ABSTRACT
Objective
Study the impact of ultrasound-assessed diaphragmatic impairment (DI) on predicting need for invasive mechanical ventilation (IMV) in acute exacerbation of chronic obstructive pulmonary disease (AECOPD) patients treated by non-invasive ventilation (NIV).
Design
A prospective observational study.
Setting
Critical Care Units of Alexandria Main University Hospital.
Patients
75 adult AECOPD patients of both sexes according to sample size calculation.
Methods
Ultrasound was used to measure diaphragmatic thickness (DT), diaphragmatic thickness fraction (DTF) was calculated, and DI was diagnosed when fraction was less than 20%. Patients were treated by NIV. Switching to IMV was done after NIV failure. Primary outcome was value of DI to predict need for IMV after NIV failure. Secondary outcome was impact of DI on fate of patients.
Results
According to fate of NIV, patients were categorized into successful and failed NIV groups. DTF in both groups were ≥33–38% and ≤16–18%. DTF with a cut-off value of <26–29% on both sides was able to predict NIV failure with 96.67% sensitivity and 80–82.22% specificity. Days of MV and ICU stay were significantly lower in the successful NIV group, p < 0.001. 28-day mortality was significantly less encountered in successful NIV group, p = 0.018.
Conclusion
DTF was a good indicator of DI that could predict need for IMV after NIV failure in AECOPD patients with good sensitivity and moderate specificity. MV Days, ICU stay, and 28-day mortality were significantly higher in patients with DI who needed IMV.
1. Introduction
According to the Global Burden of Disease (GBD), chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide [Citation1,Citation2]. Acute exacerbations of COPD (AECOPD) can be precipitated by infectious or noninfectious causes. However, up to 30% is of unknown etiology [Citation3]. Indications for intensive care unit (ICU) admission in COPD patients include severe dyspnea that responds inadequately to initial emergency therapy, changes in mental status, persistent or worsening hypoxemia and/or severe worsening respiratory acidosis despite supplemental oxygen, need for invasive mechanical ventilation, hemodynamic instability, or need for vasopressors [Citation4,Citation5].
Treatment options include medical treatment and respiratory support. In case of respiratory failure, ventilator treatment is necessary either in the form of non-invasive ventilation (NIV) or invasive mechanical ventilation (IMV) through endotracheal intubation. Early use of NIV in patients with AECOPD is preferable due to its positive effects in recruitment of collapsed alveoli, improvement of ventilation-perfusion matching, with consecutive improved oxygenation and respiratory acidosis, and in decreasing the work of breathing with reduction of intubation risk. Despite its important role, there are many contraindications that necessitate immediate intubation and IMV [Citation6–8].
The criteria for intubation include no improvement or worsening of pH and/or PaCO2, emerging need for endotracheal intubation like cardiac arrest, hemodynamic instability, agitation, Glasgow coma score (GCS) deterioration, convulsive seizures, copious tracheal secretions, and severe NIV mask intolerance. The decision to institute IMV should be based on clinical judgment that integrates many clinical variables. The use of invasive ventilation in severe COPD patients is influenced by the likely reversibility of the precipitating event. Major hazards include the risk of ventilator associated pneumonia, barotrauma, and failure to wean to spontaneous ventilation [Citation8–11].
Ultrasonography became an extension of the physical examination and is increasingly used by intensivists to guide a lot of procedures. It has been considered a non-invasive tool for quantification of diaphragmatic contractility through measuring its thickness (DT) or the variation of diaphragmatic thickness fraction (DTF) between end-inspiration and end-expiration [Citation12–16]. AECOPD patients have a risk of diaphragmatic dysfunction/impairment (DD/DI), which may affect ventilatory management. This makes ultrasound diaphragmatic assessment a reasonable tool during management plan [Citation17–19]. Aim of this work was to study impact of ultrasound-assessed diaphragmatic impairment (DI) on predicting need for IMV in AECOPD patients treated by NIV. Primary outcome was value of DI to predict need for IMV after NIV failure. Secondary outcome was impact of DI on MV days, ICU stay, and 28-day mortality.
2. Patients and methods
This prospective observational study was carried out on 75 adult AECOPD patients of both sexes according to sample size calculation. A minimal total sample size of 56 patients with AECOPD (28 patients improved with non-invasive ventilation and another 28 patients were intubated) was needed to detect an AUC of 0.84 for using diaphragmatic ultrasonography to predict need for immediate invasive mechanical ventilation in AECOPD patients through assessment of diaphragmatic function during AECOPD using ROC curve analysis with a significance level (alpha) of 0.05 and power of 90% [Citation20]. Sample size was calculated using MedCalc Statistical Software version 16.4.3.
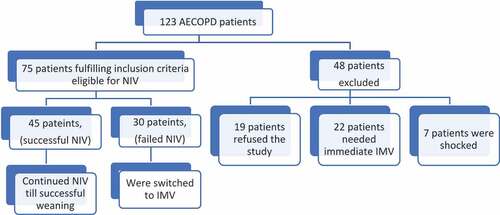
Patients were admitted to Critical Care Medicine Department in Alexandria Main University Hospital. Approval of the Medical Ethics Committee of Alexandria Faculty of Medicine was obtained. An informed consent was taken from the patients’ next of kin before their enrollment in the study. Inclusion criteria were adult (≥18 years) patients with AECOPD and eligible for NIV. Exclusion criteria were pregnant females and contraindications of NIV (unconscious uncooperative patients, shock, severe hemodynamic instability, neuromuscular disease, chest wall deformities, diaphragmatic palsy, or intra-abdominal hypertension).
All patients were subjected on admission to collection of demographic data (age and sex), patients’ comorbidities, possible causes of AECOPD, Acute Physiologic Assessment and Chronic Health Evaluation II (APACHE II) score, level of consciousness by Glasgow Coma Scale (GCS), non-invasive blood pressure (mmHg), pulse rate (beats per minute), respiratory rate (breaths per minute), oxygen saturation (%) using pulse oximeter), routine laboratory tests, and radiological investigations as needed. Arterial blood gases (ABGs) were performed on admission and then hourly or earlier until stabilization or worsening clinical parameters.
U/S assessment of the diaphragm was performed on admission and before starting NIV by a physician experienced in lung/chest US assessment who was blinded to the study. Motility of the diaphragm was assessed using a Sonosite Mindray DP10 2015–08 (China) with a 3–5-MHz linear probe to assess the diaphragm at the zone of apposition, between the 8th and 10th intercostal space in the mid-axillary or anterior axillary line, 0.5–2 cm below the costophrenic sinus. Measurements were performed with the patient in supine position at an average inclination of 45 degrees. Two parallel echogenic layers could be identified at a depth of 1.5–3 cm, the nearest was the parietal pleura and the deeper was the peritoneum. The diaphragm is the less echogenic structure in between these two lines () [Citation18,Citation21].
Figure 1. Diaphragmatic Ultrasonography for measuring DT [Citation21]. Diaphragmatic Thickness (DT) at zone of apposition in a B-mode, b M-mode. 1 Thickness at end-expiration, 2 thickness at end-inspiration. Right subcostal view in c B-mode [Citation21].
![Figure 1. Diaphragmatic Ultrasonography for measuring DT [Citation21]. Diaphragmatic Thickness (DT) at zone of apposition in a B-mode, b M-mode. 1 Thickness at end-expiration, 2 thickness at end-inspiration. Right subcostal view in c B-mode [Citation21].](/cms/asset/52e7a9bf-2158-42e7-bb95-d1a1d90a502e/teja_a_2085975_f0001_oc.jpg)
This approach was utilized to assess diaphragmatic thickness (DT) and thickening with inspiration, in B-mode (). In the subcostal area, between the mid-clavicular and anterior axillary lines, using liver or spleen as acoustic windows, diaphragm was identified as a hyperechoic line (produced by the pleura tightly adherent to the muscle) that approaches the probe during inspiration (). The thickness of the diaphragm was measured bilaterally at end-inspiration and end-expiration. Measurements were performed three times on both sides of the diaphragm, and the best value was recorded [Citation18,Citation21].
In healthy, spontaneously breathing subjects the normal thickness of the diaphragm at the zone of apposition is 1.7 ± 0.2 mm while relaxing, increasing to 4.5 ± 0.9 mm on deep inspiration [Citation18,Citation19]. The change in diaphragmatic thickness (ΔTdi) during spontaneous breathing from functional residual capacity (FRC) to tidal volume (Vt) is called diaphragmatic thickness fraction (DTF) [Citation22]. It was calculated using the formula: (end-inspiratory DT – end expiratory DT)/end expiratory DT × 100. Diaphragmatic impairment (DI) was diagnosed when bilateral DTF was less than 20% [Citation17,Citation23,Citation24].
Non-invasive mechanical ventilation (NIV) was started and set by an ICU physician who was blinded to the study, with specific software for NIV to display pressure and flow curves. Fitting face mask with proper size was used to allow using proper pressures without patient discomfort or any air leaks. Used mode was pressure support ventilation (PSV) with backup apnea ventilation. Continuous positive airway pressure (CPAP) was started at 4–6 cmH2O to be adjusted according to clinical parameters and ventilator waveforms. Pressure support (PS) was set at 10 cmH2O and gradually increased to reach tidal volume of 6–8 ml/kg (ideal body weight) and respiratory rate less than 25/minute. Inspired oxygen fraction (FiO2) was adjusted to achieve oxygen saturation of 88–92% [Citation20].
NIV was delivered as long as needed over next days and was then discontinued based on fulfillment of weaning criteria and clinical judgment. Patients were monitored and NIV was gradually discontinued when there was a general improvement in the patient’s condition with RR < 25/minute, pH > 7.35, SpO2 > 90%, and FiO2 < 0.35. This was considered as successful NIV [Citation20].
Switching to IMV was performed at any time by the attending physician who was blinded to diaphragmatic assessment of these patients according to these indications: respiratory arrest, persistent or severe respiratory distress (respiratory pauses or gasping for air, massive aspiration, or life-threatening hypoxemia), deterioration of pH, persistent respiratory acidosis despite NIV, worsening neurologic status (deteriorating GCS or agitation), intolerance to NIV, and hemodynamic instability (without response to fluids and vasoactive drugs or severe ventricular arrhythmias). This was considered as failed NIV [Citation20].
All enrolled patients were managed and followed up according to local policies and guidelines for managing AECOPD patients all through their ICU stay. Primary outcome was value of diaphragmatic impairment to predict need for IMV after NIV failure. Secondary outcome was impact of DI on MV days, ICU stay, and 28-day mortality.
2.1. Statistical analysis
Data were fed to the computer and analyzed using IBM SPSS software package version 20.0 (Armonk, NY: IBM Corp). Qualitative data were described using number and percent. Quantitative data were described using range (minimum and maximum), mean, and standard deviation. Significance of the obtained results was judged at the 5% level. Chi-square test was used for categorical variables, to compare between different groups. Fisher’s exact test was used for correction of chi-square. Student t-test was used for normally distributed quantitative variables. Paired t-test and Mann–Whitney test were used for normally and abnormally distributed quantitative variables, respectively. Receiver operating characteristic curve (ROC) was generated. Area under the ROC curve more than 50% gave acceptable performance, and area near 100% was best performance for the test.
3. Results
shows diaphragmatic thickness (DT) at zone of apposition in a: B-mode, b: M-mode. 1: Thickness at end-expiration, 2: thickness at end-inspiration, c: right subcostal view in B-mode.
draws patients’ flow chart. Current study was carried out on 75 adult AECOPD patients. According to fate of NIV, they were categorized into successful NIV group; 45 patients (60%) and failed NIV group; 30 patients (40%).
refers to baseline patients’ criteria on admission in both groups. No statistically significant difference was found between both groups regarding mean values of age, sex, comorbidities, possible causes of AECOPD, GCS, or respiratory rate/minute (p > 0.05). There was statistically significant difference between both groups in their mean APACHE II score (p = 0.005); however, its values in both groups were abnormally high (16.29 ± 2.81 and 18.20 ± 2.77, respectively).
Table 1. Comparison between both groups according to baseline patient’s criteria on admission
illustrates mean ABG parameters on admission and after NIV in both groups. Admitting individual ABG readings were comparable without statistical significance between both groups (p > 0.05). PaCO2 and SaO2 despite showed statistically significant difference between both groups on admission (p = 0.016 and 0.004, respectively); however, this difference was of no clinical importance. After NIV, all ABG parameters (except HCO3; p = 0.255) showed statistically significant improvement in their measured values regarding the successful NIV group when compared to failed NIV group (p < 0.001). When comparing before and after NIV trial in both groups, all ABG parameters (except HCO3; p1 = 0.168) showed statistically significant improvement in the successful NIV group (p1 < 0.001), while only pH and PaCO2 values showed statistically significant deterioration in the failed NIV group (p1 < 0.001).
Table 2. Comparison between both groups according to ABG on admission and after NIV
demonstrates impact of DI on need for IMV after NIV failure as our primary outcome. Regarding successful NIV group, mean right and left DTF% were 0.38 ± 0.10 and 0.33 ± 0.09, respectively. DI was evident in failed NIV group (DTF <20%), mean right and left readings were 0.18 ± 0.05 and 0.16 ± 0.05, respectively. Such bilateral DTF difference between both groups was statistically significant (p < 0.001).
Table 3. Comparison between both groups according to DTF on admission
compares between both groups according to secondary outcome (MV days, ICU stay, and 28-day mortality). Mean MV days (6.38 ± 1.90 days) in successful NIV group were significantly less than failed NIV group (14.25 ± 2.80 days) (p < 0.001). Mean ICU stay (10.98 ± 2.67 days) in successful NIV group was significantly less than failed NIV group (15.80 ± 3.10 days) (p < 0.001). Regarding 28-day mortality, non-survivors in successful NIV group (4 patients) were less than in failed NIV group (9 patients). This was statistically significant (p = 0.018).
Table 4. Comparison between both groups according to secondary outcome
and show agreement (sensitivity and specificity) data after plotting receiver operating characteristics (ROC) curve for DTF to predict need for IMV after NIV failure in all patients. Cut-off value of DTF % (<29%) at the right side was associated with NIV failure with 96.67% sensitivity, 82.22% specificity, 78.4 positive predictive value (PPV), and 97.4 negative predictive value (NPV). On the left side, cut-off value of DTF% (<26%) was associated with NIV failure with 96.67% sensitivity, 80% specificity, 76.3 PPV, and 97.3 NPV.
Figure 3. Receiver operating characteristic (ROC) curve for diaphragmatic thickness fraction (DTF) to predict need for invasive mechanical ventilation (IMV) after non-invasive ventilation (NIV) failure.
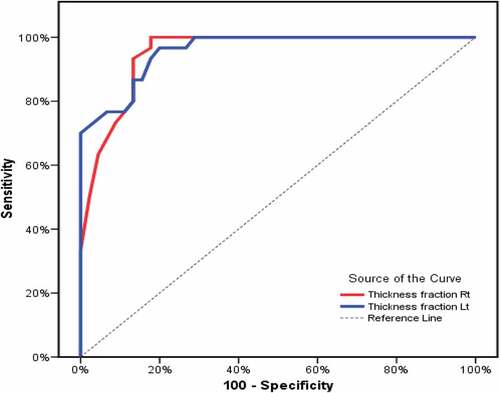
Table 5. Validity (AUC, sensitivity, specificity) of DTF to predict need for IMV after NIV failure
4. Discussion
Baseline patients’ criteria on admission including age, sex, associated comorbidities, precipitating cause (s) of AECOPD, GCS, and respiratory rate per minute were homogenous without statistical significance in both studied groups. APACHE II score was the only statistically significant variable; however, its mean values in both groups were nearly fitting within the same category with approximated in-hospital mortality rates of 25%. D. Christopher et al. [Citation25] in their review about scoring systems in critically ill patients reported that APACHE II score when calculated during the first 24 hours of ICU admission, a score of 25 represents a predicted mortality of 50%.
ABG parameters on admission in the current study were comparable. In both groups, they nearly showed uncompensated acute on top of chronic respiratory acidosis with relative hypoxia. After NIV, ABG parameters improved significantly toward normalization in the successful group, while they showed significant deterioration in the failed group. Confalonieri et al. [Citation26] suggested a chart for failure risk of NIV in AECOPD patients. They reported that patients with a GCS < 11, APACHE II ≥ 29, RR ≥ 30 breaths/min, and pH on admission < 7.25 have a predicted risk of failure > 70%. A pH < 7.25 after 2 hours greatly increased the risk of failure of NIV > 90%.
Pejkovska et al. [Citation27], in their study about predictive factors for the effect of NIV in AECOPD patients, concluded that measuring certain parameters like, pH, PaCO2, RR, and GCS may be valuable in predicting success of NIV treatment if these parameters improved within 2 hours. Fan et al. [Citation28] examined semiquantitative cough strength score (SCSS) and associated outcomes in NIV for AECOPD. They concluded that sum of SCSS, APACHE II score, and total proteins had increased power to predict NIV failure. Meanwhile, they found no statistical significance of pH, PaCO2, or PaO2 to predict NIV failure. Of note was that they enrolled ABG only on admission without follow-up after NIV treatment.
DTF in our study was measured and calculated bilaterally in both groups. Diaphragmatic dysfunction (diaphragmatic impairment) was diagnosed when DTF was < 20%. Readings in the successful NIV group were ≥ 33–38% while failed NIV readings were ≤ 16–18% indicating significant diaphragmatic impairment. Cut-off value of DTF < 26–29% on both sides was associated with NIV failure with 96.67% sensitivity and 80–82.22% specificity.
Qian et al. [Citation29] reviewed ultrasound assessment of diaphragmatic dysfunction (DD) as a predictor of weaning outcome from MV. They concluded that both diaphragmatic excursion and DTF showed good diagnostic performance to predict weaning outcomes. They defined DD to be a predictor of weaning failure in critically ill patients.
Antenora et al. [Citation30] underwent a pilot study on the prevalence and clinical consequences of diaphragmatic dysfunction (DD) diagnosed by ultrasonography during AECOPD. They considered DTF < 20% as diaphragmatic impairment (DD). They reported that NIV failure was found to be strongly associated with DD. Kocyigit et al. [Citation31] in their prospective cohort study about diaphragmatic impairment detected by ultrasound to predict NIV failure concluded that DD has high sensitivity and specificity in predicting NIV failure in patients admitted to the emergency department with AECOPD.
Marchioni et al. [Citation20] in their prospective observational study investigated ultrasound-assessed diaphragmatic impairment as a predictor of outcome in AECOPD patients on NIV. Also, they investigated the correlation between US-assessed DD and the transdiaphragmatic pressure (Pdi) assessed using invasive sniff maneuver. They found that DTF < 20% demonstrated the same accuracy as Pdi sniff in identifying diaphragmatic dysfunction. They concluded that AECOPD patients admitted for NIV treatment have a high risk of NIV failure and mortality if they had DD as assessed by US.
Mercurio et al. [Citation32] performed a preliminary physiological study to evaluate DTF and RR/DTF ratio as predictors of NIV outcome in 18 acute respiratory failure (ARF) patients. They found that the cut-off value of DTF < 36.3% significantly predicted NIV failure with sensitivity of 71.7% and specificity of 94.3%. They concluded that DTF and RR/DTF ratio may both represent valid, feasible, and non-invasive tools to predict NIV outcome in patients with de-novo ARF.
Days of MV and ICU stay in the current study were significantly lower in the successful NIV group when compared to the failed NIV group of patients. This may be attributed to major hazards of invasive mechanical ventilation including the risk of ventilator associated pneumonia, barotrauma, and failure to wean to spontaneous ventilation, all such complications could prolong duration of MV, and ICU stay [Citation11]. Such increased morbidity may have added to the increased 28-day mortality that was significantly less encountered in successful NIV group (4 patients) when compared to the failed NIV group (9 patients) who were intubated and mechanically ventilated.
Stefan et al. [Citation33] in their retrospective study of prospectively collected data compared between NIV and IMV in critically ill patients with AECOPD as regards their outcomes. More than three thousand patients were enrolled into the study. They found that NIV failure was associated with the worst outcomes. They concluded that promoting NIV was associated with a lower risk of in-hospital mortality compared with that of IMV.
Ansari et al. [Citation34] conducted a study on 104 AECOPD patients to assess frequency, predictors, and outcome of NIV failure after switching to IMV. showed statistical significance between success and failed group according to ICU stay. They found that tachycardia, hypotension, hypoxia, and respiratory acidosis when corrected by NIV within first 24 hours can predict successful NIV. They concluded also that failed NIV within first 24 hours carries poor outcome and higher mortality.
Lindenauer et al. [Citation35] conducted a retrospective study on 25,628 AECOPD patients to compare patients’ outcomes when treated with NIV or IMV. They concluded that COPD patients treated by immediate NIV had lower mortality, less morbidity, shorter ICU stay, and lower costs compared to those who failed NIV and were treated with IMV.
This study had some limitations. First, we didn’t perform comparison with other methods that are considered gold standard in the diaphragmatic function assessment like transdiaphragmatic pressure, phrenic nerve stimulation, and electromyography. These methods are expensive, invasive, require special equipment, and specialized team. Another point is that ultrasonography is an operator dependent technique that needs a lot of training.
5. Conclusion
Based on the results of this prospective observational study, DTF was a good indicator of DI that could predict need for IMV after NIV failure in AECOPD patients with 96.67% sensitivity and 80–82.22% specificity. Days of MV, ICU stay, and 28-day mortality were significantly higher in patients with DI who needed IMV after NIV failure.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Quaderi SA, Hurst JR. The unmet global burden of COPD. Glob Health Epidemiol Genom. 2018;3:e4.
- López-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology. 2016;21(1):14–23.
- Albertson TE, Louie S, Chan AL. The diagnosis and treatment of elderly patients with acute exacerbation of chronic obstructive pulmonary disease and chronic bronchitis. J Am Geriatr Soc. 2010;58(3):570–579.
- Molinari N, Briand C, Vachier I, et al. Hospitalizations for COPD exacerbations: trends and determinants of death. Copd. 2015;12(6):621–627.
- MacIntyre N, Huang YC. Acute exacerbations and respiratory failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(4):530–535.
- Fromer L, Cooper CB. A review of the GOLD guidelines for the diagnosis and treatment of patients with COPD. Int J Clin Pract. 2008;62(8):1219–1236.
- Lief L, McSparron J. Acute Exacerbation of COPD. In: Hyzy RC, McSparron J, editors. Evidence-based critical care. London: Springer; 2020. p. 169–173.
- Duca A, Rosti V, Brambilla AM, et al. Non-invasive ventilation in COPD exacerbation: how and why. Intern Emerg Med. 2019;14(1):139–142.
- Pacilli AM, Valentini I, Carbonara P, et al. Determinants of noninvasive ventilation outcomes during an episode of acute hypercapnic respiratory failure in chronic obstructive pulmonary disease: the effects of comorbidities and causes of respiratory failure. Biomed Res Int. 2014;2014:976783.
- Carratù P, Bonfitto P, Dragonieri S, et al. Early and late failure of noninvasive ventilation in chronic obstructive pulmonary disease with acute exacerbation. Eur J Clin Invest. 2005;35(6):404–409.
- Rose L. Management of critically ill patients receiving noninvasive and invasive mechanical ventilation in the emergency department. Open Access Emerg Med. 2012;4:5–15.
- Saugel B, Scheeren TWL, Teboul JL. Ultrasound-guided central venous catheter placement: a structured review and recommendations for clinical practice. Crit Care. 2017;21(1):225.
- Cimsit C, Bekir M, Karakurt S, et al. Ultrasound assessment of diaphragm thickness in COPD. Mmj. 2016;29:8–13.
- El Aziz AA A, Elwahsh RA, Abdelaal GA, et al. Diaphragmatic assessment in COPD patients by different modalities. Egypt J Chest Dis Tuberculosis. 2017;66(2):247–250.
- Baria MR, Shahgholi L, Sorenson EJ, et al. B-mode ultrasound assessment of diaphragm structure and function in patients with COPD. Chest. 2014;146(3):680–685.
- Lim SY, Lim G, Lee YJ, et al. Ultrasound assessment of diaphragmatic function during acute exacerbation of chronic obstructive pulmonary disease: a pilot study. Int J Chron Obstruct Pulmon Dis. 2019;14:2479–2484.
- Hermans G, Agten A, Testelmans D, et al. Increased duration of mechanical ventilation is associated with decreased diaphragmatic force: a prospective observational study. Crit Care. 2010;14:R127.
- Zambon M, Greco M, Bocchino S, et al. Assessment of diaphragmatic dysfunction in the critically ill patient with ultrasound: a systematic review. Intensive Care Med. 2017;43:29–38.
- Thimmaiah VT, G MJ, Jain KP. Evaluation of thickness of normal diaphragm by B mode ultrasound. Int J Contemp Med Res. 2016;3:2658–2660.
- Marchioni A, Castaniere I, Tonelli R, et al. Ultrasound-assessed diaphragmatic impairment is a predictor of outcomes in patients with acute exacerbation of chronic obstructive pulmonary disease undergoing noninvasive ventilation. Crit Care. 2018;22(1):109.
- Bello G, Blanco P. Lung ultrasonography for assessing lung aeration in acute respiratory distress syndrome: a narrative review. J Ultras Med. 2019;38(1):27–37.
- Suzuki M, Makita H, Ito YM, et al. Clinical features and determinants of COPD exacerbation in the Hokkaido COPD cohort study. Eur Respir J. 2014;43(5):1289–1297.
- Ali ER, Mohamad AM. Diaphragm ultrasound as a new functional and morphological index of outcome, prognosis, and discontinuation from mechanical ventilation in critically ill patients and evaluating the possible protective indices against VIDD. Egypt J Chest Dis Tuberc. 2017;66:339–351.
- Kim WY, Suh HJ, Hong SB, et al. Diaphragm dysfunction assessed by ultrasonography: influence on weaning from mechanical ventilation. Crit Care Med. 2011;39:2627–2630.
- Bouch DC, Thompson JP. Severity scoring systems in the critically ill. Contin Educ Anaesth Crit Care Pain. 2008;8(5):181–185.
- Confalonieri M, Garuti G, Cattaruzza MS, et al. A chart of failure risk for noninvasive ventilation in patients with COPD exacerbation. Eur Respir J. 2005;25(2):348–355.
- Pejkovska S, Kaeva BJ, Goseva Z, et al. Predictive factors for the effect of treatment by noninvasive ventilation in patients with respiratory failure as a result of acute exacerbation of the chronic obstructive pulmonary disease. Open Access Maced J Med Sci. 2015;3(4):655–660.
- Fan L, Zhao Q, Liu Y, et al. Semiquantitative cough strength score and associated outcomes in noninvasive positive pressure ventilation patients with acute exacerbation of chronic obstructive pulmonary disease. Respir Med. 2014;108(12):1801–1807.
- Qian Z, Yang M, Li L, et al. Ultrasound assessment of diaphragmatic dysfunction as a predictor of weaning outcome from mechanical ventilation: a systematic review and meta-analysis. BMJ Open. 2018;8(9):e021189.
- Antenora F, Fantini R, Iattoni A, et al. Prevalence and outcomes of diaphragmatic dysfunction assessed by ultrasound technology during acute exacerbation of COPD: a pilot study. Respirology. 2017;22(2):338–344.
- Kocyigit H, Gunalp M, Genc S, et al. Diaphragm dysfunction detected with ultrasound to predict noninvasive mechanical ventilation failure: a prospective cohort study. Am J Emerg Med. 2021;45:202–207.
- Mercurio G, D’Arrigo S, Moroni R, et al. Diaphragm thickening fraction predicts noninvasive ventilation outcome: a preliminary physiological study. Crit Care. 2021;25(1):219.
- Stefan MS, Nathanson BH, Higgins TL, et al. Comparative effectiveness of noninvasive and invasive ventilation in critically Ill patients with acute exacerbation of chronic obstructive pulmonary disease. Crit Care Med. 2015;43(7):1386–1394.
- Ansari SF, Memon M, Brohi N, et al. Noninvasive positive pressure ventilation in patients with acute respiratory failure secondary to acute exacerbation of chronic obstructive pulmonary disease. Cureus. 2019;11(10):e5820.
- Lindenauer PK, Stefan MS, Shieh MS, et al. Outcomes associated with invasive and noninvasive ventilation among patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med. 2014;174(12):1982–1993.