ABSTRACT
Background
This study aimed to assess the safety and efficacy of a neostigmine/ketorolac combination as an adjuvant to a low-dose local anesthetic mixture used for peribulbar block during vitrectomy surgeries.
Methods
This double-blinded, parallel-group, randomized trial enrolled 50 adult patients of both genders who were American Society of Anesthesiologists physical status I or II and were scheduled for vitrectomy surgeries under regional anesthesia. All patients underwent peribulbar block through a single medial canthus injection technique. In the neostigmine and ketorolac (NK) group, 25 patients received a combined mixture of neostigmine (300 μg) and ketorolac (4 mg/ml) as adjuvants to a mixture of lidocaine (2%, 2.5 ml) and bupivacaine (0.5%, 3.5 ml) containing 75 units of hyaluronidase. The control (C) group received 1.5 ml of normal saline instead of neostigmine/ketorolac. The primary outcomes were the onsets and durations of both sensory and motor blocks. Secondary outcomes included the time to first analgesic dose, hemodynamics, adverse effects, and patient’s and surgeon’s satisfaction.
Results
The onsets of sensory and motor blocks were significantly shorter in the NK group compared to the C group. A significantly longer anesthesia and akinesia duration were observed in the NK group in comparison to the C group. The meantime to the first analgesic dose showed a significant difference between the NK and C groups. The NK group had a higher rate of adverse effects than the C group, though not reaching statistical significance. Patients’ and surgeons’ satisfactions were significantly higher in the NK group than the C group.
Conclusions
In peribulbar anesthesia for vitrectomy surgery, adding neostigmine/ketorolac combination to a low-dose local anesthetic mixture was effective in reducing the onset and prolonging the duration of both sensory and motor block, and it was associated with higher safety and better patients’ and surgeons’ satisfaction.
1. Introduction
Regional anesthesia is widely used in ophthalmic surgeries, especially for the elderly with other co-morbidities. Block approaches can achieve optimal surgical circumstances while avoiding the hazards of general anesthesia. In addition, the block techniques allow for a faster patient turnover at a lower cost [Citation1].
Vitrectomy is the surgical removal of the vitreous humor that is commonly done under local anesthesia. It is used to treat retinal detachment, diabetic vitreous hemorrhage, epiretinal membrane, macular hole, and retinopathies [Citation2]. However, patients who had a vitrectomy may still experience stress and pain throughout the procedure because local anesthetics usually have a limited analgesic impact [Citation3].
For peribulbar anesthesia, the single medial canthal route is a potential technique for ocular surgery. With the eye fixated on the primary gaze, the needle is inserted into the medial canthus. The needle’s route along the medial wall of the orbit is basically perpendicular to the coronal plane. The local anesthetic is injected in the peribulbar, extraconal area beyond the equator of the globe and around the posterior pole. With a considerable amount of the local anesthetic, the injection offers a high quality of analgesia and akinesia of the globe and the lids. This is due to episceral or subtenon local anesthetic distribution [Citation4]. The single medial injection technique is as effective as the two injections technique in analgesia and akinesia. Furthermore, the medial canthus is considered the safest site with few anatomical structures [Citation5–7].
Lidocaine is approved by the United States Food and Drug Administration and is usually used for local anesthesia. Lidocaine produces analgesia and akinesia quickly, but it has a short duration [Citation8]. It has several adverse effects, such as dizziness, nystagmus, drowsiness, decreased blood pressure, heart rate, and cardiac output, and sometimes cardiovascular collapse [Citation9].
Neostigmine is a parasympathomimetic drug that binds to the active side of the acetylcholine esterase enzyme, preventing it from hydrolyzing the acetylcholine molecule, increasing its level at peripheral muscarinic receptors present in peripheral nerve endings, activating cholinergic-mediated antinocieption, and thus prolonging postoperative analgesia. Neostigmine has been co-administered with local anesthetics and other adjuvants in obstetric surgeries [Citation10].
Ketorolac belongs to the family of nonsteroidal anti-inflammatory drugs (NSAIDs) and has been confirmed a short-term analgesic that is as effective as morphine [Citation11,Citation12]. Kim et al. [Citation13] showed that preoperative ketorolac could effectively reduce postoperative pain in laser-assisted subepithelial keratectomy. Chen et al. [Citation14] reported beneficial effect of ketorolac with local anesthesia that may contribute to a wider-spread adoption of day care retinal detachment surgery
In animal studies, intrathecal neostigmine has additive analgesic effect to NSAIDs that suppose significant clinical ramifications [Citation15].
This study aimed to assess the safety and efficacy of a neostigmine/ketorolac combination as an adjuvant to a low-dose local anesthetic mixture through a single medial canthus injection peribulbar block for vitrectomy surgeries.
2. Methods
2.1. Ethical considerations
This study was carried out following approval by the Ethics Committee of the Research Institute of Ophthalmology, Giza, Egypt. The study has been conducted in accordance with the principles set forth in the Helsinki Declaration. This trial was registered at the Pan African Clinical Trial Registry (PACTR202204650206424). After explanation of the purpose and procedures of the study, a written informed consent was obtained from each participant. All participants’ data were kept confidential.
2.2. Study design, settings, and date
This double-blinded, parallel-group, randomized trial was conducted at Research Institute of Ophthalmology, Giza, Egypt during April and May 2022.
2.3. Eligibility criteria
The present study included 50 adult (25–65 year) patients of both genders, who were American Society of Anesthesiologists physical status I or II and were scheduled for vitrectomy surgery with axial eye length ranging from 20 to 29 mm. We excluded patients who had bradycardia, bronchial asthma, or severe coagulation disorders. Patients who cannot adapt supine position as those with skeletal problems and uncooperative patients were also excluded.
2.4. Randomization, allocation concealment, and blinding
Randomization and allocation concealment were performed using the sequentially numbered, opaque, sealed envelopes method [Citation16]. The allocation sequence was concealed from the physician assessing and enrolling participants. To prevent subversion of the allocation sequence, the name and hospital admission number of the participant were written on the envelope. A carbon paper transferred the information onto the allocation card inside the envelope. The corresponding envelope was opened only after the enrolled participant completed all baseline assessments and it was time to allocate the intervention. The block was administered by an anesthesiologist who was blinded to the drugs he was injecting. The patients and the outcome evaluators were also blinded to group allocation.
2.5. Interventions
Fifty patients were randomly allocated into two groups (25 patients each). All patients received peribulbar block in the form of single medial canthus injection using a local anesthetic mixture of 2.5 ml of lidocaine 2% and 3.5 ml of bupivacaine 0.5% containing 75 units of hyaluronidase.
Patients in the neostigmine and ketorolac (NK) group received the local anesthetic mixture besides a combination of 300 μg of neostigmine (Epistigmin® 0.05%, 50 μg/ml, Egyptian International Pharmaceutical Industries Company, Egypt) and 4 mg/ml of ketorolac (Ketolac®, Amryia Pharmaceutical Industries Company, Egypt). Patients in the control (C) group received the local anesthetic mixture in addition to 1.5 ml of normal saline instead of the neostigmine/ketorolac mixture.
All patients were subjected to full history taking, clinical examination, and laboratory investigations including complete blood count, coagulation profile, liver and kidney function tests as well as ECG and chest radiography. Details of the anesthetic technique were explained to the patient on the preoperative visit.
Patients were fasting for at least 6 h preoperatively. Routine monitoring was done in the form of automated noninvasive blood pressure, pulse oximetry, and ECG. All the baseline parameters were observed and recorded. A good venous access was secured with 20-G cannula; oxygen (2 L/min) was administered through a nasal cannula. Premedications (2 mg of midazolam, 0.03 mg of fentanyl, and 20 mg of propofol) were given to all patients.
The eye was cleansed with 5% povidone iodine and sterile gauze before being dried. For the peribulbar block, single injection technique through the medial canthus with a 25-G, one-inch-long disposable needle was used. It was inserted at the caruncle (as medial as possible), passing between the medial orbital wall and the globe into the medial orbital compartment. The needle was advanced into the orbit to a depth of approximately 20 mm perpendicular to the sagittal plane. After gentle negative aspiration, the anesthetic solution was injected. After needle withdrawal, a digital massage was applied for 2 minutes [Citation5].
To assess ocular anesthesia, corneal anesthesia was also evaluated using a small piece of cotton. The onset of corneal anesthesia was recorded in seconds from the time of injection till complete loss of corneal sensation. The duration of sensory block was calculated in minutes from the time of sensory loss till the beginning of return of sensation.
To assess ocular akinesia, the patients were asked to look in four directions: lateral, medial, superior, and inferior. The ocular movement in each direction was scored as 2 if it was normal, 1 if it was limited, and 0 if there was no movement (total score: 0–8). The patient was also asked to forcefully close his eyes to assess the orbicularis oculi muscle on a scale of 0–2 (0, complete akinesia; 1, partial; 2, normal movement). The signs of successful block were dropping of the upper lid with inability to open the eyes (ptosis), absent eye movement in all four directions (akinesia), and inability to fully close the eye once opened. The onset of akinesia was calculated in seconds from the time of injection till complete loss of movement, while the duration of akinesia was calculated in minutes from the time of movement loss till full return of movement.
Additionally, the time to the first analgesic dose requirement, heart rate (HR), systolic and diastolic blood pressures (SBP, DBP), and oxygen saturation (SpO2) were recorded every 10 minutes for 120 minutes.
Local and systemic complications, such as subconjunctival hemorrhage, bradycardia, or vomiting were recorded. The patients’ satisfaction score was assessed by asking the patient at the end of the surgery (1, complete dissatisfaction; 2, some dissatisfaction; 3, complete satisfaction). The surgeons’ (who were blinded to group allocation) satisfaction was assessed by asking the surgeons at the end of surgery (0, total dissatisfaction; 1, poor; 2, acceptable; 3, total satisfaction).
2.6. Outcomes
The primary outcomes were the onsets and durations of both motor and sensory blocks. The secondary outcomes included the time to first analgesic requirement, patients’ hemodynamics (heart rate, blood pressure, and oxygen saturation), adverse effects, and patient’s and surgeon’s satisfaction.
2.7. Sample size
The sample size was calculated using the G power 3.1.9.2 software after setting the used statistical test to one-way analysis of variance, with a unilateral α of 0.05, power to 0.80, and the allocation ratio to 1:1. According to Mirkheshti et al. [Citation17], an estimated 25 patients per group would be needed to provide 80% power for independent populations, assuming large Cohen’s effect sizes (0.8 to 0.9) regarding the onset of sensory block and the time to first analgesic request. The effect size for sample size calculation was set to 0.4, which was considered the threshold of a large effect size for this test. The calculated sample size was 22 subjects per group. We added 10% to account for the loss to follow up. The final sample size was then 25 subjects per group (total sample size was 50 patients).
2.8. Statistical analysis
The Statistical Package for Social Sciences (SPSS) version 26 for Windows (IBM© Corp., Armonk, N.Y., USA) was used for performing the analysis. The distribution of the numerical data was tested using the Shapiro–Wilk test for normality. All data were normally distributed and were summarized as mean ± standard deviation (SD). Comparisons between the two groups were done using the independent samples T-test. Categorical data were summarized as frequencies (count and percentage), and the associations between the studied groups were tested using the Pearson’s Chi-square test or Fisher’s exact test. A p-value <0.05 was adopted to interpret the significance of statistical tests.
3. Results
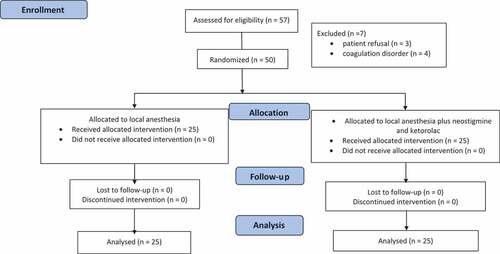
Fifty-seven patients were recruited, three patients refused to participate, and four patients were excluded due to coagulation disorders. Fifty patients received single medial canthus peribulbar block during vitrectomy surgery and were randomly allocated into two groups (25 patients each) with a 1:1 allocation ratio. The NK group received combined neostigmine and ketorolac as adjuvants to mixture of 2,5 ml of lidocaine 2%, 75 unit of hyaluronidase and 3,5 ml of bupivacaine 0.5%. Group C patients received the same local anesthetic mixture and 1.5 ml of normal saline instead of the neostigmine and ketorolac combination ().
The mean age of the enrolled patients was 60.2 ± 8.1 years in the control group and 49.9 ± 13.2 in the NK group. Compared to the control group, the NK group had a significantly more rapid onset of anesthesia (40.0 ± 8.2 vs. 23.0 ± 5.6 seconds, p < 0.001) and akinesia (87.4 ± 12.7 vs. 66.6 ± 10.8 seconds, p < 0.001). Meanwhile, the mean duration of anesthesia and akinesia was significantly longer in the NK group than in the control group (105.6 ± 14.2 & 133.6 ± 15.0 minutes vs. 86.8 ± 12.5 & 101.6 ± 11.8 min, respectively, p < 0.001). The time to the first analgesic demand was significantly longer in the NK group than in the controls (4.9 ± 0.9 vs. 2.9 ± 0.7 hours, p < 0.001; ).
Table 1. Age, onset, duration of anesthesia and akinesia, and time to the first analgesic demand (total n = 50).
Monitoring after induction of anesthesia revealed slight fluctuations of the mean heart rate in the control group, whereas a gradual decrease was observed in the NK group. However, no significant differences were observed between the two groups during the observation period (all p > 0.05; ).
Table 2. Heart rate following the induction of anesthesia (total n = 50).
Recording of the blood pressure after induction of anesthesia showed that the mean systolic blood pressure was significantly lower in the NK group compared to the control group during the observation period up to 120 minutes post-induction (all p < 0.05; ). On the contrary, the diastolic blood pressure was significantly higher in the NK group than in the control group (all p < 0.05; ).
Table 3. Systolic & diastolic blood pressure following the induction of anesthesia (total n = 50).
The oxygen saturation showed slight fluctuations during the observation period after induction and was 97% or above in all patients. There was a significant difference between the two groups at 30 and 40 minutes but without an apparent clinical significance ().
Table 4. Oxygen saturation following the induction of anesthesia (total n = 50).
As regards the safety of the evaluated techniques, the NK group had a higher rate of adverse effects compared to the control group, though not reaching statistical significance (16 vs. 8%, p = 0.667). The encountered adverse effects comprised bradycardia (n = 4, 16% of cases) in the NK group, as well as pain requiring more anesthesia (n = 1, 4%) and subconjunctival hemorrhage (n = 1, 4%) in the control group. A significantly higher percentage of patients expressed complete satisfaction in the NK group than in the control group (88 vs. 37.5%, p < 0.001). In addition, a significantly higher percentage of surgeons considered the operative conditions perfect in the NK group (88 vs. 41.7%, p = 0.001; ).
Table 5. The adverse effects as well as patients’ and surgeons’ satisfaction (total n = 50).
4. Discussion
In local anesthesia for ophthalmic surgery, a rapid onset of anesthesia with sustained intraoperative akinesia, analgesia as well as improved postoperative comfort are a desired goal. Hence, our study aimed to assess the safety and efficacy of the combined administration of neostigmine and ketorolac as adjuvants to a mixture of lidocaine 2% and bupivacaine 0.5% through single medial canthus injection peribulbar block during vitrectomy surgeries.
The current study showed that the addition of neostigmine and ketorolac to the mixture of local anesthetics lidocaine 2% and bupivacaine 0.5% had significantly more rapid onset and longer duration of sensory and motor block, as well as longer time to first analgesic demand compared to the local anesthetic mixture only. Also, the adjuvant administration of neostigmine and ketorolac produced a lower heart rate and systolic blood pressure, meanwhile slightly higher diastolic blood pressure. Oxygen saturation showed no apparent clinically significant difference. However, bradycardia was observed among patients receiving combined neostigmine and ketorolac but with non-significant difference between groups. Both patients and surgeons were satisfied with the neostigmine and ketorolac combination as they provided the sedation that enables full cooperation.
Our results agrees with Aboul Fetouh et al. [Citation18] who studied the effect of 0.5 mg and 0.25 mg of neostigmine coupled with lidocaine in peribulbar anesthesia for cataract surgery. Neostigmine in 0.5 mg was superior to 0.25 mg in terms of sensory and motor block as well as postoperative analgesia. Furthermore, neostigmine had good postoperative analgesia with a longer duration to the first analgesic requirement without adverse effects. Neostigmine 0.5 mg was safe with a non-significant rise in postoperative nausea and vomiting. Our result showed a significant difference in age between the studied groups, which could be attributed to the small number of our patients. Similarly, Elkins [Citation19] noted that small trials were liable for baseline imbalance.
Another study by Elbahrawy and El-Deeb [Citation20] found that the use of neostigmine with supraclavicular brachial plexus block in chronic renal failure patients did not affect the duration of the block. However, 500 µg of neostigmine shortened the onsets of sensory and motor blockade and enhanced the postoperative analgesia with no significant side effects.
Our study revealed that ketorolac had a beneficial role, which agrees with an earlier study [Citation14] where ketorolac addition to local anesthesia contributed to a wider-spread adoption of daycare retinal detachment surgery. Reinhart et al. [Citation21] found that adding ketorolac to lidocaine for ankle block improved the quality and duration of postoperative analgesia. Ethanol, which is employed as a diluent for ketorolac, was unlikely to be the cause of ketorolac’s improved postoperative analgesia. Furthermore, when ketorolac and local anesthetics were blended, chemical stability and shelf life were retained.
In an earlier animal study, Miranda et al. [Citation15] reported a synergistic effect between neostigmine and NSAIDs, such as ketoprofen, paracetamol, and diclofenac. The increased acetylcholine concentration in the synaptic cleft of cholinergic interneurons could have modulated supraspinal antinociception.
Perioperative eye discomfort is caused by several causes. Primary phase injury can be due to high-intensity noxious stimulation caused by tissue division, repeated ocular muscular traction, manipulation, and trauma to the globe and nearby tissues when planting segment silicone explants and encircling bands, cryopexy, and drainage of subretinal fluid. When noxious impulses from surgery-induced tissue trauma reach the spinal cord, they cause central neural sensitization, which intensifies pain sensations [Citation22]. Inflammation is mostly responsible for the secondary phase of injury. Tissue manipulation and cryopexy may cause the blood-retinal barrier to break down, releasing prostaglandins and other inflammatory mediators [Citation23]. The inflammatory substances and released enzymes lower the threshold for nociceptor neuron activity, resulting in pain [Citation24].
Lidocaine’s anesthetic effect has been connected to the suppression of substance P binding as well as substance P-evoked intracellular calcium rise [Citation25]. It produces analgesia and akinesia quickly, but it has a short duration [Citation8]. Hence, ocular pain may be caused by insufficient afferent blocking of the local anesthetic [Citation26]. However, bupivacaine, when used alone, has the benefit of a prolonged duration of the block, although it is delayed for the onset of anesthesia [Citation27]. Therefore, a mixture of bupivacaine and lidocaine is frequently used for a rapid onset of analgesia and a prolonged duration of action; nevertheless, the mixture may reduce the benefits of both agents [Citation28]. The usage of lidocaine is associated with dose-dependent adverse effects on the cardiac and central nervous systems. Hence, several adjuvants have been used with local anesthesia to prolong the duration of sensory-motor block limiting the cumulative dose requirement of the local anesthetic [Citation1].
Neostigmine’s analgesic properties have been determined by nitric oxide release and the reversible inhibition of cholinesterase enzyme, resulting in an increased concentration of acetylcholine and subsequent binding to both muscarinic and nicotinic receptors [Citation29]. Ketorolac is a powerful analgesic NSAID where the main site of action is at the nerve terminal by blocking the peripheral synthesis of prostaglandins. Ketorolac inhibits the activities of cyclooxygenase-(COX) 1 and 2 responsible for production of inflammatory mediators including prostaglandins [Citation30]. As a result, ketorolac lowers the afferent sensitivities and painful experiences. Hence, the combination of neostigmine, ketorolac, lidocaine, and bupivacaine were supposed to produce effective analgesia.
In contrast to our findings, Mirkheshti et al. [Citation17] reported that ketorolac could not decrease sensory and motor block onset in infraclavicular brachial plexus block. While the time to the first analgesic request in the ketorolac group was significantly higher than that in the dexmedetomidine and the lidocaine groups. This discordance in sensory block onset was attributed to that those researchers used lidocaine as the main local anesthetic, but we used a mixture of lidocaine 2% and bupivacaine 0.5% as our main local anesthetics, which may have caused different sensory and motor block effects.
Regarding safety, animal studies showed that neostigmine produces perineural toxicity, nausea, and vomiting. Additionally, ketorolac, like other NSAIDs, produces gastrointestinal bleeding, dyspepsia, and headaches. However, few non-significant problems were discovered in our study. These findings were in accordance with an earlier phase I safety assessment of intrathecal neostigmine methyl sulfate that showed a dose-dependent incidence of some adverse effects [Citation31]. Further studies reported neostigmine with doses less than 50 μg were not associated with any adverse effects [Citation32,Citation33]. Ketorolac was also reported as a safe NSAID when used as adjuvant to local anesthetics [Citation14,Citation21].
The patient and surgeon were significantly satisfied with neostigmine and ketorolac addition to the local anesthetic mixture where full cooperation during eye surgery is very important. Patient satisfaction was assessed by the incidence of postoperative pain and the level of preoperative anxiety. Meanwhile, Sadlak et al. [Citation34] reported a modest amount of connection between patient satisfaction with anesthesia and physician satisfaction. This signifies that doctors might be poor predictors of anesthetic satisfaction and inconsistent evaluators of perioperative patient comfort.
The current research was a single-center study with a small sample size. Hence, larger, multicenter RCTs are needed.
5. Conclusions
Through a single medial canthus peribulbar block, the addition of neostigmine/ketorolac combination to a low-dose lidocaine/bupivacaine local anesthetic mixtures induced more rapid onsets and longer durations of both sensory and motor blocks and lengthened the time to first analgesic requirement after vitrectomy surgery. Furthermore, the use of these adjuvants was associated with non-significant adverse effects, better safety, hemodynamic stability, and significant patients’ and surgeons’ satisfaction. Thus, addition of neostigmine and ketorolac adjuncts to local anesthetic mixtures could produce more appropriate surgical conditions.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Lodhi O, Tripathy K. Anesthesia for eye surgery. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC; 2022.
- De Maria M, Panchal B, Coassin M. Update on indications for diabetic vitrectomy and management of complications. Annals Eye Sci. 2018;3:51.
- Raczyńska D, Glasner L, Serkies-Minuth E, et al. Eye surgery in the elderly. Clin Interv Aging. 2016;11:407–414.
- Vohra SB, Good PA. Altered globe dimensions of axial myopia as risk factors for penetrating ocular injury during peribulbar anaesthesia. Br J Anaesth. 2000;85(2):242–245.
- Brahma AK, Pemberton CJ, Ayeko M, et al. Single medial injection peribulbar anaesthesia using prilocaine. Anaesthesia. 1994;49(11):1003–1005.
- Nouvellon E, Cuvillon P, Ripart J. Regional anesthesia and eye surgery. Anesthesiology. 2010;113(5):1236–1242.
- Demediuk OM, Dhaliwal RS, Papworth DP, et al. A comparison of peribulbar and retrobulbar anesthesia for vitreoretinal surgical procedures. Arch ophthalmol ( Chicago, Ill.: 1960 . 1995;113:908–913.
- Oji E, Oji A. Bupivacaine and lignocaine for ophthalmic surgery. Br J Ophthalmol. 1987;71(1):66–68.
- Kalra P. Miller’s anesthesia, volumes 1 and 2, 7th edition. Anesthesiology. 2010;112(1):260–261.
- Cossu AP, De Giudici LM, Piras D, et al. A systematic review of the effects of adding neostigmine to local anesthetics for neuraxial administration in obstetric anesthesia and analgesia. Int J Obstet Anesth. 2015;24(3):237–246.
- Pathan SA, Mitra B, Straney LD, et al. Delivering safe and effective analgesia for management of renal colic in the emergency department: a double-blind, multigroup, randomised controlled trial. Lancet. 2016;387(10032):1999–2007.
- Grimsby GM, Conley SP, Trentman TL, et al. A double-blind randomized controlled trial of continuous intravenous ketorolac vs placebo for adjuvant pain control after renal surgery. Mayo Clin Proc. 2012;87(11):1089–1097.
- Kim SK, Hong JP, Nam SM, et al. Analgesic effect of preoperative topical nonsteroidal antiinflammatory drugs on postoperative pain after laser-assisted subepithelial keratectomy. J Cataract Refract Surg. 2015;41:749–55.
- Chen X, Liu B, Liang X, et al. The combination of ketorolac with local anesthesia for pain control in day care retinal detachment surgery: a randomized controlled trial. J Ophthalmol. 2017;2017:3464693.
- Miranda HF, Sierralta F, Pinardi G. Neostigmine interactions with non steroidal anti-inflammatory drugs. Br J Pharmacol. 2002;135(7):1591–1597.
- Doig GS, Simpson F, Delaney A. A review of the true methodological quality of nutritional support trials conducted in the critically ill: time for improvement. Anesth Analg. 2005;100(2):527–533.
- Mirkheshti A, Saadatniaki A, Salimi A, et al. Effects of dexmedetomidine versus ketorolac as local anesthetic adjuvants on the onset and duration of infraclavicular brachial plexus block. Anesth Pain Med. 2014;4(3):e17620–e.
- Aboul Fetouh IS, Sherif NA, Osama NA, et al. Safety and efficacy of adding different doses of neostigmine as an adjuvant in peribulbar block for cataract surgery: a randomized controlled trial. Egypt J Anaesth. 2021;37(1):349–355.
- Elkins MR. Assessing baseline comparability in randomised trials. J Physiother. 2015;61(4):228–230.
- Elbahrawy K, El-Deeb A. The effects of adding neostigmine to supraclavicular brachial plexus block for postoperative analgesia in chronic renal failure patients: a prospective randomized double-blinded study. Res Opin Anesth Intensive Care. 2016;3:36–41.
- Reinhart DJ, Stagg KS, Walker KG, et al. Postoperative analgesia after peripheral nerve block for podiatric surgery: clinical efficacy and chemical stability of lidocaine alone versus lidocaine plus ketorolac. Reg Anesth Pain Med. 2000;25(5):506–513.
- Marzak S, Miloudi Y, El Harrar N, et al. Postoperative pain in retinal detachment surgery. J Fr Ophtalmol. 2007;30(10):992–997.
- Massicotte E, Hammamji K, Landry T, et al. Postoperative Pain Management in Vitreoretinal Surgery for Retinal Detachment: a Systematic Review of Randomized Controlled Trials. Canadian Journal of Pain. 2018;2(1):160–175
- Perl ER. Pain mechanisms: a commentary on concepts and issues. Prog Neurobiol. 2011;94(1):20–38.
- Li YM, Wingrove DE, Too HP, et al. Local anesthetics inhibit substance P binding and evoked increases in intracellular Ca2+. Anesthesiology. 1995;82(1):166–173.
- Sadiq SA, Stevenson L, Gorman C, et al. Use of indomethacin for pain relief following scleral buckling surgery. Br J Ophthalmol. 1998;82(4):429.
- Zand F, Azemati S. Comparative study of onset and duration of action of 0.5% bupivacaine and a mixture of 0.5% bupivacaine and 2% lidocaine for epidural anesthesia. Acta Med Iran. 2004;42:256–258.
- Chin GN, Almquist HT. Bupivacaine and lidocaine retrobulbar anesthesia. a double-blind clinical study. Ophthalmol. 1983;90:369–372.
- Lauretti GR. The evolution of spinal/epidural neostigmine in clinical application: thoughts after two decades. Saudi J Anaesth. 2015;9(1):71–81.
- Dong L, Smith JR, Winkelstein BA. Ketorolac reduces spinal astrocytic activation and PAR1 expression associated with attenuation of pain after facet joint injury. J Neurotrauma. 2013;30(10):818–825.
- Hood DD, Eisenach JC, Tuttle R. Phase I safety assessment of intrathecal neostigmine methylsulfate in humans. Anesthesiology. 1995;82(2):331–343.
- Swain A, Nag DS, Sahu S, et al. Adjuvants to local anesthetics: current understanding and future trends. World J Clin Cases. 2017;5(8):307–323.
- Ho KM, Ismail H, Lee KC, et al. Use of Intrathecal Neostigmine as an Adjunct to Other Spinal Medications in Perioperative and peripartum analgesia: a meta-analysis. Anaesth Intensive Care. 2005;33(1):41–53.
- Sadlak N, Fiorello MG, Cabral HJ, et al. Poor correlation of provider and patient satisfaction with anesthesia in ophthalmic surgeries: a secondary analysis of a clinical trial. Clin Ophthalmol. 2022;16:677–683.