ABSTRACT
A pot experiment was conducted to examine the ability of Blue Panic grass (Panicum antidotale) to grow in slightly saline soils (2.40 dS m–1) under different levels of saline irrigation water in the presence or absence of compost. Eight treatments were set up in a randomized block design with five replicates as follows: T1 (Freshwater), T2 (Freshwater + compost at 20%), T3 (Saline water 5000 mg L–1), T4 (T3 + compost at 20%), T5 (Saline water 10000 mg L–1), T6 (T5 + compost at 20%), T7 (Saline water 15000 mg L–1) and T8 (T7 + compost at 20%). Growth parameters of Blue Panic Grass were evaluated at the end of the experimental period as plant and root length, shoot, and root fresh and dry weights, total chlorophyll, and total carbohydrates. In general, tested Blue Panic Grass appeared to be tolerant to high salt concentrations in irrigation water, and slightly significant differences were found for all the measured parameters. A remarkable growth increase occurred in plants grown in compost-amended soils, with respect to the unamended soils. The results demonstrate the possibility to stabilize the yield of blue panic grass, an important feed crop in Egypt, irrigated with saline water, which can secure animal feed resources without reducing the already limited freshwater availability.
Introduction
The availability of freshwater for agricultural use became a limiting factor worldwide. Egypt, particularly, is facing a significant shortage in the availability of freshwater resources for irrigation of the current farmland and for expansion of the farmland in the reclaimed areas to secure food for the rapidly growing populations. The agricultural sector consumes about 80% of the total freshwater in Egypt, which indicates a potential increase in the gap between limited water supply and water demands. To lessen the gap, water from nonconventional sources must be used in irrigation, e.g. saline water, agriculture drainage water, treated sewage effluent, and any other available alternative. Therefore, saline water blended with freshwater could be a promising solution to irrigate some agricultural crops but salinity stress and its adverse effects on crop and yield should be evaluated before using saline water in irrigation.
Salinity stress is one of the most serious environmental problems affecting soils in arid and semiarid areas around the world, affecting more than 50% of the world’s irrigated land and 20% of the cultivated land (Hasegawa, Bressan, Zhu, & Bohnert, Citation2000). The adverse effects of saline irrigation water directly affect soil-water-plant relations, including the severe restriction of plant physiological activities and productive capacity of crops (De Pascale, Orsini, & Pardossi, Citation2013; Plaut, Edelstein, & Ben-Hur, Citation2013). Wu, Guo, and Harivandi (Citation2001) and Katerji, van Hoorn, Hamdy, and Mastrorilli (Citation2003) reported that the crop's sensitivity and tolerance to salinity level may vary depending on meteorological and soil conditions in the region, as well as the irrigation method. Accordingly, a proper management strategy is required to allow acceptable relative yield and efficient use of saline water in irrigation and to prevent the development of excessive soil salinization (Wang, Kang, Wang, Liu, & Feng, Citation2007).
Low-cost and efficient treatment strategies are required to reduce salt toxicity of soils and to improve soil properties (Shaaban, Abid, & Abou-Shanab, Citation2013). Application of organic amendments to the soil under salinity stress could be considered a means to reduce the negative effects of salts on plant growth. Clark, Dodgshun, Sale, and Tang (Citation2007) reported that application of organic matter (OM) improved the physical, chemical, and biological properties of salt-affected soil beside its importance in sustainable land use and crop productivity (Cha-um & Kirdmanee, Citation2011; Wong, Dalal, & Greene, Citation2009). Several organic materials, such as farmyard manure, agro-industrial byproducts, and composts can be used as amendments for soil remediation in the salt-affected areas due to their high organic matter content (Diacono & Montemurro, Citation2010; Montemurro et al., Citation2010). Cha-um and Kirdmanee (Citation2011) studied the effect of applied OM on saline soil cultivated with rice and found that OM application improved photosynthetic abilities and increased chlorophyll content and total chlorophyll pigments in rice grown in saline soil with OM treatment compared to unamended control.
Blue Panic (Panicum antidotale) or Giant panic is a native of Southeast Asia and is an excellent sand binder that favors arid and semiarid conditions (Cope, Citation1982). Blue Panic is also considered as an ideal fodder grass due to its high protein contents (15–18%; Bokhari, AlyaeeshL, & Al Noori, Citation1988). Several authors reported that Blue Panic has ability to adapt to a variety of climatic conditions including severe environmental stresses like drought, salinity, toxic nutrients (e.g. Ahmed, Ashraf, & Ali, Citation2010; Ashraf, Citation2004; Zhang, Irving, Tian, & Zhou, Citation2012). Nevertheless, it was reported that Blue Panic can tolerate salinity up to 15000 mg L–1 and drought using almost 50% less water than alfalfa does (Bokhari et al., Citation1988). Moreover, Jacobs and Wall (Citation1993) reported that blue panic grass can grow up to 1.5 m with a very deep root system. Based on the reported facts above, the objective of this study was to investigate the response of blue panic grass to different salinity levels of irrigation water compared to freshwater in the presence/absence of compost under greenhouse conditions.
Materials and methods
Experimental procedure
Seeds of Blue Panic (Panicum antidotale) were purchased from a commercial supplier in Egypt. Plants were grown from the 10th April to the 25 May 2018, under natural conditions in the research facilities of the Central Laboratory for Environmental Quality Monitoring (CLEQM), with the National Water Research Center, Qalubiya (30° 11´ 53.89” N; 31° 7´ 21.57” E). The temperature ranged from 40°C (day) to 16°C (night) with an average of 26°C and an average of 47% relative humidity, with an average photoperiod of 12 h per day. To prevent emergence failures, more than 20 seeds were sown in each pot; then, when the first pair of true leaves appeared, seedlings were thinned out and 10 uniform ones per pot were allowed to continue the growth period.
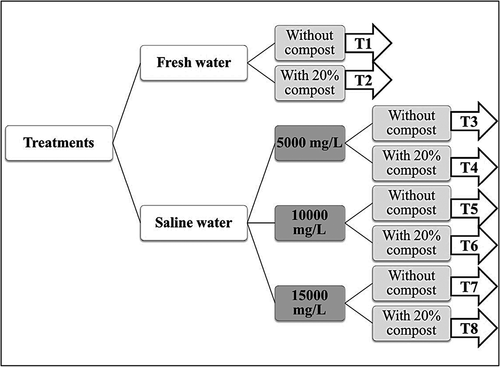
Seeds of blue panic were planted in a loamy sand soil in pots made of polyvinyl chloride having a diameter of 20 cm and a depth of 20 cm. The experiment was set up as a randomized block design with five replicates and included eight treatments (40 pots), as follows: T1 (Freshwater), T2 (T1 + compost at 20%), T3 (Saline water 5000 mg L–1), T4 (T3 + compost at 20%), T5 (Saline water 10000 mg L–1), T6 (T5 + compost at 20%), T7 (Saline water 15000 mg L–1), and T8 (T7 + compost at 20%). All pots were irrigated and kept at the field capacity moisture throughout the growing season. The experiment layout is presented in .
Figure 1. Layout of the different treatments used to test blue panic growth under different saline conditions
The saline water was prepared by dissolving Rashidi salt (a Mediterranean Sea salt with 93.5% NaCl; 2.5% MgCl, 1.5% other salts, and 2.5% humidity) in freshwater (0.15–0.25 dS m–1) then applied to the pots. Soils were collected from new-reclaimed land of Wadi El Rayan area, El Fayoum Governorate, after grass cover removal from the top 20 cm, air-dried, gently ground to pass through a 2-mm sieve, homogenized, and used to fill the pots (2 kg soil per pot). A Commercial compost (20% w/w) was used as amendments by thorough mixing with the soil. Characteristics of fresh and saline water used in this experiment are presented in . Chemical and physical characteristics of the used soil and compost prior to the experiment are presented in .
Table 1. Characteristics of fresh and saline water used in irrigation
Table 2. Selected chemical and physical characteristics of tested soil and compost
Plants were equally irrigated and every pot received a total of 8.0 g of mineral fertilization of ammonium nitrate (33.5% N), calcium superphosphate (15.5% P2O5), and potassium sulfate (48% K2O); every 10 days at a rate of 2.0 g pot−1per application time. At the end of the experimental period (45 days), growth parameters were determined in form of plant and root length, shoot, and root fresh and dry weights, total chlorophyll, and total carbohydrates. Shoot and root fresh and dry weights were weighed separately and then oven-dried at 65°C for 1 week.
Analytical procedure
Chemical and physical characteristics of soils were determined prior to the study using standard methods reported in George, Rolf, and John (Citation2013) and Jackson (Citation1962). Soil samples were analyzed for pH, the electrical conductivity of the saturated soil extract (ECe), total nitrogen (N), total phosphorus (P), total potassium (K), soil organic matter (OM), as well as soluble cations (Ca+2, Mg+2, Na+, and K+) and soluble anions (Cl−, SO4−2, NO3−, HCO3−, and PO4−3). The characteristics of the compost used were determined according to the standard procedures described by Bertran-Kerhres, and Andrease (Citation1994) and El-Kouny (Citation1999). Water samples were analyzed according to the standard methods of APHA (Citation1995) for pH, electric conductivity (EC), total-dissolved solids (TDS), soluble cations, and soluble anions. Plants' total chlorophyll and carbohydrate concentrations were determined following the method described in Shabala, Shabala, Martynenko, Babourina, and Newman (Citation1998) and Dubois, Gilles, Robers, and Smith (Citation1956), respectively.
Statistical analysis
Data were subject to analysis of variance (ANOVA) based on randomized block design (RBD) with five replications using the MSTAT program. One-way ANOVA was used was to determine the difference between the treatment's means. Then, the least significant difference (LSD) test was used for appraising the significant difference between the mean values at 5%.
Results and discussion
Physico-chemical properties of water, soil and compost
The physicochemical analysis of the used water is presented in . The pH values were moderately alkaline and within FAO limits (6.5–8.5) for irrigation water. The EC values of saline water used ranged from 8 to 24 (dS m−1), which are much higher than the range reported by FAO guidelines (>3.0 dS m−1) with severe restriction to be used for irrigation. The TDS values (5312, 10316, 15424 mg L−1) of saline water exceeded the range described by FAO guidelines (>2000 mg L−1). The major salt ions except NO3−, Cl−, K+, and Na+ were within the acceptable limits for saline water samples (). The high concentration of these ions is attributed to the EC and TDS values in which reflect the ingredients of used sea salt.
The results of the physical and chemical properties of soil and compost are presented in (). Soil samples had a loamy sand soil texture, with a slightly alkaline pH, which is a type of arid areas. Analysis of OM amended soil (at 20% compost) in comparison with the reference soil showed higher OM, N, and K content. These results are consistent with many studies, which indicated that compost addition increase OM and nutrients in amended soils (Adugna, Citation2018; Bhogal et al., Citation2018, Naba et al., Citation2020). In addition, compost, naturally, contributes to the stabilization and increase of crop productivity and crop quality (Adugna, Citation2018).
Plant and root length
shows the impact of increasing salinity levels in irrigation water on plant length, regardless of applying 20% compost to some treatments. In general, plant shoot length decreased with increasing the salinity levels in irrigation water from 5000 mg L–1 to 15000 mg L–1 by 8–32% compared with irrigation with freshwater. Plant shoot length decreased by 5.5, 10.6, and 21.4 cm for T4, T6, and T8, respectively, with increasing the concentration of salts in irrigation water from 5000 mg L–1 to 15000 mg L–1, compared to freshwater treatment. However, the impact of saline water irrigation was lessened by adding 20% compost to the tested soil irrigated with saline water as plant shoot length increased by 4.5–10%. The plant length increased due to compost application corresponded to 2.8, 3, and 5 cm, for T4, T6, and T8, respectively, compared to the treatments without compost application (i.e. T3, T5, and T7). The plant shoot length decreased following the order: T2 > T1 > T4 > T3 > T6 > T5 > T8 > T7, which may indicate that Blue Panic has a certain ability to grow under high levels of salt concentration in irrigation water despite the decrease in plant length.
Table 3. Growth parameters of Blue Panic (Panicum antidotale) after different treatments in the pot experiment
Similarly, increasing salt concentration in irrigation water adversely affected Blue Panic root length. The root length decreased from 15.50 to 11.90 cm with increasing salt concentration in irrigation water from 5000 mg L–1 to 15000 mg L–1. This decrease in the root length indicates that the Blue Panic plant can endure the considerable level of salinity stress without an extreme reduction in the root system. Moreover, treating the soil with 20% compost increased the root length in treatments irrigated with freshwater and in treatments irrigated with saline water (5000 mg L–1 and 15000 mg L–1). Compost application did not show effects when added to soil irrigated with 10000 mg L–1 saline water when compared to the same treatment with no compost application. The Blue Panic root growth response to the various treatments followed the order: T2 > T4 > T1 > T3 > T5 = T6 > T8 > T7.
Plant shoots length and root length were meaningfully affected by increasing salinity stress (), which agrees with previous studies that reported that increasing salt concentration negatively affects root and shoot development (Ashraf & Tufail, Citation1995; Dash & Panda, Citation2001; Delgado & Sanchez-Raya, Citation2007; Munns et al., Citation2002; Reinhardt & Rost, Citation1995). Similarly, Jamil and Rha (Citation2004) reported that root length and plant height provide important insights into the response of plants to salt stress because roots are directly in contact with soil, absorb water, and shoot supply it to the rest of the plant. The inhibition occurred in cytokinesis and cell expansion, in addition to the toxic effect of salts, are thought to be the main reasons responsible for the negative impacts of salt stress. Additionally, the increase in osmotic pressure around the roots as a result of saline environment can also prevent water absorption by roots, resulting in shorter root length and plant height (Al-Karaki, Citation2001; Aydinşakir, Ulukapı, Kurum, & Büyüktaş, Citation2013; Bohnert, Nelson, & Jensen, Citation1995; Mensah, Akomeah, Ikhajiagbe, & Ekpekurede, Citation2006; Sadat-Noori, Mottaghi, & Lotfifar, Citation2008; Werner & Finkelstein, Citation1995). Furthermore, the decrease in hormones that stimulate the growth and increase in hormones that hinder growth can cause shorter root and shoot lengths (Ashraf & O’leary, Citation1997; Foolad Citation1996; Prakash & Prathapasenan, Citation1990; Taiz & Zeiger, Citation1998).
Different works reported different explanations for Blue Panic growth under adverse conditions; e.g. Ashraf (Citation2003) associated the adaptation of blue panic with saline habitats to the CO2 assimilation and stomatal conductance whereas Eshghizadeh, Kafi, and Nezami (Citation2012) showed that leaf area, chlorophyll a content, and shoot K+ content are the main components of salt tolerance.
Fresh and dry weight
shows that the fresh weight of Blue Panic decreased significantly from 25.9 to 15.3 g pot–1 with increasing salt content in irrigation water with a decreasing percentage reached about 41.1% compared to the irrigation treatment with freshwater. Compost application to treatments irrigated with saline water improved the fresh weight of the Blue Panic plant with a percent ranged 6.3–8.1%. However, compost application in the freshwater irrigated treatment showed a different trend as the fresh weight decreased by about 9% compared to the same treatment without compost application, which might be justified by the nutrients supplied from compost. In general, the fresh weight of Blue Panic was in the following order: T1 > T2 = T4 > T3 > T6 > T5 > T8 > T7.
Similar to fresh weight, the dry weight of the Blue Panic plant decreased with increasing salt concentration in irrigation water (). The dry weight values decreased gradually from 4.6 g pot–1 (irrigated with freshwater only) to 3.9, 3.1, and 2.6 g pot–1 with increasing salinity of irrigation water by 5000, 10000, and 15000 mg L–1, respectively, regardless of the presence/absence of compost. However, applying 20% compost to some treatments seems to have improved the efficiency of Blue Panic resistance to high salinity levels, which consequently resulted in increased dry biomass. The dry matter of blue panic resulted from the different treatments was in the following order: T2 > T1 > T4 > T3 > T6 > T5 > T8 > T7.
Several authors also agreed with our results, which showed that salinity stress had adverse effects on other plant growth parameters such as plant and root fresh weight for other crops, such as soybean (Zaidi & Sing, Citation1993), chickpea (Khalid, Iqbal, Tahir, & Ahmad, Citation2001), cowpea (Düzdemir, Ünlükara, & Kurunç, Citation2009), broad bean (De Pascale & Barbieri, Citation1997), black cumin (Hajar, Zidan, & Al-Zahrani, Citation1996), melon (Sivritepe, Sivritepe, Eris, & Turhan, Citation2005), tomato (Yurtseven, Kesmez, & Ünlükara, Citation2005), watermelon (Yu-feng, Citation2006), and okra (Ünlükara, Kurunç, Kesmez, & Yurtseven, Citation2008).
In general, the decrease in dry biomass of Blue Panic in T3, T5, T6, and T7 fluctuated around 20% of the T1, which is a very acceptable decline in biomass when compared to the benefits provided by saving freshwater and replacing it with saline water (up to 10000 mg L–1).
Total chlorophyll
shows the concentrations of total chlorophyll content (mg 100–1 g plant). Total chlorophyll concentrations in the Blue Panic plant varied from one treatment to another; the largest significantly different values of total chlorophyll concentration was observed in T2 and T4. There were no significant differences between T4, T5, and T6 and between T1 and T5. A similar observation was observed between T7, T8, and T3 and between T3 and T7. Accordingly, it seems that blue panic has the ability to form and produce chlorophyll regardless of salinity stress. However, some treatments that were adversely affected by increasing salt concentration were positively affected by compost application (T4, T5, and T6; ).
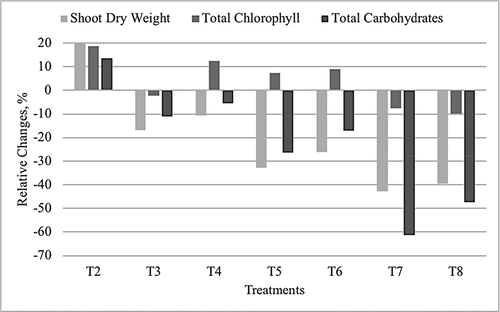
Figure 2. The relative changes in shoot dry weight (g pot–1), total chlorophyll (mg 100–1 g plant) and total carbohydrates (mg 100–1 g plant) as a percent after the freshwater treatment
Applying organic matter to salt-affected soil may function as salt ion binding agents, which detoxify the growing medium from toxic ions, especially Na+ and Cl–, as shown by low electrical conductivity (ECe) in some soils treated with compost (Hanay, Büyüksönmez, Kiziloglu, & Canbolat, Citation2004; Tejada, Garcia, Gonzalez, & Hernandez, Citation2006; Zahid & Niazi, Citation2006; Zaka, Mujeeb, Sarwar, Hassan, & Hassan, Citation2003). Similarly, Cha-um and Kirdmanee (Citation2011) reported that remediation of salt-affected soil in paddy fields using OM should be considered further as an effective way of enhancing food crop productivity. They found that photosynthetic abilities, including total chlorophyll pigment, in plants grown with organic matter-amended treatment were greater than in those cultivated in soil without OM application, especially in high salt (1–2% salt) levels. The degree of reduced photosynthetic pigments in plants was dependent on the level of salt contamination. Similarly, compost application in the growth experiment of Blue Panic might have sustained the photosynthetic abilities of the plant.
Total carbohydrates
Increasing salt concentration in irrigation water significantly affected the production of total carbohydrates in the blue panic plant (). The concentration of total carbohydrates significantly decreased from 1.60 to 0.62 mg 100–1 g when the concentration of salts in irrigation water increased to 15000 mg L–1. In addition, compost application slightly increased the ability of plants to form carbohydrates by 6%, 11%, and 26% under the concentrations of saline water of 5000, 10000, and 15000 mg L–1, respectively. Total carbohydrates were in the following order: T2 > T1 > T4 > T3 > T6 > T5 > T8 > T7.
In a summary, illustrates the relative changes in short dry weight, total chlorophyll, and total carbohydrates as a percent of the freshwater treatment. It shows that irrigating with up to 10000 mg L–1 saline water would reduce the total dry mass by 20%, did not change meaningfully the chlorophyll content, and reduced the carbohydrate content by a maximum of 60%. Given that Blue Panic is rich in fibers, the reduction in carbohydrate contents might not affect the animal acceptance and digestibility of Blue Panic, thus, suggesting to grow Blue Panic in salt-affected soils and/or irrigate it with low-quality irrigation water.
Conclusion
This study examined the ability of the Blue Panic plant to grow under different saline water levels in the presence/absence of compost. The results indicated that Blue Panic can grow under the high saline conditions with slight but acceptable differences compared to the control (freshwater) treatment. Application of organic amendments such as compost-enhanced plant growth and decreased the effect of saline water on plant growth. The ability of Blue Panic to grow under saline conditions suggests that it could be used as an animal feed in a partial or full substitution of the typical Egyptian fodder. Blue Panic has shown notable regrowth after cutting of the crop and is well relished by animals as it is rich in protein (>10%), fibers (>32%), and minerals (e.g. Calcium 0.4%). Cultivation of Blue Panic as an animal feed will allow the reuse of saline-recycled water and saves freshwater for other strategic crops, such as wheat production, in a period of water shortage in Egypt and the world. Further field studies are needed to elucidate the feasibility and productivity of a promising plant, Blue Panic, under saline conditions to tackle the problems of shortage in good quality irrigation water and productive farming land.
Additional information
Funding
References
- Adugna, G. (2018). A review on impact of compost on soil properties, water use and crop productivity. Agricultural Science Research Journal, 4(3), 93–104.
- Ahmed, M., Ashraf, M., & Ali, Q. (2010). Soil salinity as a selection pressure is a key determinant for the evolution of salt tolerance in blue panicgrass (Panicum antidotale Retz.). Flora, 205, 37–45.
- Al-Karaki, G. N. (2001). Germination, sodium, and potassium concentrations of barley seeds as influenced by salinity. Journal of Plant Nutrition, 24, 511–512.
- APHA (American Public Health Association). (1995). Standard methods for the examination of water and wastewater. Washington, DC: USA.
- Ashraf, M. (2003). Relationships between leaf gas exchange characteristics and growth of differently adapted populations of blue panic grass (Panicum antidotale Retz.) under salinity or water logging. Plant Science, 165, 69–75.
- Ashraf, M. (2004). Some important physiological selection criteria for salt tolerance in plants. Flora, 199, 361–376.
- Ashraf, M., & O’leary, J. W. (1997). Response of a salt-tolerant and a salt-sensitive line of sunflower to varying sodium/calcium ratios in saline sand culture. Journal of Plant Nutrition, 20, 361–377.
- Ashraf, M., & Tufail, M. (1995). Variation in salinity tolerance in sunflower (Helianthus annus L.). Journal of Agronomy and Crop Science, 175, 351–362.
- Aydinşakir, K., Ulukapı, K., Kurum, R., & Büyüktaş, D. (2013). The effects of different salt source and concentrations on germination and seedling growth of some pumpkin seeds used as rootstock. Journal of Food Agriculture & Environment, 11(1), 503–510.
- Bertran-Kerhres, and Andrease. (1994). Methods book for analysis of compost (pp. 14–60). Köln-Gremberghoven: Federation Compost Quality Assurance Organization (FCQAO), Cologne, Germany.
- Bhogal, A., Nicholson, F. A., Rollett, A., Taylor, M., Litterick, A., Whittingham, M. J., & Williams, J. R. (2018). Improvements in the quality of agricultural soils following organic material additions depend on both the quantity and quality of the materials applied. Frontiers in Sustainable Food Systems, 2, 9. doi:https://doi.org/10.3389/fsufs.2018.00009
- Bohnert, H. J., Nelson, D. E., & Jensen, R. G. (1995). Adaptations to environmental stresses. The Plant Cell, 7, 1099–1111.
- Bokhari, U., AlyaeeshL, F., & Al Noori, M. (1988). Potentials of forage crops. Saudi Arabian Journal of Scientific Research, 6, 359–367.
- Cha-um, S., & Kirdmanee, C. (2011). Remediation of salt-affected soil by the addition of organic matter - an investigation into improving glutinous rice productivity. Scientia Agricola, 68(4), 406–410.
- Clark, G. J., Dodgshun, N., Sale, P. W., & Tang, C. (2007). Changes in chemical and biological properties of a sodic clay subsoil with addition of organic amendments. Soil Biology & Biochemistry, 39, 2806–2817.
- Cope, T. A. (1982). Flora of Pakistan. In E. Nasir & S. I. Ali (Eds.), Family poaceae (Vol. 143, pp. 678–682). Karachi, Pakistan: Department of Botany, University of Karachi.
- Dash, M., & Panda, S. K. (2001). Salt stress induced changes in growth and enzyme activities in germinating Phaseolus mungo seeds. Biologia Plantarum, 44(4), 587–589.
- De Pascale, S., & Barbieri, G. (1997). Effects of salinity and top removal on growth and yield of broadbean as a green vegetable. Scientia Horticultureae, 71, 147–165.
- De Pascale, S., Orsini, F., & Pardossi, A., 2013. Irrigation water quality for greenhouse horticulture. In Good Agricultural Practices for Greenhouse Vegetable Crops; FAO Plant Production and Protection Paper 217; Food and Agriculture Organization of the United Nations: Rome, Italy; pp. 169–204.
- Delgado, I. C., & Sanchez-Raya, A. J. (2007). Effects of sodium chloride and mineral nutrients on initial stages of development of sunflower life. Communications in Soil Science and Plant Analysis, 38, 2013–2027.
- Diacono, M., & Montemurro, F. (2010). Long-term effects of organic amendments on soil fertility: A review. Agronomy for Sustainable Development, 30, 401–422.
- Dubois, M., Gilles, K. A., Robers, J. H., & Smith, F. (1956). Colorimetric methods for determination of sugar and related substances. Analytical Chemistry, 28, 350–356.
- Düzdemir, O., Ünlükara, A., & Kurunç, A. (2009). Response of cowpea (Vigna unguiculata) to salinity and irrigation regimes. New Zeland Journal of Crop and Horticultural Science, 37, 271–280.
- El-Kouny, H. M., 1999. Evaluation of compost production and its properties with special reference to compost extract. [PhD. Thesis.] Faculty of Agriculture, Alexandria University, Alexandria, Egypt.
- Eshghizadeh, H. R., Kafi, M., & Nezami, A. (2012). The mechanisms of salinity tolerance in the xero-halophyte blue panicgrass (Panicum antidotale Retz). Notulae Scientia Biologicae, 4(2), 59–64. doi:https://doi.org/10.15835/nsb427363
- FAO (Food and Agriculture Organization). (1985). Water Quality for Agriculture, R. S. Ayers, D. W. Wescot. Irrigation and Drainage Paper 29 Rev. 1 (p. 174) ISBN 92-5-102263-1, Rome, Italy: FAO.
- Foolad, M. (1996). Genetic analysis of salt tolerance during vegetative growth in tomato, Lycopersicon esculentum Mill. Plant Breeding, 115(4), 245–250. doi:https://doi.org/10.1111/j.1439-0523.1996.tb00911.x
- George, E., Rolf, S., & John, R. (2013). Methods of soil, plant, and water analysis: A manual for the West Asia and North Africa region. International Center for Agricultural Research in Dry Areas (ICARDA), 65, 120.
- Hajar, A. S., Zidan, M. A., & Al-Zahrani, H. S. (1996). Effect of salinity stress on the germination, growth and some physiological activities of black cumin (Nigella sativa L.). Arab Gulf Journal of Scientific Research, 14(2), 445–454.
- Hanay, A., Büyüksönmez, F., Kiziloglu, F. M., & Canbolat, M. Y. (2004). Reclamation of saline-sodic soils with gypsum and MSW compost. Compost Science & Utilization, 12, 175–179.
- Hasegawa, P. M., Bressan, R. A., Zhu, J. K., & Bohnert, H. J. (2000). Plant cellular and molecular responses to high salinity. Annual Review of Plant Physiology and Molecular Biology, 51, 463–499.
- Jackson, M. L. (1962). Soil chemical analysis. London, UK: Constable and Co. Ltd.
- Jacobs, S. W., & Wall, C. A. (1993). Poaceae. In G. J. Harden (Ed.), Flora of New South Wales (pp. 281–589). Kensington, Australia: New South Wales Univ. Press.
- Jamil, M., & Rha, E. S. (2004). The effect of salinity (NaCl) on the germination and seedling of suger beet (Beta vulgaris L.) and cabbage (Brassica oleracea capitata L.). Korean Journal of Plant Research, 7, 226–232. https://www.koreascience.or.kr/article/JAKO200414714198880.pdf
- Katerji, N., van Hoorn, J. W., Hamdy, A., & Mastrorilli, M. (2003). Salinity effect on crop development and yield, analysis of salt tolerance according to several classification methods. Agricultural Water Management, 62, 37–66.
- Khalid, M. N., Iqbal, H. F., Tahir, A., & Ahmad, A. N. (2001). Germination potential of chickpeas (Cicer arietinum L.) under saline conditions. Pakistan Journal of Biological Sciences, 4(4), 1395–1396 and Soil 75:75–80.
- Mensah, J. K., Akomeah, P. A., Ikhajiagbe, B., & Ekpekurede, E. O. (2006). Effects of salinity on germination, growth and yield of five groundnut genotypes. African Journal of Biotechnology, 5(20), 1973–1979.
- Montemurro, F., Vitti, C., Diacono, M., Canali, S., Tittarelli, F., & Ferri, D. (2010). A three-year field anaerobic digestates application: Effects on fodder crops performance and soil properties. Fresenius Environmental Bulletin, 19, 2087–2093.
- Munns, R., Husain, S., Rivelli, A. R., James, R. A., Condon, A. G., Lindsay, M. P., … Hare, R. A. (2002). A venue for increasing salt tolerance of crops, and the role of physiologically based selection traits. Plant and Soil, 247, 93–105.
- Naba, R. P., Hans, P. S., Jan, M., Sarah, E. H., Olivier, H., & Gerard, C. (2020). Nutrient effect of various composting methods with and without biochar on soil fertility and maize growth. Archives of Agronomy and Soil Science, 66(2), 250–265. doi:https://doi.org/10.1080/03650340.2019.1610168
- Plaut, Z., Edelstein, M., & Ben-Hur, M. (2013). Overcoming salinity barriers to crop production using traditional methods. Critical Reviews in Plant Sciences, 32, 250–291.
- Prakash, L., & Prathapasenan, G. (1990). Interactive effect of NaCI salinity and gibberellic acid on shoot growth, content of abscisic acid and gibberellins-like substances and yield of rice (Oryza sativa L. var. G.R.3). Proceding Indian Academy Science, 100, 173–181.
- Reinhardt, D. H., & Rost, T. L. (1995). On the correlation of the primary root growth and treachery element size and distance from the tip in cotton seedlings grown under salinity. Environmental and Experimental Botany, 35, 575–588.
- Sadat-Noori, S. A., Mottaghi, S., & Lotfifar, O. (2008). Salinity tolerance of maize in embryo and adult stage. American-Eurasian Journal of Agricultural & Environmental Sciences, 3(5), 717–725.
- Shaaban, M., Abid, M., & Abou-Shanab, R. (2013). Amelioration of salt affected soils in rice paddy system by application of organic and inorganic amendments. Plant, Soil and Environment, 59, 227–233.
- Shabala, S. N., Shabala, S. I., Martynenko, A. I., Babourina, O., & Newman, I. A. (1998). Salinity effect on bioelectric activity, growth, Na+ accumulation and chlorophyll fluorescence of maize leaves: A comparative survey and prospects for screening. Australian Journal of Plant Physiology, 25, 609–616.
- Sivritepe, H. O., Sivritepe, N., Eris, A., & Turhan, E. (2005). The effects of NaCl pre-treatments on salt tolerance of melons grown under long-term salinity. Scientia Horticulturae, 106(4), 568–581.
- Taiz, L., & Zeiger, E. (1998). Plant physiology (2nd ed.). Sunderland, Massachusetts, USA: Sinauer Associates Ins. Publisher.
- Tejada, M., Garcia, C., Gonzalez, J. L., & Hernandez, M. T., . (2006). Use of organic amendment as a strategy for saline soil remediation: Influence on the physical, chemical and biological properties of soil. Soil Biology & Biochemistry, 38, 1413–1421.
- Ünlükara, A., Kurunç, A., Kesmez, D. G., & Yurtseven, E. (2008). Effects of salinity on eggplant (Solanum melongena L.) growth and evapotranspıration. Irrigation and Drainage, 59, 203–214.
- Wang, S., Kang, Y., Wang, D., Liu, S. P., & Feng, L. P. (2007). Effect of drip irrigation with saline water on tomato (Lycopersicon esculentum Mill) yield and water use in semi-humid area. Agricultural Water Management, 90, 63–74.
- Werner, J. E., & Finkelstein, R. R. (1995). Arabidopsis mutants with reduced response to NaCl and osmotic stress. Physiologia Plantarum, 93(4), 659–666.
- Wong, V. N., Dalal, R. C., & Greene, R. S. (2009). Carbon dynamics of sodic and saline soils following gypsum and organic material additions: A laboratory incubation. Applied Soil Ecology, 41, 29–40.
- Wu, L., Guo, X., & Harivandi, A. (2001). Salt tolerance and salt accumulation of landscape plants irrigated by sprinkler and drip irrigation systems. Journal of Plant Nutrition, 24, 1473–1490.
- Yu-feng, W. (2006). Effect of NaCl stress on seed germination of watermelon. Journal of Anhui Agriculture Science, 34(24), 6497–6499.
- Yurtseven, E., Kesmez, G. D., & Ünlükara, A. (2005). The effects of water salinity and potassium levels on yield, fruit quality and water consumption of a native central Anatolian tomato species (Lycopersicon esculentum). Agriculture Water Management, 78, 128–135.
- Zahid, L., & Niazi, M. F. K. (2006). Role of ristech material in the reclamation of saline-sodic soils. Pakistan Journal of Water Resources, 10, 43–49.
- Zaidi, P. H., & Sing, B. B. (1993). Dry matter partitioning and yield attributes of soybean as affected by soil salinity and growth regulators. Legume Research, 16, 3–4.
- Zaka, M. A., Mujeeb, F., Sarwar, G., Hassan, N. M., & Hassan, G. (2003). Agromelioration of saline sodic soil. OnLine Journal of Biological Science, 3, 329–334.
- Zhang, H., Irving, L., Tian, Y., & Zhou, D. (2012). Influence of salinity and temperature on seed germination rate and the hydrotime model parameters for the halophyte, Chloris virgata, and the glycophyte, Digitaria sanguinalis. South African Journal of Botany, 78, 203–210.
