ABSTRACT
Purpose
To compare impact of intranasal infusion of dexmedetomidine, ketamine, or combination of both on IOP in children.
Patient & methods
This prospective, randomized, observational study was conducted at Benha University Hospital, Egypt and included ASA I or II children aged 1–6 years who underwent examination under sedation. They were randomly divided into three groups: Group D (dexmedetomidine 3 μg/kg); Group DK (dexmedetomidine 1 μg/kg with ketamine 2 mg/kg) & Group K (ketamine 4 mg/kg). We assessed IOP difference before and after sedation. Secondary outcomes were sedation scale assessment (Ramsay Sedation Score), emergency agitation and medication side effects.
Results
We studied 118 children divided into Group D (36 patients), Group DK (42 patients) & Group K (40 patients). IOP was significantly lower in group D (13 ± 3 mmHg) than in groups DK (16 ± 4 mmHg) and K (17 ± 3 mmHg), with no significant difference between groups DK and K. Ramsay 3 was higher in group K (65%) compared to groups D and DK (22.2% and 9.5%, respectively), while Ramsay 4 was higher in group D and DK (52.8% and 52.4%, respectively) compared to group K (35%). Post-sedation nausea and vomiting were higher in group K (25%) compared to groups D and DK (0% for each). Agitation was higher in group K (62.5%) than in groups D and DK (0% for each) (p < 0.001).
Conclusion
Intranasal dexmedetomidine and ketamine combination are viable for achieving optimal sedation in pediatric patients undergoing surgeries or medical procedures with no significant change in the IOP.
1. Introduction
Pediatric glaucoma is a disease that could potentially cause blindness [Citation1]. Intraocular pressure (IOP) measurement is a cornerstone in the diagnosis and follow-up of pediatric glaucoma. Accurate measurement of the IOP in younger children can pose a challenge [Citation2].
Successfully examining children’s eyes can be challenging due to their lack of cooperation. This poses a significant obstacle to achieving positive results. Administering sedative agents is a crucial step in conducting thorough examinations [Citation3].
When undergoing anaesthesia, it can be challenging to determine the best time to measure the IOP due to the possibility of anaesthesia decreasing the IOP in different ways. To get accurate measurements of the IOP, it’s best to measure it while the patient is awake, as the effects of sedation or general anaesthesia can vary depending on the agent used [Citation4].
Chloral hydrate is a potent sedative frequently utilized for its strong sedative effects. It is a common choice for sedating children [Citation5]. Despite its potential benefits, there are some challenges with using chloral hydrate for pediatric sedation. For instance, its solid and unpleasant odour and its bitter taste can make it difficult for some children to take it orally. Additionally, this drug can cause irritation and discomfort in the gastrointestinal tract, leading to symptoms such as nausea and vomiting. Moreover, as with many sedative medications, chloral hydrate can cause irritability upon waking. Over time, its use has led to reported instances of severe adverse reactions in children, including laryngospasm and respiratory depression [Citation6].
Dexmedetomidine is a pharmacological agent that selectively binds to α2-adrenergic receptors, resulting in sedation and analgesia [Citation7]. Dexmedetomidine has been applied safely and successfully in pediatric sedation outside the theatre for noninvasive diagnostic procedures, including CT scanning and MRI [Citation8]. Its IOP-lowering properties make it a premedication often used in ophthalmic surgery [Citation9].
Intranasal ketamine is a pharmaceutical agent utilized for its sedative and analgesic properties and a premedication before anaesthetic induction. When administered intranasally, it has been observed to effectively produce sedation with doses ranging from 0.5 mg/kg to 5 mg/kg. However, this medication’s most frequently reported adverse effects are nausea and vomiting [Citation10].
The impact of administering dexmedetomidine with ketamine through the nose on children’s intraocular pressure (IOP) has not been researched yet. This study aimed to conduct a randomized, controlled, double-masked investigation to compare the impact of intranasal infusion of dexmedetomidine, ketamine, or a combination of both on the IOP in children.
2. Patients and methods
This prospective, double-blinded, randomized, observational study was conducted at Benha University Hospital, Egypt. Patients were recruited from July 2023 to September 2023. The study was approved by the ethical review board of Benha University (RC 13 June 2023). All children’s parents or legal guardians were required to provide informed consent before participation in the study. It is crucial to note that the principles of the Declaration of Helsinki were strictly followed throughout the study.
The study recruited children between the ages of one and six years, classified as American Society of Anesthesiologists (ASA) I or II, who were scheduled to receive sedation for medical or surgical purposes other than IOP check.
Children with conditions such as bradycardia, cardiorespiratory distress, seizures, upper respiratory tract infection, hypersensitivity to drugs, neurologic deficits, liver disease or any acute medical condition were not included in the study.
Before the procedure, the patient’s weight was measured for proper dosing. Additionally, their body temperature, heart rate, respiratory rate, blood pressure, and oxygen saturation were evaluated before and during the procedure.
We randomly divided the study patients into three groups using computer-generated random numbers. The allocation sequence was kept confidential from the study investigators in sealed envelopes to ensure that the allocation of participants to the study arms remained unbiased. The patient selected the envelopes containing the allocation data sequentially in the presence of the study nurse.
Patients were provided with pharmaceuticals that an independent investigator prepared during the study. Intranasal drugs were administered via a one-milliliter tuberculin syringe.
Group D: received intranasal dexmedetomidine 3 μg/kg
Group DK: received intranasal dexmedetomidine 1 μg/kg with Intranasal ketamine 2 mg/kg
Group K: received intranasal ketamine 4 mg/kg
The children in our study were monitored every 15 minutes to track the start and duration of their sedation and full recovery. The study also evaluated potential side effects related to the gastrointestinal, respiratory, and neurological systems and compared them between groups. The level of sedation was evaluated using the Ramsay Sedation Score [Citation11], which classified patients as alert and calm, drowsy, or sedated based on the level of sedation. To ensure unbiased results, two anesthetists MAE & MSME evaluated the children for the onset of sedation at the 15th, 30th, 45th, and 60th-minute marks. The study has defined sedation as a state of complete unconsciousness with a lack of body movements. Sedation success was achieved when the patient reached Ramsay Sedation Score 3 or more.
We used the iCare (IC100) device, manufactured by iCare Finland Oy in Vantaa, Finland, to measure children’s Intraocular Pressure (IOP). We selected iCare for our study because it is a handheld device that provides reliable readings and is easy to use with children. The measurements were taken before and after administering sedatives upon reaching complete sedation.
—Our study’s primary outcome was assessing the IOP difference before and after sedation.
—The secondary outcomes were sedation scale assessment, emergency agitation and medication side effects.
We conducted a preliminary pilot study to establish the optimal sample size for our research while ensuring its accuracy and reliability. The pilot study involved 45 patients, with 15 randomly assigned to each group. We assessed the study’s outcomes and incorporated the findings into the final analysis. Ophthalmologist, MAA, who checked the children’s IOP, was blinded to the nature of the drug used.
2.1. Sample size calculation
The sample size calculation was performed using G*power software version 3.1.9.2, which relied on data from the pilot study carried out as part of the current research project. The pilot revealed a large effect size of IOP between the studied groups (d = 0.4). The total sample size calculated was 105 patients (35 per group). Alpha and power were adjusted at 0.05 and 0.95, respectively.
2.2. Statistical methods
The data management and statistical analysis were performed using SPSS version 28 (IBM, Armonk, New York, United States). Quantitative data were assessed using the Shapiro-Wilk test and direct data visualization methods to check for normality. Quantitative data were presented as means and standard deviations, while categorical data were presented as numbers and percentages. The one-way ANOVA test was used to compare quantitative data between the studied groups, and all post hoc analyses were adjusted for multiple comparisons. Our study compared categorical data using either Chi-square or Fisher’s exact test. Multivariate linear regression analysis was performed to predict post-sedation IOP, and the regression coefficients (B) with 95% confidence intervals were calculated. All statistical tests were two-sided, and p values less than 0.05 were deemed significant.
3. Results
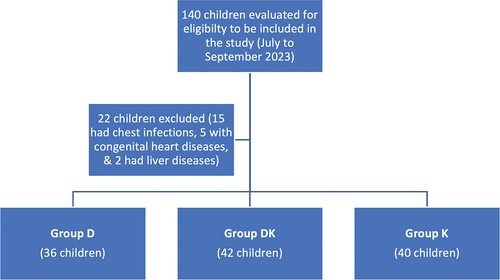
We evaluated 140 children to determine their eligibility to participate in our study. After careful consideration, we excluded 22 children from the sample because they did not meet the inclusion criteria. Specifically, 15 children had chest infections, five were diagnosed with congenital heart diseases, and two had liver diseases. Our final study population included 118 ASA I or II children aged 1–6 years. We randomly divided our participants into three distinct groups: Group D, which comprised 36 children; Group DK, which had 42 children; and Group K, which had 40 children. ()
3.1. General characteristics
As shown in , the studied groups were comparable regarding age (p = 0.675), sex (p = 0.941), and ASA (p = 0.896)
Table 1. General characteristics of the studied groups.
3.2. Intraocular pressure
No significant differences were observed in the IOP before sedation in the right (p = 0.654) and left (p = 0.799) eyes. After sedation, IOP significantly differed between groups in both eyes (p < 0.001 for each). Post hoc analysis revealed that in both eyes, IOP was significantly lower in group D (13 ± 3 mmHg) than in groups DK (16 ± 4 mmHg) and K (17 ± 3 mmHg), with no significant difference between groups DK and K (, ).
Figure 2. Intraocular pressure (mmHg) of the right and left eyes before and after sedation in the studied groups.
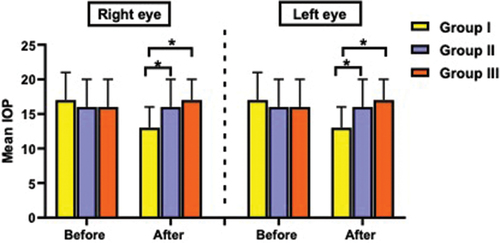
Table 2. Intraocular pressure of the right and left eyes before and after sedation in the studied groups.
3.3. Secondary outcomes
A significant association was observed between the studied groups and sedation score (p < 0.001), with Ramsay 3 being higher in group K (65%) compared to groups D and DK (22.2% and 9.5%, respectively). Ramsay 4 was higher in group D and DK (52.8% and 52.4%, respectively) compared to group K (35%). No patients had Ramsay 5 in group K (0%) compared to 25% and 38.1% in groups D and DK, respectively ().
Table 3. Secondary outcomes in the studied groups.
Post-sedation nausea and vomiting significantly differed between the studied groups (p < 0.001). It was higher in group K (25%) compared to groups D and DK (0% for each). Additionally, agitation was higher in group K (62.5%) than in groups D and DK (0% for each) (p < 0.001) ().
Heart rate before sedation was comparable between the studied groups (p = 0.878). After sedation, it significantly differed between groups (p < 0.001). It was significantly lower in group D (89 ± 7) than in groups DK (97 ± 6) and K (99 ± 7), with no significant difference between groups DK and K ().
All patients in our study achieved a Ramsay Sedation Score of 3 or more. However, sedation onset revealed a significant difference between the studied groups (p < 0.001). It was significantly higher in group K (25 ± 5) than in groups D (19 ± 3) and DK (10 ± 1). Additionally, it was significantly higher in group D than in group DK ().
3.4. Prediction of intraocular pressure after sedation
Multivariate linear regression analysis was done to predict IOP after sedation in both eyes. The model revealed that GI was a significant predictor for IOP in the right eye (B = -4.614, 95% CI = -6.28 - -2.948, p < 0.001) and left eye (B = -4.334, 95% CI = -5.907 - -2.751, p < 0.001), controlling for age, sex, and ASA ().
Table 4. Multivariate linear regression analysis to predict intraocular pressure after sedation.
4. Discussion
It is common for children who undergo elective surgery to feel scared and anxious in the hospital environment, particularly in the waiting area before the operation. During the induction period, children experience anxiety due to separation from parents, the clinical environment, and interactions with masked personnel [Citation12]. Studies indicate that around 60–70% of children exhibit significant anxiety levels before surgery [Citation13].
For many years, Ketamine has served as a useful premedication drug. Despite this, it is not the ideal choice due to its negative effects, such as paradoxical reactions, postoperative behavioral changes, cognitive impairment, and slow recovery [Citation14].
Dexmedetomidine is a type of medication that selectively stimulates alpha-2 adrenergic receptors. It is becoming increasingly popular as a premedication for children due to its sedative and anxiolytic effects, which have minimal impact on respiratory function. Several studies have shown that dexmedetomidine premedication can provide adequate sedation before surgery, reduce separation anxiety in parents, decrease the incidence of emergence delirium, and improve the acceptance of facemask induction [Citation15].
Administering intranasal doses of dexmedetomidine and ketamine before surgery has greatly enhanced children’s ability to tolerate the inhalation of anesthesia masks. This sedative approach has a high success rate and is capable of effectively mitigating the incidence and severity of emergence agitation [Citation14].
The study conducted by Jianxia Liu and colleagues aimed to evaluate the sedative effects of intranasal 2 μg/kg dexmedetomidine combined with 1 mg/kg ketamine on young children undergoing transthoracic echocardiography. Their findings revealed that successful sedation was achieved in 96% of the participants. Additionally, they identified several independent risk factors for sedation failure, such as cyanotic heart disease, history of congenital heart disease surgery, history of sedation failure, and fever. The research findings suggest that the use of an intranasal combination of dexmedetomidine and ketamine is a safe and effective method for administering sedation to young children during echocardiography [Citation16].
In a study conducted by Prakhar Gyanesh and colleagues, they compared the effectiveness of two intranasal drugs, namely 1 μg/kg dexmedetomidine and 5 mg/kg ketamine, as a premedication for children undergoing MRI. The researchers found that most of the children involved in the study had no issues with taking the intranasal drugs, with 90.4% of anesthesiologists in the dexmedetomidine group and 82.7% in the ketamine group expressing satisfaction with the conditions for IV cannulations. Based on their findings, the researchers concluded that dexmedetomidine and ketamine were equally effective when administered intranasally as a premedication for children undergoing MRI [Citation17].
In a recent study, Xinlei Lu and colleagues compared the effectiveness of intranasal 2 μg/kg dexmedetomidine, 1 mg/kg ketamine, and a combination of dexmedetomidine 1 μg/kg and ketamine 0.5 mg/kg in inducing anesthesia for children. The results showed that the combination of dexmedetomidine and ketamine had a higher success rate of sedation (90%) compared to the dexmedetomidine group (70%) and the ketamine group (53.3%) (p = 0.007). The anesthesiologist satisfaction was also higher in the combination group. The study concluded that administering a combination of dexmedetomidine and ketamine intranasally before surgery significantly improves children’s cooperation with inhalation anesthesia masks. The utilization of this specific sedation method has demonstrated a commendable success rate and has been shown to diminish the incidence and intensity of emergence agitation effectively [Citation18].
A study led by Hayrullah Alp and his team discovered that intranasal midazolam with a dosage of 0.2 mg/kg, intranasal ketamine with a dosage of 4 mg/kg, and oral chloral hydrate with a dosage of 50 mg/kg are all effective in achieving conscious sedation during pediatric echocardiography. The study revealed that intranasal midazolam has a faster onset of sedation, while intranasal ketamine has a shorter duration of sedation compared to the other two sedatives. However, all three agents have effectively provided adequate sedation for successful transthoracic echocardiography. The study also revealed that children undergoing transthoracic echocardiography may experience side effects [Citation19].
Another study evaluated the effectiveness of intranasal ketamine (5 mg/kg) and dexmedetomidine (2.5 μg/kg) for providing sedation in children before shifting to the radiotherapy suite. They demonstrated that intranasal dexmedetomidine is superior to intranasal ketamine in providing procedural sedation. Children who had dexmedetomidine required more time to awaken, but this difference was not clinically significant [Citation20].
The impact of dexmedetomidine on the IOP has extensively been studied [Citation21,Citation22]. Dexmedetomidine may affect the IOP by constricting the blood vessels in the ciliary body, decreasing aqueous humour production. This medication may also enhance the aqueous humour outflow by reducing the ocular drainage system vasomotor tone, which the sympathetic nervous system controls. Furthermore, the haemodynamic response associated with dexmedetomidine may help to decrease the IOP [Citation23].
A study by Deepika D et al. evaluated the efficacy of two doses of intranasal dexmedetomidine (3.0 and 3.5 µg/kg) as a sedative for postoperative glaucoma exams in children. The study included 61 children. The findings showed that a 3.5 µg/kg dose was more effective and eliminated the need for recurring general anesthesia. However, one patient in the 3.5 µg/kg group experienced ventricular arrhythmia, which was treated with dextrose-saline infusion and glycopyrrolate injection. That study did not compare pre- and post-sedation IOP [Citation24].
There is an apprehension that ketamine may increase IOP, which is based on animal studies, studies involving multiple anesthetics simultaneously, and studies using higher doses of ketamine than what is typically used for procedural sedation and pain relief. On the other hand, several other studies have found no significant increase in IOP [Citation25].
A study evaluated the effect of ketamine on intraocular pressure (IOP) in children receiving ketamine for procedural sedation and analgesia for reasons other than eye injury. The study included children between 1 and 5 years of age receiving a mean total ketamine dosage of 1.6 mg/kg. The study stated that there was no significant increase in the IOP of pediatric patients without eye injuries [Citation26].
Furthermore, Nagdeve NG and their colleagues found that administering a low dose (3 mg/kg) of intramuscular ketamine did not cause a significant change in the IOP of children in their study. However, administering a higher dose (6 mg/kg) of ketamine significantly increased IOP just 5 minutes after injection [Citation27].
The administration of intranasal 1 μg/kg Dexmedetomidine and 2 mg/kg Ketamine combination to patients in our study resulted in a faster onset of sedation without significant changes in heart rate, agitation, or post-sedation nausea or vomiting. Over half of the patients in that group ranked at a grade 4 level on the Ramsay sedation scale. Additionally, there were no notable disparities in the IOP before and after sedation, indicating that this combination is favorable for pediatric patients undergoing sedation to evaluate their IOP.
Our research was carried out at Benha University Hospital and was limited to a specific group of patients, which is a significant constraint. Nevertheless, we are proud to have been the first to investigate the impact of intranasal sedatives, Dexmedetomidine and Ketamine, on IOP fluctuations. We are confident that our findings have immense value.
Our research indicates that this combination of sedatives is viable for optimal sedation in pediatric patients undergoing surgeries or medical procedures with no significant change in the IOP and with minimal side effects. However, it is important to note that further research is necessary to confirm its efficacy and safety in larger patient populations.
Author contributions
MSME: has a major role in the conception and design of work, performing anaesthetic tasks, data acquisition, interpretation, analysis, drafting and review of the manuscript. MAE: participated in anaesthetic tasks, data analysis, interpretation, drafting and final manuscript review. MAA: performed the ophthalmic part of the work and participated in data analysis, manuscript drafting and review. All authors have read and approved the manuscript in its current state.
Consent for publication
Consent for the publication of identifying patient/clinical data, as well as identifying images, was obtained from all relevant patients in writing.
Ethics approval and consent to participate
This study was approved by the ethical committee of Benha Faculty of Medicine, Benha University (Approval number: RC 13 June 2023). A written informed consent, including the aim of surgery, detailed steps, and potential complications, was signed by each patient’s guardian before surgery.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets of this study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Strouthidis NG, Papadopoulos M. Clinical evaluation of glaucoma in children. Curr Ophthalmol Rep. 2013;1(2):106–112. doi: 10.1007/s40135-013-0012-6
- Brusini P, Salvetat ML, Zeppieri M. How to measure intraocular pressure: an updated review of various tonometers. J Clin Med. 2021 27;10(17):3860. doi: 10.3390/jcm10173860
- Pirlich N, Grehn F, Mohnke K, et al. Anaesthetic protocol for paediatric glaucoma examinations: the prospective EyeBIS study protocol. BMJ Open. 2021;11(10):e045906. doi: 10.1136/bmjopen-2020-045906
- Thanapaisal S, Oatts J, Zhao J, et al. Effect of general anaesthesia on intraocular pressure in paediatric patients: a systematic review. Eye (Lond). 2021;35(4):1205–1212. doi: 10.1038/s41433-020-1093-8
- Chen ML, Chen Q, Xu F, et al. Safety and efficacy of chloral hydrate for conscious sedation of infants in the pediatric cardiovascular intensive care unit. Medicine (Baltimore). 2017;96(1):e5842. doi: 10.1097/MD.0000000000005842
- Ratnapalan S. Chloral hydrate sedation in children. Clin Pediatr (Phila). 2014;53(10):933–936. doi: 10.1177/0009922813508000
- Kaur M, Singh PM. Current role of dexmedetomidine in clinical anesthesia and intensive care. Anesth Essays Res. 2011;5(2):128–133. doi: 10.4103/0259-1162.94750
- Lyu X, Tao Y, Dang X. Efficacy and safety of intranasal dexmedetomidine vs oral chloral hydrate for sedation in children undergoing computed tomography/magnetic resonance imaging: a meta-analysis. Front Pediatr. 2022;10(10):872900. doi: 10.3389/fped.2022.872900
- Jones JH, Aldwinckle R. Perioperative dexmedetomidine for outpatient cataract surgery: a systematic review. BMC Anesthesiol. 2020;20(1):75. doi: 10.1186/s12871-020-00973-4
- Poonai N, Canton K, Ali S, et al. Intranasal ketamine for procedural sedation and analgesia in children: a systematic review. PLoS One. 2017 20;12(3):e0173253. doi: 10.1371/journal.pone.0173253
- Riessen R, Pech R, Tränkle P, et al. Comparison of the RAMSAY score and the Richmond Agitation Sedation Score for the measurement of sedation depth. Crit Care. 2012;16(S1):326. doi: 10.1186/cc10933
- Liu W, Xu R, Shen Y, et al. Research progress on risk factors of preoperative anxiety in children: a scoping review. Int J Environ Res Public Health. 2022;19(16):9828. doi: 10.3390/ijerph19169828
- Fortier MA, Kain ZN, Morton N. Treating perioperative anxiety and pain in children: a tailored and innovative approach. Paediatr Anaesth. 2015 Jan;25(1):27–35. doi: 10.1111/pan.12546 Epub 2014 Sep 30. PMID: 25266082; PMCID: PMC4261033.
- Qian B, Zheng W, Shi J, et al. Ketamine enhances intranasal dexmedetomidine-induced sedation in children: a randomized,double-blind trial. Drug Des Devel Ther. 2020;14:3559–3565. doi: 10.2147/DDDT.S269765
- Arun N, Choudhary A, Kumar M. Comparative study of intranasal dexmedetomidine versus intranasal ketamine as premedicant in children. Cureus. 2022 July 05;14:7. doi: 10.7759/cureus.26572
- Liu J, Du M, Liu L, et al. Sedation effects of intranasal dexmedetomidine combined with ketamine and risk factors for sedation failure in young children during transthoracic echocardiography. Paediatr Anaesth. 2019;29(1):77–84. doi: 10.1111/pan.13529
- Gyanesh P, Haldar R, Srivastava D, et al. Comparison between intranasal dexmedetomidine and intranasal ketamine as premedication for procedural sedation in children undergoing MRI: a double-blind, randomized, placebo-controlled trial. J Anesth. 2014;28(1):12–18. doi: 10.1007/s00540-013-1657-x
- Lu X, Tang L, Lan H, et al. A comparison of intranasal dexmedetomidine, esketamine or a dexmedetomidine-esketamine combination for induction of anaesthesia in children: a randomized controlled double-blind trial. Front Pharmacol. 2021;12:808930. doi: 10.3389/fphar.2021.808930
- Alp H, Elmacı AM, Alp EK, et al. Comparison of intranasal midazolam, intranasal ketamine, and oral chloral hydrate for conscious sedation during paediatric echocardiography: results of a prospective randomised study. Cardiol Young. 2019;29(9):1189–1195. doi: 10.1017/S1047951119001835
- Suvvari P. Comparison of intranasal dexmedetomidine versus intranasal ketamine as premedication for level of sedation in children undergoing radiation therapy: a prospective, randomised, double-blind study. Turk J Anaesthesiol Reanim. 2020 [cited 2024 May 18];48(3):215–222. doi: 10.5152/TJAR.2019.45087
- Abdalla MI, Al Mansouri F, Bener A. Dexmedetomidine during local anesthesia. J Anesth. 2006;20(1):54–56. doi: 10.1007/s00540-005-0351-z
- Virkkila M, Ali-Melkkila T, Kanto J, et al. Dexmedetomidine as intramuscular premedication for day-case cataract surgery. A comparative study of dexmedetomidine, midazolam and placebo. Anaesthesia. 1994;49(10):853–858. doi: 10.1111/j.1365-2044.1994.tb04257.x
- Mowafi HA, Aldossary N, Ismail SA, et al. Effect of dexmedetomidine premedication on the intraocular pressure changes after succinylcholine and intubation. Br J Anaesth. 2008;100(4):485–489. doi: 10.1093/bja/aen020
- Deepika D, Babita G, Pranshuta S, et al. Evaluation of intranasal dexmedetomidine as a procedural sedative for ophthalmic examination of children with glaucoma. J Glaucoma. 2020;29(11):1043–1049. doi: 10.1097/IJG.0000000000001607
- Ruiz-Villa JOA, Jaramillo-Rivera DAB, Pineda-Gutierrez LMA. Ketamine impact on intraocular pressure of children: a systematic review and qualitative synthesis of evidence. Colombian J Anesthesiol. 2019 Oct-Dec;47(4):226–235. doi: 10.1097/CJ9.0000000000000141
- Halstead SM, Deakyne SJ, Bajaj L, et al. The effect of ketamine on intraocular pressure in pediatric patients during procedural sedation. Acad Emerg Med. 2012 Oct;19(10):1145–1150. doi: 10.1111/j.1553-2712.2012.01450.x Epub 2012 Sep 25. PMID: 23009160.
- Nagdeve N, Yaddanapudi S, Surinder P. The effect of different doses of ketamine on intraocular pressure in anesthetized children. J Pediatr Ophthalmol Strabismus. 2006;43:219–223.