?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Hypospadias repair surgery, though necessary, can be painful after the procedure, especially for children. Effective pain management is essential in all surgeries, but particularly in pediatric procedures. The current study was conducted to evaluate the analgesic efficacy of intravenous ketorolac versus dexmedetomidine after hypospadias repair surgery.
Patients and methods
A total of 60 children aged between 2 and 7 years old undergoing hypospadias repair under general anesthesia enrolled in the study. Those children were randomly divided into either group Ketorolac (group A) or Dexmedetomidine group (group B). Group (A) included 30 patients who received IV ketorolac in a dose 0.9 mg/kg after intubation for general anesthesia while group (B) included 30 patients who received IV dexmedetomidine in a dose 1 μg/kg after intubation. Pain scores (FLACC), sedation and emergence agitation (EA) scores, 1st analgesic dose, hemodynamics, and any side events were recorded.
Results
The main finding in this study was that group (B) had significantly lower FLACC at different postoperative times compared to group n(A)with p < 0.05; with exception at 4th and 12th hours. It was found that FLACC was zero in group (B) till the 6th hour postoperatively. All patients in the studied groups had sedation scale was 3 starting from the 4th postoperative hour. Immediate and 2-hour postoperatively, the score was significantly higher among the dexmedetomidine group.Two patients developed EA in group A but non in group B.
Conclusion
Adjuvant intravenous dexmedetomidine is more effective than intravenous ketorolac in postoperative analgesia children after hypospadias repair surgery under general anaesthesia.
1. Introduction
A painful and intrusive treatment is hypospadias correction surgery. When it comes to pediatric and neonatal operations, effective pain management is essential. There is a moderate to severe amount of discomfort following surgery in up to 40% of youngsters [Citation1].
Non-steroidal anti-inflammatory drugs (NSAIDs) like ketorolac are commonly used postoperatively and offer analgesia similar to that of opioids. It helps lessen opioid-related side effects as respiratory depression, pruritus, and drowsiness while also relieving moderate pain and supplementing severe pain with opioids [Citation2,Citation3].
An α2-adrenergic receptor agonist, dexmedetomidine, helps stabilize hemodynamics and preserve neuroprotection during neurosurgery. In addition to inhibiting glutamate, pro-apoptotic proteins, and pro-inflammatory cytokines, it lowers the release of catecholamines during surgery. With fewer adverse effects than opioids, dexmedetomidine also lessens sedation and the need for opioids [Citation4].
This study attempted to discover the best course of action for pain control and postoperative decrease of analgesic use by comparing the effectiveness of intravenous ketorolac against dexmedetomidine as analgesia following hypospadias correction surgery.
2. Patients and Methods
A randomized controlled clinical trial was conducted at Anesthesia and ICU Department of Assiut University Hospitals. It was done in the period between 2022 and 2023. This work was conducted after obtaining approval from the Medical Ethics Committee of the Faculty of Medicine at Assiut University Medical Ethics number (17101788). Also, a written informed consent was obtained from all legal guardians before being enrolled in the study. The study was registered on ww w. Clinicaltrials.gov Identifier: NCT05194904.
2.1. Inclusion criteria
• American society of anesthesiologists I or II.
• Age ranged between two and seven years old.
• Child body weight below 30 kilograms.
2.2. Exclusion criteria
• If there are contraindications of the drugs used in the study.
• Parental refusal
• Recurrent or previous surgery in the same site.
• Sixty patients were randomly subdivided into two groups: Ketorolac group (group A) included 30 patients who received IV ketorolac in a dose 0.9 mg/kg. Dexmedetomidine group (group B) included 30 patients who received IV dexmedetomidine in a dose 1 μg/kg.
3. Randomization
Each patient was be randomly assigned to his group using quick Calcs method for randomization with 1:1 ratio either group A or group B.
3.1. Procedure Preoperative assessment
Before surgery, all patients received a preoperative visit where the entire procedure was thoroughly explained to them and their legal guardians, and informed consent was obtained. Comprehensive patient histories were recorded, and a thorough general examination was conducted, which included airway assessment, chest examination, and cardiac auscultation. Patients adhered to appropriate fasting guidelines, abstaining from food for 6 hours and water for 2 hours prior to surgery.
3.2. Operative assessment
General anesthesia was induced through inhalation of sevoflurane at a concentration of 6–8%, with maintenance achieved using sevoflurane at 2–3%. Intravenous access was established via insertion of a 22 G cannula. Participants were randomly assigned to receive either 0.9 mg/kg of ketorolac or 1 μg/kg of Dexmedetomidine via intravenous injection based on their respective groups. Intravenous fluids, specifically Hartmann’s solution, were administered according to body weight: 4 ml/kg for the first 10 kg, 2 ml/kg for the second 10 kg, and 1 ml/kg for each kilogram above 20 kg. Standard monitoring (ECG, body temperature pulse oximetry non-invasive blood pressure and capnography) was carried and checked every five minutes. An IV dose of ephedrine 0.2 mg/kg was used to treat significant hypotension, which was defined as a drop in mean arterial blood pressure (MAP) of 20% or more below the baseline value. Atropine 0.01 mg/kg IV was used to treat significant bradycardia, which was defined as an heart rate (HR) of less than 60 beats per minute. In order to avoid postoperative nausea and vomiting (PONV), ondansetron 0.1 mg/kg IV was administered.
At the end of operation extubation was done after full recovery and the patients transported to the post-anesthesia care unit (PACU). While the patients in the PACU, the patient’s vital signs were closely monitored. Pain was assisted using the Face, Legs, Activity, Cry, Consolability (FLACC) pain score [Citation5]. This score has a 0–10 range and used to measure pain after the surgery at 2, 4, 6, 8, 12, 18, and 24 hours. Intravenous acetaminophen HCL 15 mg/kg/6-hour fentanyl 0.5–1 µg/kg was administered when the FLACC score was ≥ 4. The duration, kind, frequency, and total amount of analgesics consumed were all recorded. The Ramsay Sedation Scale (RSS) [Citation6] used to measure sedation. It was requested of bedside nurses to record the patient’s level of drowsiness on extra sedation scales. Emergence Agitation (EA) was measured using Aono’s Four-Point Scale [Citation7], where a score of 1 indicated calmness, a score of 2 indicated not calm but readily soothed, a score of 3 indicated not easily calmed but moderately agitated or restless, and a score of 4 indicated excitement or disorientation. Post-operative complications and any reported side effects were also recorded.
3.3. Outcome of the study
Primary outcome was to evaluate the efficacy of intravenous ketorolac versus dexmedetomidine on pain score after hypospadias repair surgery.
Secondary outcomes were the 1st analgesic dose, RSS, Aono’s Four-Point sedation Scale and any complications.
3.4. Sample size calculation
A total of 26 patients in each group were needed to be able to detect 20% difference pain score assuming α error of 0.5, β error of 0.8 and 1:1 allocation ratio. Another 4 patients were added to each group to compensate for violation of the study protocol, this made the total sample size of 60 patients.
3.5. Statistical analysis
The data was collected and analyzed utilizing SPSS (Statistical Package for the Social Sciences, version 20, IBM, Armonk, New York). Quantitative data were presented as mean ± standard deviation (SD). Student’s t-test was employed to compare quantitative data with a normal distribution. Nominal data were presented as number (n) and percentage (%). A confidence level of 95% was maintained, thus, a P-value less than 0.05 was deemed significant.
4. Results
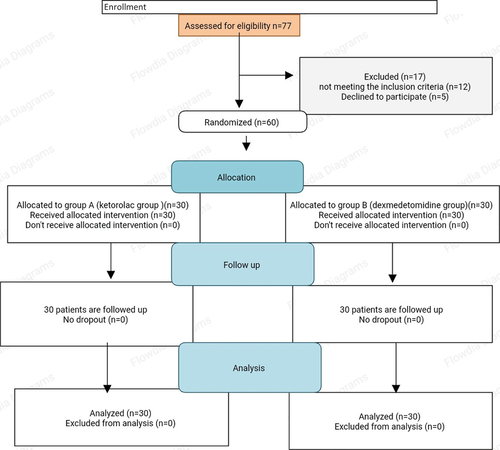
This study was performed between January 2022 and November 2023 in Assiut University hospitals, 77 children were screened for participate in this study, 17 children were excluded due to not meeting the inclusion criteria or declined to participate, 60 children were enrolled and divided into group(A), and group(B), all children continued the study ().
4.1. Baseline data of the studied groups
Both groups had insignificant differences as regard patients’ and surgical data (age, weight, operative time, ASA and intraoperative fluid intake). Majority of both groups had ASA class-I with no significant difference between both groups ().
Table 1. Baseline data of the studied groups.
As regard to postoperative FLACC pain score, dexmedetomidine group had significantly lower FLACC pain score at different postoperative times (P with exception at 4th and 12th hours there were insignificantly differences. It was found that FLACC pain score was zero in dexmedetomidine group till the 6th hour postoperatively (, ). Time to 1st analgesia dose and total analgesia consumption in both groups showed that group (B) had significantly longer duration till 1st analgesia dose (5.56 ± 2.21 vs. 2.22 ± 0.79 (hour); (p < 0.001) and less total analgesia consumption (248.11 ± 58.56 vs. 207.54 ± 23.87 (mg); p < 0.001) compared to the ketorolac group (). RSS and frequency of emergency agitation showed all patients in the studied groups had sedation scale was 3 starting from the 4th postoperative hour. Immediate and 2-hour postoperatively, the score was significantly higher among the group (B). Two patients in group (A) developed EA and no patient had EA in group (B) (, ).
Figure 2. Change in postoperative FLACC pain scale at different times in both groups. FLACC: The Face, Legs, Activity, Cry, Consolability.
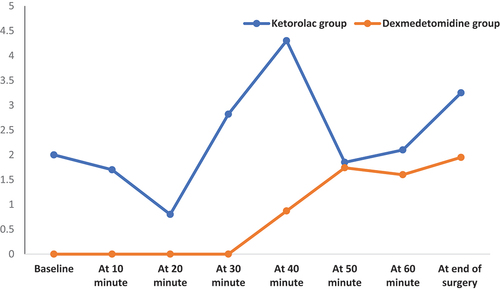
Figure 3. Change in postoperative RSS at different times in both groups. RSS: Ramsay sedation score.
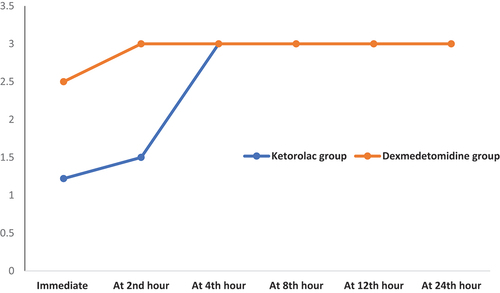
Table 2. Time to 1st analgesia request and total analgesia consumption.
Table 3. Postoperative FLACC pain score at different times in both groups.
Table 4. RSS and frequency of emergency agitation in both groups.
Both groups had insignificant differences as regard assessment of MAP, oxygen saturation, HR at different times either intraoperatively and postoperatively with exception significantly lower intraoperative HR in group (B)compared to group(A) with p < 0.001.
Adverse events among the studied groups: Majority (90% vs. 86.7%) of both groups developed no adverse events. Bradycardia was reported in two patients of dexmedetomidine and one patient in ketorolac group. Three patients in ketorolac and only one patient in dexmedetomidine group suffered from nausea and vomiting ().
5. Discussion
Postoperative pain following children lower abdomen surgery has been managed by a variety of techniques, including the use of opioids, non-opioids, peripheral nerve blocks, and central nerve blocks. There has been a growing trend in pediatric anesthesia to utilize α-2 adrenergic agonists (dexmedetomidine)as adjuvant medications, especially to extend the duration of caudal analgesia [Citation8,Citation9]. The goal of the current study was to compare the effectiveness of intravenous ketorolac and dexmedetomidine as analgesics following hypospadias correction surgery.
In the result of the current study, we found that group of dexmedetomidine (group B) had significantly lower FLACC pain score at different postoperative times with exception at 4th and 12th hours compared to group of ketorolac (group A). As regard to 1st analgesia dose; group (B) had significantly longer duration and statistically significant less total analgesia consumption in comparison to the group (A). Although the exact mechanism underlying dexmedetomidine’s analgesic action is unknown, it may involve protein kinase B/Akt (Li SS). Consistent with the findings of this investigation, according to the findings of Al-Zaben KR et al., intravenous dexmedetomidine administration during hypospadias repair in children decreases the need for analgesics both during and after surgery (9). Furthermore, Yuan Zhang et al. [Citation10] discovered that testing would be done to see how dexmedetomidine or lidocaine affects pediatric patients’ postoperative analgesia. Additionally, the results aligned with our findings and will offer other options for children’s multimodal perioperative pain relief. In another study by Wang, X.X. et al concluded that dexmedetomidine has been considerably extend the time until pain relief medicine is first administered [Citation11]. Unlugenc H. et al. reported that postoperative morphine consumption was significantly reduced at equal pain levels when a single intravenous dose of dexmedetomidine (1 microg kg (−1)) was administered 10 min before induction of anesthesia. Our study’s results were consistent with their findings [Citation12]. Alexander Schnabe et al. found that intraoperative dexmedetomidine administration produced superior postoperative analgesia when compared to placebo use, which is consistent with the findings of this investigation. Their meta-analysis of [Citation11] included randomized controlled trials [Citation13].
Emergence agitation is a condition that arises during the initial stages of anesthesia recovery. The clinical presentation of this condition is associated with multiple risk variables, including age, pediatric anesthetic behavior score, kind of surgery, and duration of anesthesia.
Hino M. Dexmedetomidine is showing promise in a variety of pediatric anesthetic applications, where its sedative qualities can be used as a premedication or as an adjuvant for balanced anesthesia, which can reduce the need for additional medications and emergent delirium [Citation14]. In this study, all patients in the studied groups had sedation scale was 3 starting from the 4th postoperative hour. Immediate and 2-hour postoperatively, the score was significantly higher among the dexmedetomidine group. We found that only two patients in ketorolac group had emergency agitation (EA). Meanwhile, none of the patient in dexmedetomidine group developed EA. Zhang X et al. in their study confirmed the beneficial effects of dexmedetomidine on EA, severe EA, and PONV in children. There was firm and high-quality evidence for the efficacy of dexmedetomidine in preventing EA in children [Citation10] In meta-analysis by Wen Tang et al. revealed that, compared with placebo, the administration of dexmedetomidine in children undergoing general anesthesia was associated with a lower incidence of emergence agitation [Citation15], these studies corresponded with our results.
In the current study, both groups had insignificant differences as regard changes in hemodynamics, respiratory rate, oxygen saturation with exception significantly lower intraoperative HR in dexmedetomidine group. This was consistent with previous meta-analysis by Tong, Y. et al. found that dexmedetomidine as an additive to local anesthetic provides no adverse effects and hemodynamic changes [Citation16]. We found that majority of both groups developed no adverse events. Bradycardia was reported in two patients of dexmedetomidine and one patient in ketorolac group. Three patients in ketorolac and only one patient in dexmedetomidine group suffered from nausea and vomiting. Zhenzhen Tu.et al said that dexmedetomidine seems to be has no side effects in children [Citation17–20].
Limitations of this study were its single-center design and somewhat small sample size. Also, to our knowledge, this is the first study that discussed such point in children who were scheduled for hypospadias repair.
Conclusion: The conclusion of this study is that intravenous adjuvant dexmedetomidine at a dose of 1 μg/kg is more efficient at extending the analgesic effects following surgery than intravenous ketorolac in children undergoing hypospadias repair under general anesthesia, with a better calming effect and a lower incidence of adverse events. We recommended future studies on large number of patients in multiple centers are warranted to draw firm conclusion.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Lönnqvist PA, Morton NS. Postoperative analgesia in infants and children. Br J Anaesth. 2005;95(1):59–68. doi: 10.1093/bja/aei065
- Forrest JB, Heitlinger EL, Revell S. Ketorolac for postoperative pain management in children. Drug Saf. 1997;16(5):309–329. doi: 10.2165/00002018-199716050-00003
- Lee JY, Jo YY. Attention to postoperative pain control in children. Korean J Anesth. 2014;66(3):183–188. doi: 10.4097/kjae.2014.66.3.183
- Gao J, Sun Z, Xiao Z, et al. Dexmedetomidine modulates neuroinflammation and improves outcome via alpha2-adrenergic receptor signaling after rat spinal cord injury. Br J Anaesth. 2019;123(6):827–838. doi: 10.1016/j.bja.2019.08.026
- Fares KM, Othman AH, Alieldin NH. Efficacy and safety of dexmedetomidine added to caudal bupivacaine in pediatric major abdominal cancer surgery. Pain Physician. 2014;17(5):393–400. doi: 10.36076/ppj.2014/17/493
- Rasheed AM, Amirah MF, Abdallah M, et al. Ramsay sedation scale and Richmond agitation sedation scale: a cross-sectional study. Dimens Crit Care Nurs. 2019;38(2):90–95. doi: 10.1097/DCC.0000000000000346
- Zhong Q, Qu X, Xu C. Effect of preoperative visiting operation room on emergence agitation in preschool children under sevoflurane anesthesia. Int J Pediatr Otorhinolaryngol. 2018;104:32–35. doi: 10.1016/j.ijporl.2017.10.038
- Zahra SW, Okab ME, Eldaba AA, et al. Comparative study between bupivacaine, bupivacaine plus dexamethasone and bupivacaine plus dexmedetomidine in caudal anesthesia for pediatric patients undergoing inguinoscrotal surgery.
- Al-Zaben KR, Qudaisat IY, Alja’bari AN, et al. The effects of caudal or intravenous dexmedetomidine on postoperative analgesia produced by caudal bupivacaine in children: a randomized controlled double-blinded study. J Clin Anesth. 2016;33:386–394. doi: 10.1016/j.jclinane.2016.04.049
- Zhang X, Bai Y, Shi M, et al. Effect of different administration and dosage of dexmedetomidine in the reduction of emergence agitation in children: a meta-analysis of randomized controlled trials with sequential trial analysis. Transl Pediatr. 2021;10(4):929–9564. doi: 10.21037/tp-21-105
- Wang XX, Dai J, Dai L, et al. Caudal dexmedetomidine in pediatric caudal anesthesia: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2020;99(31):e21397. doi: 10.1097/MD.0000000000021397
- Unlugenc H, Gunduz M, Guler T, et al. The effect of pre-anaesthetic administration of intravenous dexmedetomidine on postoperative pain in patients receiving patient-controlled morphine. Eur J Anaesthesiol. 2005;22(5):386–391. doi: 10.1017/S0265021505000669
- Schnabel A, Reichl SU, Poepping DM, et al. Efficacy and safety of intraoperative dexmedetomidine for acute postoperative pain in children: a meta‐analysis of randomized controlled trials. Pediatr Anesthesia. 2013;23(2):170–179. doi: 10.1111/pan.12030
- Hino M, Mihara T, Miyazaki S, et al. Development and validation of a risk scale for emergence agitation after general anesthesia in children: a prospective observational study. Anesth Analg. 2017;125(2):550–555. doi: 10.1213/ANE.0000000000002126
- Wen T, DongWei H, YuLin L. Effect of dexmedetomidine in children undergoing general anaesthesia with sevoflurane: a meta-analysis and systematic review. J Int Med Res. 2020 Jun;48(6):030006052092753. doi: 10.1177/0300060520927530
- Tong Y, Ren H, Ding X, et al. Analgesic effect and adverse events of dexmedetomidine as additive for pediatric caudal anesthesia: a meta-analysis. Pediatr Anesthesia. 2014;24(12):1224–1230. doi: 10.1111/pan.12519
- Tu Z, Tan X, Li S, et al. The efficacy and safety of dexmedetomidine combined with bupivacaine on caudal epidural block in children: a meta-analysis. Med Sci Monit. 2019;25:165–173. doi: 10.12659/MSM.913098
- Li SS, Zhang WS, Yang JL, et al. Xu H.Involvement of Protein Kinase B/Akt in analgesic effect of dexmedetomidine on neuropathic pain. CNS Neurosci Ther. 2013;19(5):364–366. doi: 10.1111/cns.12100
- Al-Zaben KR, Qudaisat IY, Al-Ghanem SM, et al. Intraoperative administration of dexmedetomidine reduces the analgesic requirements for children undergoing hypospadias surgery. Eur J Anaesthesiol. 2010 Mar;27(3):247–252. doi: 10.1097/EJA.0b013e32833522bf
- Sottas CE, Anderson BJ, Anderson BJ. Dexmedetomidine: the new all-in-one drug in paediatric anaesthesia? Curr Opin Anaesthesiol. 2017 Aug;30(4):441–451. doi: 10.1097/ACO.0000000000000488