ABSTRACT
Background
Non-invasive Hb (SpHb) may allow for a faster detection of clinically important blood loss, improve perioperative transfusion practices significantly and enable a faster evaluation of a patient’s condition and a better blood treatment and may even reduce needless transfusions. This study determined the accuracy of continuous SpHb monitoring as a guide for fluid and blood transfusion practice using Masimo pulse co-oximetry in comparison with invasive Hb during elective cesarean section (CS).
Methods
This prospective cohort study was carried out on 60 pregnant women candidates for elective CS under general anesthesia. Participants were multigravida, aged between 18 and 45 years and carry a singleton fetus with ASA I–II. All had antepartum hemorrhage and were candidates for blood transfusion. Pre-induction of anesthesia (basal), pre-transfusion and post-transfusion SpHb and invasive Hb were assessed. The primary outcome parameter was the correlation between SpHb and invasive hemoglobin (Hb).
Results
There was a significant positive correlation between SpHb and Invasive Hb in baseline, pretransfusion and posttransfusion (r = 0.946, 0.902 and 0.698, respectively). Bland–Altman analysis between SpHb and invasive Hb reported low bias with moderate limits of agreement at baseline, pre-transfusion and post-transfusion [mean bias (limits of agreement): 0.348 (−0.584 and 1.280) g/dl, 0.314 (−0.561 and 1.188) g/dl and 0.348 (−0.584 and 1.280) g/dl, respectively]
Conclusions
In patients undergoing CS with antepartum hemorrhage, continuous SpHb through Masimo pulse co–oximetry demonstrated clinically acceptable accuracy of Hb measurement compared with Invasive Hb, even at low hemoglobin levels.
1. Introduction
Antepartum hemorrhage is associated with adverse maternal and neonatal outcomes. Placenta previa accounts for one-third of causes of antepartum hemorrhage. Other causes include placental abruption and uterine rupture. As these causes are very hazardous and can jeopardize both maternal and fetal lives, it is compulsory and lifesaving to monitor tissue perfusion, intravascular volume status supported by intraoperative hemoglobin levels as well as fluid and blood demands [Citation1].
Blood transfusions continue to present a risk to patients as adverse effects such as postoperative pneumonia, reduced lung function, and increased duration of hospital stay and mortality. Transfusion is a major contributor to the cost of surgical management of cases [Citation2].
The laboratory hemoglobin (Invasive Hb) values are a crucial indicator of blood transfusion requirement, but testing is intermittent, and results are often delayed. Therefore, blood transfusion initially and subsequently during surgery can be made without recent Hb level [Citation3].
Nowadays, continuous monitoring with noninvasive Hb (SpHb), is applicable and could be achieved using pulse Co-Oximeter and multiwavelength adhesive sensor [Citation4].
The SpHb may enable a more rapid detection of clinically significant blood loss, improve peri-operative transfusion practices, allow patient condition to be assessed more quickly and blood management more adequately, and perhaps even reduce needless transfusions [Citation5].
The purpose of this study was to assess the use of Masimo pulse co-oximetry for continuous SpHb monitoring in patients with antepartum hemorrhage as a guideline for fluid and blood transfusion practice and compare it to Invasive Hb during elective CS.
2. Methods
This prospective cohort study included 60 pregnant women candidates for elective CS under general anesthesia at Kasr Alainy maternity Hospital, Cairo University between April 2016 and December 2017 after being approved by the institutional ethical committee (Number: N7-G1-2015/MD) and registered at clinical trial registry (Code: NCT03408938). Informed consent was obtained from all participants included.
Inclusion criteria were multigravida aged between 18 and 45 years and carry a singleton fetus with ASA I–II. All had antepartum hemorrhage and were candidates for blood transfusion. They were candidates for elective CS under general anesthesia.
Exclusion criteria: known dysrhythmia, intracardiac shunts, significant coagulopathy, left or right ventricular dysfunction, significant hepatic dysfunction (ALT and AST>3 times of the normal) and significant renal disorders (creatinine clearance < 40 ml/min and/or serum creatinine>1.5 mg/dl).
Patient’s preoperative evaluation included: history, clinical examination, and routine laboratory investigations. Premedication (ondansetron 4 mg intravenously (IV) and ranitidine 150 mg orally) were received. Insertion of the peripheral cannula (18 G) was performed under local anesthesia, followed by infusion of 15 ml/kg normal saline for 0.25–0.5 h [Citation6]. Standard monitoring (pulse oximetry, blood pressure (non-invasive), ECG, temperature probe and capnogram) have been used for all patients. In compliance with best practice guidance, the Masimo sensor was installed, and the automatic data collection (ADC) was initiated.
To avoid the variance induced by the use of multiple lab devices, all blood samples were analyzed on the same calibrated hematology laboratory analyzer (Coulter, Sysmex). Blood transfusion was determined when Lab Hb decreased by more than 20% than the basal assay [Citation7].
All the patients obtained GA after 5-minutes of preoxygenation. Rapid-sequence induction was done by 3–5 mg/kg thiopental sodium and suxamethonium 1 mg/kg with cricoid pressure [Citation8]. Then a wide bore venous access with 18 gauge canula was inserted and urine was collected via Foley catheter.
Maintenance of anesthesia was achieved by 0.8–1 MAC isoflurane and 100% oxygen. For muscle relaxation after suxamethonium activity fades, atracurium 0.5 mg/kg was administered, and the patients were ventilated for 4–4.6 kPa EtCO2 [Citation9].
Following clamping of the umbilical cord, 10 U of oxytocin IV infusion and fentanyl l-2 μg/kg IV were administered [Citation10]. Inhalational anesthetic was stopped at the end, and a residual neuromuscular block was antagonized with atropine 20 μg/kg and neostigmine 50 μg/kg and the trachea extubation was performed and patients were sent after extubation to PACU for observation till full recovery then sent to the ward.
Data reported included demographic characteristics, the parity, medical disorders, surgical details (CS duration, complications, blood loss) and neonatal outcome (NICU admission and APGAR score). Pre-induction of anesthesia (basal), pre-transfusion and post-transfusion hemoglobin were assessed by both Invasive Hb and SpHb.
The primary outcome was the correlation between SpHb and Invasive Hb and the secondary outcome was the accuracy of SpHb in relation to Invasive Hb.
Sample size was estimated by using G.power 3.1.9.2 (Universitat Kiel, Germany) based on that 80% power and 0.05 α error of the study and a mean bias (±SD) was −0.02 ± 1.39 g/dl of SpHb according to a previous study [Citation11]). Fifty-eight cases were required. Sixty participants were recruited to compensate for any dropout cases.
2.1. Statistical analysis
SPSS v26 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The mean and standard deviation (SD) presented quantitative variables and compared using student t-test. Qualitative variables were presented as number and percentage. Pearson correlation was done to check the relationship between two numerical variables. To evaluate agreement amongst approaches over the range of haemoglobin levels, modified Bland-Altman graphs with limits of agreement (defined as bias ± SD) were drawn. The bounds of agreement specify the range within which 95% of the discrepancies between measurements using the two methods. P value less than 0.05 was considered to be statistically significant.
3. Results
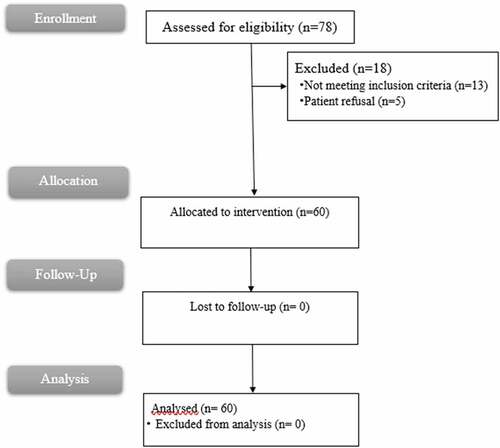
Seventy-eight cases were assessed for eligibility, 18 were excluded (13 cases not meeting inclusion criteria and 5 refused to contribute, 60 signed informed consent); therefore, sixty patients were analyzed (.
Participants’ characteristics are illustrated in .
Table 1. Characteristics of the study population.
Differences between SpHb and invasive Hb were insignificant at baseline, pre- and post-transfusion times (P = 0.196, 0.092, 0.570 respectively) (.
Table 2. Comparison between noninvasive Hb (SpHb) and laboratory hemoglobin (Invasive Hb).
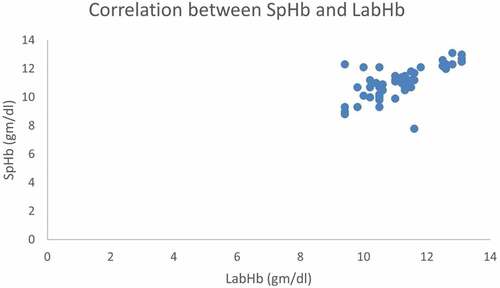
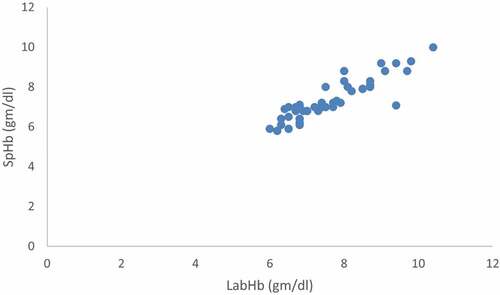
There was a significant positive relationship between SpHb and Invasive Hb at baseline, pre-transfusion and post-transfusion (r = 0.946, 0.902 and 0.698 respectively and P < 0.001).
Figure 2. Correlation between baseline noninvasive Hb (SpHb) and Laboratory hemoglobin (Invasive Hb).
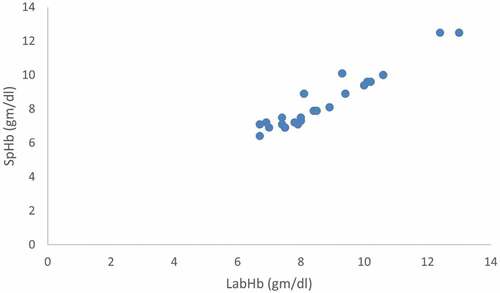
Figure 3. Correlation between pretransfusion noninvasive Hb (SpHb) and Laboratory hemoglobin (Invasive Hb).
HR and MAP changes among studied patients are shown in .
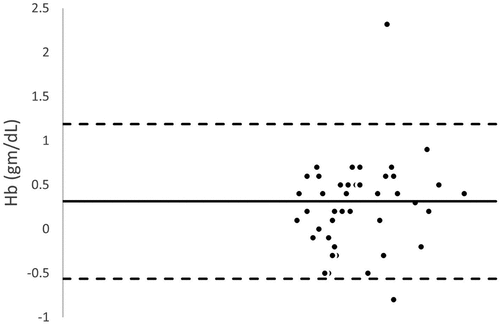
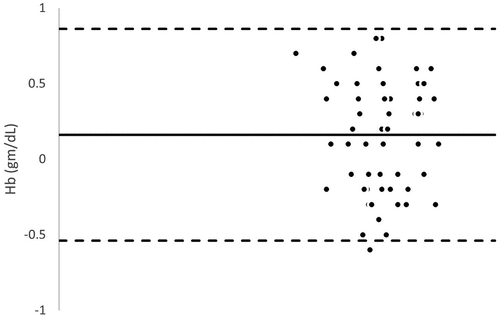
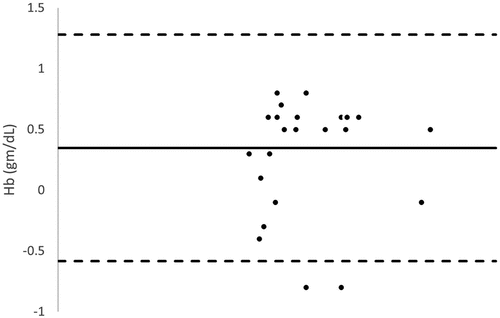
Bland – Altman analysis between SpHb and Invasive Hb demonstrated low bias with moderate limits of agreement at baseline, pretransfusion and posttransfusion [Mean bias (limits of agreement): 0.348 (−0.584 and 1.280) g/dl, 0.314 (−0.561 and 1.188) g/dl and 0.348 (−0.584 and 1.280) g/dl respectively].
Figure 5. Bland–Altman analysis between baseline noninvasive Hb (SpHb) and Laboratory hemoglobin (Invasive Hb).
4. Discussion
Pulse CO-Oximetry is a multiwavelength spectrophotometric method for the continuous, noninvasive detection of total Hb (SpHb). This method has FDA 510(k) clearance and is based on detecting the differential optical density of seven distinct wavelengths of light passing through the finger [Citation4].
Confirming our results, Adel et al. [Citation12] reported that SpHb displayed significant correlation with Invasive Hb and given the beneficial mean bias of 0.01 g/dl for SpHb, even with poor perfusions and low hemoglobin in patients undergoing major orthopedic operations with expected major blood loss.
Also, Gamal et al. [Citation13] who investigated trauma patients scheduled with low hemoglobin (<8 g/dL). In trauma patients with weak hemoglobin levels and with limits of agreement of −0.56 g/dL and 0.79 g/dL and a mean bias of 0.12 g/dL.
In contrast to our results, Kapoor et al. [Citation14] who studied 30 patients undergoing GA pituitary surgery reported a low correlation between Invasive Hb and SpHb. This difference may be due to small sample size than ours, that cannot distinguish minor difference.
Also, Panda et al. [Citation15] demonstrated that SpHb is possible in clinical situations and can offer comparable Invasive Hb values in neonates up to young adults. This can be explained by that they included younger population (neonates and children).
Raikhel [Citation16] reported that patients presented at the clinician for outpatient research and showed that the SpHb bias was −0.5 g/dL and limits of agreement of −2.5 to 1.5 g/dL compared to Invasive Hb and concluded that it has the potential to confer the additional benefits of patient comfort, increased safety, and decreased complexity for health care providers who are not exposed to blood-spill contaminations and needle-stick injury risks.
Also, Miller et al. [Citation17] studied 20 people who performed prone spine surgery under GA. Their data confirmed the correlation between SpHb and Invasive Hb values well. But their study shows that the SpHb in some patients may not be as reliable as clinically required. This could be explained by that as the technology improved with new different versions of the sensor and different devices developed. Therefore, we may get a better knowledge of the relationship between perfusion, acute Hb changes, and the efficacy of this noninvasive sensor.
Dermaco et al. [Citation18] results differ from our results. They demonstrated that SpHb was unable to measure blood loss during CS. SpHb demonstrated poor precision and no trends in comparison to Invasive Hb. This disagreement was due to smaller sample size compared to ours.
Moreover, Murphy et al. [Citation19] found that SpHb has significant bias relative to Invasive Hb since the mean bias for SpHb was +1.64 with limits of agreement of −1.03 to 4.31. The bias for SpHb increased with increased average hemoglobin levels. This due to different population (dark-skinned critically ill patients)
Also, Skelton et al. [Citation20] showed that the co-oximeter was less reliable than experimental tests and less sensitive when compared to HemoCue cuvette system.
Also, Gayat et al. [Citation21] demonstrated that hemoglobin monitoring device was biased and too unreliable to guide transfusion decisions.
Butwick et al. [Citation22] examined 50 elective CS patients for 48 hours after surgery. SpHb showed significant positive bias at baseline and at 24 hours post-CS. The prejudice directly after CS contributed to 0.14 g dl(−1) (95% CI:–0.18 to 0.46). The limits of agreement were: −0.9 and 3.33, −2.35 and 2.56, and −0.55 and 3.27 g dl (−1), respectively. This is due to their earlier study since 2012 and during this time gap several modifications were applied in devices used with novel different versions of the sensor.
Also, in disagreement with our study, Lamhaut et al. [Citation11] who stated that SpHb displayed a bias of −0.02 ± 1.39 g/dl (−1) and a precision of 1.11 ± 0,83 g/dl (−1) relative to Invasive Hb. Despite the appropriate ± 1.0 g/dl (−1) gap to Invasive Hb, the outliers of SpHb were significantly higher (46% P < 0.05).
The strength of our study is that we employed a laboratory hematology analyzer as the gold standard. However, it may not be as accurate as the cyano-hemoglobin test [Citation23].
Our study has some limitation as it was a single-center study, and we included antepartum hemorrhage pregnant women and were candidate for blood transfusion. In addition, we did not analyze the repeatability of the oximeter’s readings, which is a key metric for gauging device performance.
4.1. Clinical value of the study
SpHb® evaluation has the potential for additional benefits, including patient comfort, increased safety, and decreased complexity for healthcare staff, who are not exposed to the risks of needle-stick injury and blood-spill contamination.
5. Conclusion
Continuous SpHb Masimo pulse co-oximetry shows an appropriate clinically reliable Hb calculation in comparison to Invasive Hb even in patients undergoing CS with low hemoglobin.
Further studies are needed on larger sample size with multicenter collaboration. Furthermore, we recommend the assessment of this technique on patients with common morbidities, such as high cholesterol, high blood pressure, and diabetes. We also recommend comparing SpHb accuracy to other modalities such as HemoCue 201+®.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Mohamed Ibrahim Beleta and Hossam Eldin Mostafa Yahya. The first draft of the manuscript was written by Ashraf Abd ElMawgood Ali, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ananth CV, Demissie K, Smulian JC, et al. Placenta previa in singleton and twin births in the United States, 1989 through 1998: a comparison of risk factor profiles and associated conditions. Am J Obstet Gynecol. 2003;188:275–281.
- Al-Harbi SA, Alkhayal N, Alsehali A, et al. Impact of blood transfusion on major infection after isolated coronary artery bypass surgery: incidence and risk factors. J Saudi Heart Assoc. 2019;31:254–260.
- Awada WN, Mohmoued MF, Radwan TM, et al. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015;29:733–740.
- Tang B, Yu X, Xu L, et al. Continuous noninvasive hemoglobin monitoring estimates timing for detecting anemia better than clinicians: a randomized controlled trial. BMC Anesthesiol. 2019;19:80–85.
- Berkow L, Rotolo S, Mirski E. Continuous noninvasive hemoglobin monitoring during complex spine surgery. Anesth Analg. 2011;113:1396–1402.
- Encarnacion B, Zlatnik MG. Cesarean delivery technique: evidence or tradition? A review of the evidence-based cesarean delivery. Obstet Gynecol Surv. 2012;67:483–494.
- Abdelazim I, Farghali M, Amer OO. Routine haemoglobin assay after uncomplicated caesarean sections. Prz Menopauzalny. 2021;20:29–33.
- Oh TT, Sng BL. Rethinking the rapid sequence induction in obstetrics. Trends Anaesth Crit Care. 2014;4:42–46.
- Devroe S, Van de Velde M, Rex S. General anesthesia for caesarean section. Curr Opin Anaesthesiol. 2015;28:240–246.
- Butwick AJ, Coleman L, Cohen SE, et al. Minimum effective bolus dose of oxytocin during elective Caesarean delivery. Br J Anaesth. 2010;104(3):338–343.
- Lamhaut L, Apriotesei R, Combes X, et al. Comparison of the accuracy of noninvasive hemoglobin monitoring by spectrophotometry (SpHb) and HemoCue® with automated laboratory hemoglobin measurement. Anesthesiology. 2011;115(3):548–554.
- Adel A, Awada W, Abdelhamid B, et al. Accuracy and trending of non-invasive hemoglobin measurement during different volume and perfusion statuses. J Clin Monit Comput. 2018;32(6):1025–1031.
- Gamal M, Abdelhamid B, Zakaria D, et al. Evaluation of noninvasive hemoglobin monitoring in trauma patients with low hemoglobin levels. Shock. 2018;49(2):150–153.
- Kapoor I, Mahajan C, Mamuliya R, et al. Assessment of accuracy of continuous noninvasive versus invasive method of hemoglobin estimation in patients undergoing pituitary surgery. Journal of Neuroanaesthesiology and Critical Care. 2018;05(3):168–172.
- Panda P, Sen M. Accuracy of haemoglobin estimation by non-invasive pulse co-oximetry method: a prospective observational study among neonates, children and young adults. J Med Res. 2018;4(1):10–15.
- Raikhel M. Accuracy of noninvasive and invasive point-of-care total blood hemoglobin measurement in an outpatient setting. Postgrad Med. 2012;124(4):275–281.
- Miller RD, Ward TA, Shiboski SC, et al. A comparison of three methods of hemoglobin monitoring in patients undergoing spine surgery. Anesth Analg. 2011;112(4):858–863.
- Dermaco A, Chappelle J. Non-invasive hemoglobin monitoring: a method of measuring blood loss in cesarean delivery? Obstet Gynecol. 2017;129:189S.
- Murphy SM, Omar S. The clinical utility of noninvasive pulse co-oximetry hemoglobin measurements in dark-skinned critically ill patients. Anesth Analg. 2018;126:1519–1526.
- Skelton VA, Wijayasinghe N, Sharafudeen S, et al. Evaluation of point-of-care haemoglobin measuring devices: a comparison of radical-7 pulse co-oximetry, HemoCue((R)) and laboratory haemoglobin measurements in obstetric patients*. Anaesthesia. 2013;68:40–45.
- Gayat E, Bodin A, Sportiello C, et al. Performance evaluation of a noninvasive hemoglobin monitoring device. Ann Emerg Med. 2011;57:330–333.
- Butwick A, Hilton G, Carvalho B. Non-invasive haemoglobin measurement in patients undergoing elective Caesarean section. Br J Anaesth. 2012;108:271–277.
- Kim H, Do SH, Hwang JW, et al. Intraoperative continuous noninvasive hemoglobin monitoring in patients with placenta previa undergoing cesarean section: a prospective observational study. Anesth Pain Med (Seoul). 2019;14:423–428.