and 1.5 mg/kg/h for maintenance]. The continuous intraoperative iv infusion of each tested drug was stopped 10 min before the end the surgical procedure. This study revealed that intraoperative continuous iv infusion of dexmedetomidine had significantly more opioid sparing analgesia than lidocaine with minimal intraoperative associated complications in both.
Literature reporting the intraoperative use of dexmedetomidine and lidocaine in children are limited [Citation14,Citation15]. To date, there are no studies comparing between both drugs as opioid sparing agents for intracranial surgeries in children.
Dexmedetomidine mediates supraspinal and spinal analgesia through activation of α2 receptors in the spinal cord, inhibition of substance P and decreasing the nociceptive transmission corresponding to the gate theory. This explains its opioid sparing effect [Citation16]. While, Lidocaine mediates its analgesic effects by blocking of sodium channel and inhibition of N-methyl D-Aspartate receptors and coupled protein G [Citation17].
In a study performed on adults by Mohammed et al [Citation18], they reported that the intraoperative fentanyl consumption was significantly higher in lidocaine group compared to dexmedetomidine in patients undergoing lumbar spine fixation. This was in a correlation with the present study.
The results of the current study revealed that the number of children with states 1 and 2 sedation were significantly higher in dexmedetomidine group compared to lidocaine group. On contrary, states 3 and 4 were significantly higher in lidocaine group.
Dexmedetomidine decreases the locus coeruleus transmission and inhibits the main noradrenergic center in the central nervous system [Citation19]. So, the sedative effect of dexmedetomidine is more related to the natural sleep without respiratory depression [Citation20]. This explains why twenty two children in this study developed sedation state 2 in dexmedetomidine group compared to only seven in lidocaine group.
In a recent meta-analysis conducted on 753 children; it was demonstrated that dexmedetomidine produced high-quality sedative effect without affecting oxygen saturation for magnetic resonance imaging [Citation21]. Also, Hall et al [Citation22] reported that 1ug/kg bolus dose of dexmedetomidine followed by 0.2 or 0.6 ug/kg/h produced sedation in healthy volunteers who could be easily aroused to perform different tests and this was in a close correlation with the current study as we used the same dose.
Forster et al [Citation23] during study the sedative effect of lidocaine recorded that intravenous infusion of lidocaine at a rate of 4 mg/kg/h after 1.5 mg/kg intravenous bolus dose reduced the propofol requirements up to 50% in patients undergoing colonoscopy. That was different from the present study results as we used smaller infusion dose (1.5 mg/kg/h) than what they used.
In the present study, time to reach pain score 4 or more was around 4 hours in dexmedetomidine group and around 1 hour in lidocaine group. The mean time to first call of naluphine in dexmedetomidine group was significantly prolonged compared to lidocaine group (235.03 ± 5.02 vs 55.87 ± 6.28) min respectively with decreasing postoperative naluphine consumption.
In accordance with the present study results, Lopaz et al [Citation24] concluded that dexmedetomidine reduced postoperative pain and narcotic use in children undergoing alveolar bone graft surgery. Song et al [Citation25] revealed that intraoperative infusion of dexmedetomidine decreased the cumulative consumption of morphine and the time to first call for postoperative analgesia was 171.2 ± 31.2 min without significant side effects after craniotomy.
In a retrospective study by Lemming et al [Citation26] on 50 children ranging from 2 to 17 years, lidocaine infusion was an acceptable and tolerable pain control medication with other multimodal analgesia in children. Kaszyński et al [Citation27] reported that the median time to first call of nalbuphine was 50 min in lidocaine group compared to 40 min in control group in children undergoing laparoscopic appendectomy. This coincides with results of the present study.
The current study revealed that the occurrence of postoperative nausea and vomiting in PACU was significantly higher among children in lidocaine group (8 children) compared to dexmedetomidine group (one child). This could be explained by the stronger opioid sparing effect and lower WBFPS in dexmedetomidine group compared to lidocaine group.
Previous studies reported that dexmedetomidine reduced the incidence of nausea and vomiting by decreasing opioid requirements [Citation28,Citation29].
Otherwise, no significant side- effects between the two groups and no systemic lidocaine toxicity was recorded.
Lauder [Citation30] reported that the frequently used lidocaine infusion regimen in pediatrics was 1.5 mg/kg as a bolus followed by 1.5–2 mg/kg/h continuous infusion and the lidocaine dose in this study was bolus 1.5 mg/kg intravenous over 15 min followed by 1.5 mg/kg/h continuous infusion and it is safe dose in accordance with Kaszyński et al [Citation27] and El-Deeb et al [Citation31]’s studies.
In the present study, the mean duration of PACU stay was prolonged in lidocaine group (114.28 ± 10.56 min) compared to dexmedetomidine group (99.75 ± 14.36 min).
In a retrospective cohort study on 814 patients who received intraoperative dexmedetomidine infusion; the duration of PACU stay after lung surgery ranged from 57 to 115 min [Citation32] which was in a line with our results.
The prolonged duration of PACU stay in lidocaine group could be attributed to the early postoperative pain perception and nausea/ vomiting which was recorded in eight children in lidocaine group compared to one child in dexmedetomidine group. Moreover, the reduced number of children that needed the second dose of fentanyl in dexmedetomidine (one child) group compared to lidocaine (8 children) and most of children developed sedation state 2 in dexmedetomidine making the children calmer in the PACU that allowed their earlier discharge.
Recently, Kaye et al [Citation33] concluded that dexmedetomidine is included in enhanced recovery after surgery (ERAS) protocols in the PACU.
7. Limitation
Limitations of the present study were lack of studies that compare between intraoperative continuous infusion of dexmedetomidine and lidocaine in children undergoing intracranial surgeries and measurement of the plasma lidocaine level was not performed as the used dose of lidocaine was below the maximum recommended dose. So, we recommend further studies.
8. Conclusion
Continuous Intraoperative intravenous infusion of dexmedetomidine was superior to lidocaine in opioid sparing analgesia during intracranial surgeries in children.
Author contributions
‘‘Dr. Alshaimaa Abdel Fattah Kamel. This author helped in registration, conception, writing, and collecting data ‘‘.
”Dr.Sara Mohamed Abdel Naby. This author helped in, editing and approving final manuscript ‘‘.
‘‘Dr. Wael Abdel Rahman Ali Elmesallamy. This author helped in performing surgeries and designing the study ‘‘.
‘‘Dr. Dina Abdelhameed Elsadek Salem.This author helped in designing the study and analyzing data ”.
Supplemental Material
Download MS Word (20.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here.
References
- Lee JY, JO YY. Attention to postoperative pain control in children. Korean J Anesthesiol. 2014;66:183–188.
- Boric K, Dosenovic S, Jelicic Kadic A, et al. Intervention for postoperative pain in children: an overview of systemic reviews. Paediatr Anaesth. 2017;27:893–904.
- Sottas CE, Anderson BJ. Dexmedetomidine: the new all-in-one drug in paediatric anaesthesia? Curr Opin Anesthesiol. 2017;30:441–451.
- Van Hoorn CE, Flint RB, Skowno J, et al. Off-label use of dexmedetomidine in pediatric anesthesiology: an international survey of 791 (pediatric) anesthesiologists. Eur J Clin Pharmacol. 2021;77:625–635.
- Wang Y, Han R, Zuo Z. Dexmedetomidine post-treatment induces neuroprotection via activation of extracellular signal-regulated kinase in rats with subarachnoid haemorrhage. Br J Anaesth. 2016;116:384–392.
- Wu GJ, Chen JT, Tsai HC, et al. Protection of dexmedetomidine against ischemia/reperfusion-induced apoptotic insults to neuronal cells occurs via an intrinsic mitochondria-dependent pathway. J Cell Biochem. 2017;118:2635–2644.
- Lee S. Dexmedetomidine: present and future directions. Korean J Anesthesiol. 2019;72:323–330.
- Bellon M, Le Bot A, Michelet D, et al. Efficacy of intraoperative dexmedetomidine compared with placebo for postoperative pain management: a meta-analysis of published studies. Pain Ther. 2016;5:63–80.
- Lauder GR. A review of intravenous lidocaine infusion therapy for paediatric acute and chronic pain management, pain relief- from analgesics to alternative therapies. Cecilia Maldonado IntechOpen. 2017;5:64–109.
- Batko I, Kościelniak-Merak B, Tomasik PJ, et al. Lidocaine as an element of multimodal analgesic therapy in major spine surgical procedures in children: a prospective, randomized, double-blind study. Pharmacol Rep. 2020;72:744–755.
- Drendel AL, Kelly BT, Ali S. Pain assessment for children: overcoming challenges and optimizing care. Pediatr Emerg Care. 2011;27:773–781.
- Aldrete JA. The post-anesthesia recovery score revisited. J Clin Anesth. 1995;7(1):89–91.
- Cravero JP, Askins N, Sriswasdi P, et al. Validation of the pediatric sedation state scale. Pediatric. 2017;139(5):e20162897.
- Both CP, Thomas J, Bühler PK, et al. Factors associated with intravenous lidocaine in pediatric patients undergoing laparoscopic appendectomy – a retrospective, single-centre experience. BMC Anesthesiol. 2018;18:88.
- Yuen VM. Dexmedetomidine: perioperative applications in children. Paediatr Anaesth. 2010;20:256–264.
- Tobias JD. Dexmedetomidine: applications in pediatric critical care and pediatric anesthesiology. Pediatr Crit Care Med. 2007;8:115–131.
- Hollmann MW, Strumper D, Herroeder S, et al. Receptors, G proteins and their interactions. Anesthesiology. 2005;103:1066–1078.
- Mohammed NS, Habib MK, Abbas EA, et al. Comparative study between the effect of dexmedetomidine and lidocaine infusion in lumbar fixation on hemodynamics, fentanyl requirements, and postoperative analgesia. Ain-Shams J Anesthesiol. 2020;12:67.
- Nacif-Coelho C, Correa-Sales C, Chang LL, et al. Perturbation of ion channel conductance alters the hypnotic response to the alpha 2-adrenergic agonist dexmedetomidine in the locus coeruleus of the rat. Anesthesiology. 1994;81:1527–1534.
- Bonfield S, Garland A, and English W. Use of dexmedetomidine for sedation in adult critical care; 2021. Accessed 21 August 2021; Tutorial of World Federation Society of Anesthesiologist 454. https://resources.wfsahq.org/anaesthesia-tutorial-of-the-week
- Kim JY, Kim KN, Kim DW, et al. Effects of dexmedetomidine sedation for magnetic resonance imaging in children: a systemic review and meta-analysis. J Anesth. 2021;35:525–535.
- Hall JE, Uhrich TD, Barney JA, et al. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705.
- Foster C, Vanhaudenhuyse A, Gast P, et al. Intravenous infusion of lidocaine significantly reduces propofol dose for colonoscopy: a randomized placebo-controlled study. Br J Anaesth. 2018;121:1059–1064.
- Lopez MM, Zech D, Linton JL, et al. Dexmedetomidine decreases postoperative pain and narcotic use in children undergoing alveolar bone graft surgery. Cleft Palate Craniofac J. 2018;55:688–691.
- Song J, Ji Q, Sun Q, et al. The opioid-sparing effect of intraoperative dexmedetomidine infusion after craniotomy. J Neurosurg Anesthesiol. 2015;28:14–20.
- Lemming K, Fang G, Buck ML. Safety and tolerability of lidocaine infusion as a component of multimodal postoperative analgesia in children. J Pediatr Pharmacol Ther. 2019;24:34–38.
- Kaszyński M, Lewandowska D, Sawicki P, et al. Efficacy of intravenous lidocaine infusions for pain relief in children undergoing laparoscopic appendectomy: a randomized controlled trial. BMC Anesthesiol. 2021;21:2–13.
- Song Y, Shim JK, Song JW, et al. Dexmedetomidine added to an opioid- based analgesic regimen for the prevention of postoperative nausea and vomiting in highly susceptible patients: a randomised controlled trial. Eur J Anaesthesiol. 2016;33:761–766.
- Jin S, Liang DD, Chen C, et al. Dexmedetomidine prevent postoperative nausea and vomiting on patients during general anesthesia: a PRISMA-compliant meta- analysis of randomized controlled trials. Medicine (Baltimore). 2017;96:e5770.
- Lauder GR. A review of intravenous lidocaine therapy for paediatric acute and chronic pain management. In: Maldonado C, editor. Pain relief-from analgesics to alternative therapies [Internet]. London: IntechOpen;2017. 63–109 . cited 2022 Feb 12]. Available from; https://www.intechopen.com/chapters/53881
- El-Deeb A, El-Morsy GZ, Ghanem AAA, et al. The effect of intravenous lidocaine infusion on hospital stay after major abdominal pediatric surgery. A randomized double-blinded study. Egypt J Anaesth. 2013;29:225–230.
- Kang X, Tang X, Yu Y, et al. Intraoperative dexmedetomidine infusion is associated with reduced emergence agitation and improved recovery profiles after lung surgery: a retrospective cohort study. Drug Des Devel Ther. 2019;13:871–879.
- Kaye AD, Chernobylsky DJ, Thakur P, et al. Dexmedetomidine in Enhanced Recovery After Surgery (ERAS) protocols for postoperative pain. Curr Pain Headache Rep. 2020;24:21–34.