Abstract
Lower respiratory tract infections (LRTIs) cause high morbidity and mortality worldwide. Empiric therapy often base the choice of antibiotic treatment on antibacterial spectrum of the agent rather than on its pharmacological properties or the pathogen resistance profile. Inappropriate prescribing leads to therapeutic failure and antibiotic resistance, with increasing direct and indirect health costs. A consensus on appropriate prescribing in LRTI therapy was appraised by this Delphi exercise, based on a panel of 70 pulmonologists, coordinated by a Scientific Committee of nine experts in respiratory medical care. Full or very high consensus was reached on several issues, including the role of oral cephalosporins in first-line treatments of LRTIs and the appropriateness of cefditoren, with balanced spectrum and high intrinsic activity, in LRTI treatment. Evidence-based medicine approach and a comprehensive process of disease management, from diagnosis to therapy and follow-up, should guide antibiotic prescribing.
Introduction
Acute lower respiratory tract infections (LRTIs), like community-acquired pneumonia (CAP) and acute exacerbations of chronic bronchitis (AECB), are responsible for high morbidity and mortality worldwide. The burden of disease is greater than other infective, acute or chronic disorders (e.g. AIDS, diabetes or cancer) that are commonly considered pervasive health problems.Citation1 Bacterial infections account for the 80–90% of these respiratory infections and are the most frequent indications for antibiotic treatment: S. pneumoniae is the pathogen with highest incidence in CAP, while infections by H. influenzae and M. catarrhalis are more prevalent in AECB (Table ).Citation2–4
Table 1 Percentage incidence of bacterial species within the spectrum of lower respiratory tract infection (LRTI)-associated pathogens
Antibiotic therapy aims not only at improving the clinical outcome, but also eradicating the targeted pathogens or reducing bacterial load.Citation5 Therefore, empiric therapy should select the right dose and the most appropriate duration of treatment to obtain the optimal clinical response, to minimize toxicity and to prevent the emergence of resistant pathogens.Citation6 However, the treatment strategy often emerges from the empirical approach of clinicians, who are prone to choose the best therapeutic agent among the antibiotics available on the market. Consequently, the rationale of the choice is based predominantly on the antibacterial spectrum of the molecule without considering the profiles of pathogen resistance to the same molecule or the pharmacological properties of the different antimicrobial drugs.Citation7 This may result in an inappropriate prescribing practice, which may explain both the therapeutic failure and the proliferating resistance to antibiotics by pathogens of the respiratory tract.
The lack of appropriateness in antibiotic prescribing practices may further aggravate the epidemiologic profile of the resistant pathogens, reducing the potential of antibiotic therapy. The consequence is the increase of health costs, which are not necessarily influenced using the most expensive antibiotic, but rather by the indirect costs. Pharmacoeconomics studies conducted in U.S. and Europe demonstrated that the therapeutic failure following the incorrect choice of the antibiotic (and/or of its dosage and administration schedule) may cause worsening of patient’s conditions and hospitalization, which accounts for most of the total costs.Citation8–10
This work aims at methodically and systematically defining a consensus on the most controversial and debated issues on the diagnostic and therapeutic approaches to LRTIs. The role of oral antibiotic therapy for the management of CAPs and AECBs will be examined, in particular. The final goal of our contribution is promoting the most appropriate use of antibiotics to maximize therapeutic outcome with minimal drug toxicity and to minimize the development of antibiotic resistance, in accordance with the recommendations from the most prestigious National and International health organizations.
Material and methods
To assess the consensus on appropriate prescribing of antibacterial agents for LRTI therapy, we used the Delphi methodology. This is a group-facilitative method based on the multistage process of a series of iterative rounds (or questionnaires), each followed by feedback responses or ranking, designed to verify in a given area of uncertainty within health sciences the convergence of opinion of an expert panel in search of the most reliable group consensus according to evidence-based medicine.Citation11 This method, which is becoming increasingly popular in health care research, overcomes the potential problem of group dynamics that may occur with decision-making committees.Citation12
The Delphi process was developed over nearly eight months by the following steps: (i) establishment of a scientific steering committee of nine experts who were in charge preliminary of reviewing the literature and of developing the statements to be ranked; (ii) selection of an expert panel of specialists; (iii) first-round of online statement ranking by each panel expert; (iv) analysis of the results of round one, and second round of ranking of items that have not gained collective opinion; (v) final consensus meeting.
Scientific steering committee
Nine experts were identified among Italian institutions, as representative of different clinical specialties involved in medical care of patients with CAP, AECB and of exacerbations of chronic obstructive pulmonary disease (COPD) as well. Track record of publications, attendance to National and International meetings, participation to clinical trials, recognized expertise and/or academic rank were the selection criteria. The scientific steering committee defined 39 statements divided into the following four main topics:
| (1) | Issues in aetiology and antibiotic resistance involved in the process of prescribing antibiotics | ||||
| (2) | Pharmacologic issues involved in the practice of prescribing antibiotics | ||||
| (3) | Clinical considerations on the management of patients with CAP, AECB and COPD | ||||
| (4) | Pharmacoeconomics viewpoints on the practice of prescribing antibiotics | ||||
Panel of specialists
Seventy pulmonologists (MASTER working group) were selected from different Respiratory Units (Hospital or University based) or Respiratory Healthcare Structures as representative of the clinical practice in the field of respiratory disorder management in Italy.
Rounds
The statements developed by the steering committee were delivered to panel experts, who rated agreement or disagreement for each of the 39 statements, independently and blindly. Survey was performed online on a secured survey website (first round), using Verisign certificate SSL version 3 with 128-bit encryption. The responses of participants were collected and analyzed prior to the final consensus meeting (second round). Participants expressed their level of agreement on each statement using a five-point Likert-type scale (1: disagree, 2: somewhat disagree, 3: neither agree nor disagree, 4: somewhat agree, 5: agree).
Data analysis
The results of Delphi exercise data analysis were expressed as percentage of respondents who scored an item either 4 (somewhat agree) or 5 (agree). For the purposes of the consensus statement, agreement among the respondents of ≥70% for each statement was considered consensus, according to previous Delphi studies.Citation13–15
The scientific steering committee evaluated the responses and grouped those for which no consensus was reached and that were selected for the following step. After the individual and anonymous online survey, the participants (plus the nine members of the steering committee) attended a meeting to share the results of the first-round voting. After discussing the results, the 39 statements were voted again (once more blinded) using the same five-point scale.
Results
The panel of Italian experts performed the Delphi process and generated consensus opinions on 39 statements regarding different issues on the prescribing process of antibiotics for the management of patients with acute LRTIs. In details, the 39 statements were grouped per four areas within the following general concepts: (i) aetiology and antibiotic resistance; (ii) pharmacology; (iii) clinical practice; (iv) pharmacoeconomics (Table ).
Table 2 Statements defined by the scientific steering committee into four main topics and submitted to the expert panel of the Delphi-based consensus. The percentage of consensus reached for each statement is reported
The overall response rate of Delphi first round was 100% (70 responding participants out of 70 total panellists) and that of second round was 76% (53 out of 70). After second-round voting a positive consensus was reached on 38 of 39 statements with agreement ranging from 71 to 100%. Total agreement (100%) was reached in 8 of 39 of the statements.
In Authors’ view, the consensus opinions reached in 20 of the 39 statements appear of particular impact on appropriateness of prescribing practice and are selected for discussion, as outlined below.
Topic: issues in aetiology and antibiotic resistance involved in the process of prescribing antibiotics (Table 3)
The accurate aetiological diagnosis in LRTIs remains a difficult task still nowadays. The identification rate of the pathogen in hospitalized patients within controlled studies does not exceed 50% and these infections are often empirically treated.Citation7 The distribution of chemosensitive bacterial strains is geographically variable and updated information on a specific area is not frequently available. This leads to a risk of therapeutic failure ranging between 8 and 21% for AECB.Citation7,8 and also to underestimate the antibiotic resistance issue even if when patients are revisited for the same problem or present with AECB after a recent antibiotic treatment.
The microorganisms causing LRTIs have remained almost unchanged for the last years. S.pneumoniae, H.influenzae and M.catarrhalis are the main potential pathogens. More information has become available on the frequency of polymicrobial infections, including viral infections. Panton-Valentine leucocidin- producing S.aureus has emerged as a new cause of LRTIs, most often of severe CAP, although its occurrence remains currently uncommon.Citation16,17 Gram-negative nosocomial multidrug-resistant pathogens – such as P.aeruginosa – are more frequently isolated in patients with severe respiratory alterations or with a long history of hospitalization. Although no significant changes were identified in the aetiology of these syndromes, still frequency and clinical relevance of antimicrobial resistance remain important issues.
The expert panel reached consensus (71%) on avoiding the use in empiric therapy of antibiotics known to have a percentage of resistance above the 10–20% threshold, an opinion formulated by the study group of the Italian Group for the Study of Antibiotic Resistance in Respiratory Infections (GIARIR).Citation7 In addition, based on the observed resistance profiles, several antibiotics such as non-protected betalactams (amoxicillin), tetracyclines, trimethoprim/sulphamethoxazole and macrolides, should never be used in empiric therapy (consensus = 72%).
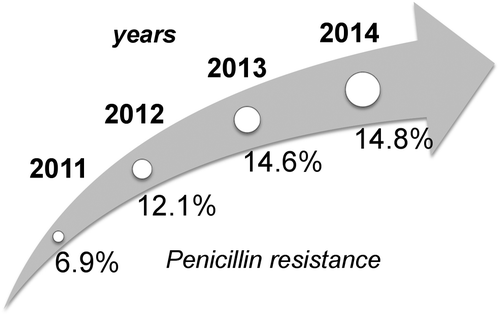
In case of S. pneumoniae in particular, a constant increase of bacterial resistance was observed in the last seven years,Citation18,19 such as in Italy, where the percentage of strains not susceptible to penicillin (intermediate and resistant) has reached values close to 15% onwards increasing in a four-year period (6.9% in 2011; 12.1% in 2012; 14.6% in 2013; 14.8% in 2014; Figure ) and, due to the acquisition of the erythromycin ribosomal methylation (ermB) gene, the incidence of resistance to macrolides is constantly growing with current values reaching 40–50%.Citation20 This high prevalence of erythromycin resistance prompted to the reassessment of the current use of this drug. High level of consensus (79%) has been reached on the major role of these species in increasing the levels of resistance.
Figure 1 Constant increase of bacterial non-susceptibility to penicillin in S. pneumoniae, expressed as percentage of intermediate and resistant strains (% I+R) observed in Italy from 2011 to 2014 and further raising trend in the following years.
On the contrary, no consensus was reached (41%) about the need of running at least one set of blood cultures in hospitalized patients with CAP, independently on how severe can be patient’s conditions (Table ). The unambiguous lack of consensus on this statement may be explained also by the existence of two inconsistent guidelines about the value of blood culture in the diagnosis of CAP. Guidelines of European Respiratory Society (ERS) and European Society for Clinical Microbiology and Infectious Diseases (ESCMID) recommend blood culture in all CAP patients who require hospitalization with a high level of evidence,Citation3 while those of the Infectious Diseases Society of America/American Thoracic Society (IDSA/ATS) recommend blood cultures for patients with severe CAP; blood cultures are also considered optional in all hospitalized patients with CAP but should be performed selectively.Citation21 The specialists involved in this Delphi consensus exercise may have been deployed in one or other of these two lines of thought.
Table 3 Consensus on the management of acute LRTIs. Percentage of agreement to the statements of topic 1: Issues in aetiology and antibiotic resistance involved in the process of prescribing antibiotics
The cephalosporin class of antimicrobial agents is known for its broad spectrum of activity, proven efficacy and favourable safety profile. However, even if all cephalosporins are equally active on Gram-negative bacteria (e.g. Haemophilus spp, Moraxella spp), remarkable differences exist among the members of this class for Gram-positive species (e.g. S. pneumoniae) (Table ). Several studies have been conducted to compare the pharmacokinetics/pharmacodynamics (PK/PD) parameters and the activity of the II- and III-generation oral cephalosporins available on the market.
Table 4 Different spectrum of activity among oral cephalosporins on S. pneumoniae
In a study aimed at comparing in vitro activity of cefditoren with several other comparators against more than 2,000 isolates from community-acquired respiratory infections in Italy,Citation22 cefditoren resulted the most active antibiotic against all penicillin-susceptible strains of S. pneumoniae and S. pyogenesis, and slightly less potent on penicillin-resistant pneumococci. Moreover, cefditoren shared with levofloxacin the highest activity against methicillin susceptible staphylococcus aureus (MSSA), K. pneumoniae and E. coli. Together with levofloxacin and cefotaxime, cefditoren displayed the most potent antimicrobial activity against all strains of H. influenza, irrespective of their ampicillin resistance.Citation22 The structural basis of this strong antimicrobial activity lies on the high affinity of the cefditoren molecule for the Penicillin Binding Protein 2X (PBP 2X), responsible for cephalosporin resistance when mutated, as demonstrated by crystallographic analysis of the antibiotic-protein complex.Citation23 Consistently, the statement declaring cefditoren pivoxil the III-generation oral cephalosporin with the highest intrinsic activity on S. pneumoniae, penicillin-resistant strains included, attained full consensus (100%) in this Delphi consensus exercise.
Moreover, in addition of S. pneumoniae strains that are resistant to penicillin, macrolides or fluoroquinolones, new phenotypes of H. influenzae emerged alongside canonical β-lactamase- negative (BLN) and β-lactamase-positive (BLP) strains: β-lactamase-negative ampicillin-resistant (BLNAR) and β-lactamase-positive amoxicillin/clavulanic acid-resistant (BLPACR). The carriage of these strains, characterized by mutations of the ftsI gene encoding the PBP 3 protein, caused recent concern for its increasing occurrence in several European countries.Citation24–26 When treating AECB, to prevent the intra-species diffusion of BLNAR and BLPACR resistant strains it is mandatory using antibiotics able to target also the emergent strains. The expert panel reached consensus (78%) also on this statement (Table ). According to a pharmacodynamics study, cefditoren displayed higher antibacterial activity than cefuroxime and amoxicillin/clavulanic acid against a mixed population of H. influenzae strains, including BLNAR and BLPACR phenotypes.Citation27
Topic: pharmacologic issues involved in the practice of prescribing antibiotics (Table 5)
The management of antimicrobial therapy benefits from knowing the PK/PD parameters of the antibiotic in use, which are useful predictors of the therapeutic efficacy. To obtain a bacterial eradication and not only a clinical result, dose and time of administration of an antibiotic must be adequate to its PK/PD profile. For time-dependent antibiotics like β-lactams, the parameter that better defines bactericidal efficacy and that allows controlling the emergence of resistant strains is T > MIC.Citation28 This is the time the serum concentration of the agent maintains above the minimum inhibitory concentration (MIC) for a given pathogen, expressed as percentage of dose interval.Citation29 On the contrary, in case of concentration-dependent antibiotics, such as fluoroquinolones and semi-synthetic macrolides (azithromycin and clarithromycin), the pharmacological goal is obtained by attaining adequate values of the ratio between either the area under the plasma concentration versus time curve (AUC) and the MIC (AUC/MIC), or the peak plasma concentration (Cmax) and the MIC (Cmax/MIC).Citation30 Even in empiric therapy, when these parameters reach optimal values, both the clinical/microbiological efficacy is ensured and the emergence of resistant bacteria is hampered.Citation31 As a matter of fact, an almost maximal consensus (98%) has been reached on the importance of the PK/PD profile of the antibiotic of choice for the patient’s treatment and to avoid an increase of microbial resistance.
An antibiotic for the effective therapy of LRTIs should be; (i) active on the most common isolates and on strains resistant to other agents; (ii) provide the highest in vitro activity; (iii) display pharmacodynamics features to make pathogen eradication very likely to occur. The structure-activity relationship of the different cephalosporins fully explains their versatility in terms of antimicrobial activity against both Gram-positive and Gram-negative species. Starting from second-generation cephalosporins, the presence of a 2-metoxyimino group (protecting from β-lactamases) and of an aminothiazole substitution in the C7 side chain, as well as a thiazole-derivative group as the C3 side chain of the cephem molecular skeleton have been shown to enhance antibacterial activity.Citation23 These substitutions also play as discriminators between Gram-positive and Gram-negative targets (Figure ). The C3 substitution is particularly important for activity against Gram-positive pathogens. The later generation ceftaroline, for example, has a broad-spectrum activity against Gram-positive species (including methicillin-resistant Staphylococcus strains), because of the presence of thiazole-pyridine rings in C3 position, but it displays a reduced activity on Gram-negative species, because of the lack of the aminothiazole substitution in the C7 side chain (Figure a). On the other hand, ceftriaxone, a third-generation molecule (Figure b), having the metoxyimino group and the aminothiazole substitution in the C7 side chain, has a good β-lactamase stability and a higher activity on Gram-negative bacteria, but lacking the thiazole substitution in C3, has a lower potency against Gram-positive species.Citation32 Among oral cephalosporins, cefditoren essentially fulfils these conditions (Figure c) and holds a balanced antimicrobial spectrum that includes the three major pathogens of community-acquired LRTIs: S. pneumoniae, H. influenzae and M. catarrhalis.Citation5 Very high consensus has been reached (96%) on the statement that amongst III-generation oral cephalosporins, the spectrum of ceftibuten and cefixime is mainly directed against Gram-negative bacteria, while the one of cefditoren is more balanced, including both Gram-positive and Gram-negative species (Table ).
Figure 2 Structural formulas of three representative cephem molecules and functional groups determining spectrum activity. Ceftaroline (Fifth generation) (a) is characterized by the presence of the thiazole-pyridine rings in C3 position conferring broad-spectrum activity against Gram-positive species. Ceftriaxone (Third generation) (b) carries the methoxyimino group and the aminothiazole substitution in the C7 side chain, granting a good β-lactamase stability and a higher activity on Gram-negative bacteria. Cefditoren (Third generation) (c) possess all these functional groups and possess full spectrum of activity both on Gram-positive and Gram-negative bacteria. Black circle, methoxyimino group (beta-lactamase stability); grey circle, aminothiazole group (activity against Gram-); dashed circle, methylthiazole group (activity against Gram+).
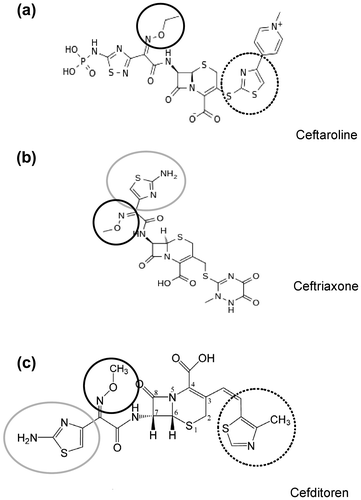
Table 5 Consensus on the management of acute LRTIs. Percentage of agreement to the statements of topic 2: Pharmacological issues involved in the practice of prescribing antibiotics
Oral administration is considered as the route of choice for the home care of mild LRTIs. Almost maximal consensus (98%) has been reached on this statement by the panel (Table ). All oral cephalosporins have a relatively short half-life, therefore – considering their time-dependent efficacy – daily dosage should provide for at least two administrations. This ensures that concentrations above the MIC should be adequately maintained for a sufficiently long time interval along the 24-h period.Citation28
The information on PK/PD features of the different classes of antibiotics offer a useful tool to the clinician for the choice of the most appropriate drug and of the most effective dose and route of administration in the therapy of LRTIs. The panel reached an almost complete agreement (97%) on the essential role of patient’s disease severity in determining the initial dose and the route of administration of cephalosporins for the treatment of LRTIs. Several studies, including the multicenter ARISE project, demonstrated that the PK/PD aspects of cefditoren, mainly at a dosage of 400 mg/12 h, can produce adequate and effective concentrations for a time-dependent antibiotic, making this cephem derivative an antimicrobial agent of choice against both AECB and CAP.Citation7,33,34
As far as adverse events and safety of antibiotics are concerned, macrolides have a quite high incidence of adverse events at gastrointestinal level, also due to the stimulatory effect on peristalsis and variable degrees of hepatotoxicity within the class.Citation35 Moreover, both macrolides and fluoroquinolones are inhibitors of cytochrome P450 enzymes, causing interactions with many different drugs, including HMG-CoA reductase inhibitors, calcium channel blockers, warfarin, benzodiazepines and other different drugs frequently used by elderly patients. In the case of fluoroquinolone class, a structure–adverse event relationship exists according to the different molecular features of these antimicrobial agents.Citation36 Inhibition of GABA system and stimulation of glutamate pathways account for the effects of fluoroquinolones on central nervous system. A QT interval prolongation has been observed at cardiac level.Citation37 Moreover, tendinopathy and Achilles tendon rupture have been associated with the use of several fluoroquinolone drugs.Citation38
Cephalosporins have fewer adverse events than other antimicrobial agents currently used, with very rare risks of anaphylactic shock. The incidence of allergic reactions is approximately 1/10 of those associated with penicillin treatment and very few interactions have been observed with other drugs, mostly because the hepatic P450 cytochrome system is not affected by these β-lactam derivatives.Citation39
Oral cephalosporins are generally well tolerated and treatment discontinuation with cefditoren, as a consequence of adverse events, has been observed only in 2.6% of patients.Citation40 The safety profile of cefditoren regarding the pharmacological interactions can be favourable in the clinical practice, especially in patients with comorbidities.Citation5 High consensus was reached (92%) on oral betalactams considered as the safest antibiotics with respect to the pharmacological interaction profile in patients under poly-pharmacotherapy (Table ).
Hospitalized patients with CAP are usually treated with intravenous administration of antibiotics to maximize the drug concentration in the tissues. However, early switch from parenteral to oral antibiotic administration (switch-therapy or sequential therapy) has been demonstrated safe and suitable to reduce hospital stay.Citation41 According to the guidelines of US IDSA/ATS and of the European Respiratory Society/European Society of Clinical Microbiology and Infectious Diseases (ERS/ESCMID), the switch from parenteral to oral therapy is safe when patients reach hemodynamic and clinical stability.Citation3,21 Approximately 70% of hospitalized CAP patients are candidates for sequential therapy after 72 h, provided that clinical stability is reached.Citation42 The characteristics of oral antibiotics to be considered for the switch therapy are: (i) similar antimicrobial spectrum; (ii) high bioavailability; (iii) administration time 12–24 h; (iv) good tolerability.Citation43 The expert panel reached a high level of consensus (93%) on cefditoren pivoxil as the most adequate option for the switch therapy from parenteral third-generation cephalosporins (like cefotaxime or ceftriaxone) to oral therapy, because of the similar spectrum and the highest intrinsic activity. A recommendation should be issued to clinicians to perform, when possible, a sequential antibiotic therapy (i.e. the switch from parenteral to oral therapy) with no dosage reduction, unless in the presence of adverse events. A very high consensus (96%) was reached by the panel on this statement (Table ). Furthermore, oral route is the ideal administration route in ‘compliant’ patients with mild LRTIs.
Topic: clinical considerations on the management of patients with CAP, AECB or COPD EXACERBATIONS (Table 6)
The choice of the most appropriate antibacterial agent, the identification and stratification of patients and the optimal duration of the therapy are main issues in the clinical management of LRTIs, while differences exist among recommendations of the different guidelines. On the basis of the antibiotic resistance emerged in recent years, some antimicrobial agents should not be considered for empirical therapy.Citation7 (Table ).
Table 6 Consensus on the management of acute LRTIs. Percentage of agreement to the statements of topic 3: Clinical considerations on the management of patients with CAP, AECB and COPD
The guidelines of the ERS/ESCMID for the management of antibiotic therapy of LRTIs indicate amoxicillin and tetracycline as ‘preferred’ antibiotics when aiming at minimal damage and based on a wide experience in clinical practice. Moreover, among cephalosporins, cefpodoxime and cefditoren prove to be more active than cefuroxime and cefprozil against S. pneumoniae.Citation3 The guidelines of the Canadian Thoracic Society distinguish the antibiotic choice according to disease severity and presence of common risk factors of COPD in the patient.Citation44
A comprehensive review of the European national guidelines for the COPD management in the last seven years revealed a remarkable diversity in clinical and functional classification of COPD. Among different countries, there is a general concordance for the choice of treatments, diagnostic criteria and use of long-lasting bronchodilators, while the definition of patient phenotype subgroups and stratification are considerably different.Citation45
It is of relevance the existence of evaluation criteria on the most appropriate antibiotic therapy based on the severity of COPD exacerbation. The guidelines of the Canadian Thoracic Society recommend the use of antibiotics in the presence of purulent sputum, while in case of mild bronchopathy the indication is to not administer antibiotics.Citation44 Further stratification criteria are based on the comorbidity and the pathogens involved. Groups are identified based on: (i) non-complicated COPD (group A) for which no antibiotic treatment is required; (ii) mild-moderate COPD with no risk factors for P. aeruginosa (group B) and with risk factors for this pathogen (group C). Indications for different antibiotics are given for the groups B and C.Citation3 In cases of COPD-bronchiectasis overlap syndrome, for the appropriate treatment of exacerbated non-cystic fibrosis-related bronchiectasis, the empirical antibiotic therapy should be adjusted or modified according to the results of sputum culture.Citation3 European guidelines and evidence from randomized controlled trials have been recently reviewed, revealing that inhaled antibiotic therapy emerges among the current approaches for non-cystic fibrosis bronchiectasis to maximize local drug concentrations and to reduce resistance risks.Citation46
The most appropriate duration of treatment was defined by the ERS/ESCMID guidelines as not exceeding eight days in CAP responsive patients. Moreover, evaluation of biomarkers as procalcitonin are recommended to verify the efficacy of the treatment and to modulate its duration.Citation3 The guidelines of the British Thoracic Society recommend seven days of appropriate antibiotics for CAP patients with mild-moderate severity, while a longer therapy, up to 14 or 21 days, can be adopted in the presence of relevant bacterial species (e.g. S. aureus), underscoring the importance of having complete microbiological information for the best management of LRTIs.Citation47 The US ATS/IDSA consensus guidelines recommend that patients with CAP should be treated for a minimum of five days, according to their clinical conditions and indicate a longer duration of therapy only after bacteremia is present and pathogens are identified.Citation21 In case of exacerbation of COPD, the most recent guidelines of Global Initiative for Chronic Obstructive Lung Disease (GOLD) and of the Italian Federation of Hospital Internal Medicine (FADOI) recommend a duration of antibiotic treatment of 5–10 days.Citation48,49 The updated guidelines of the National Institute for Health and Care Excellence (NICE) give no recommendation on treatment duration, and the criteria for antibiotics use to treat exacerbations of COPD include a history of more purulent sputum.Citation50 In an ATS/ERS position paper no criteria of duration are defined, antibiotics use is indicated as first-line treatment for ambulatory patients.Citation51
The panel of experts agreed (77% agreement) that betalactams should be contemplated among the first-choice antibiotics for the treatment of community-acquired LRTIs. Consensus was reached on the concept that the number and the duration of past year antibiotic treatments are predictive of a bacterial infection resistant to previously used antibiotics (89% agreement). Regarding the duration of antibiotic treatment, consensus was reached on a maximal eight days and a minimal five-day duration for responsive patients with CAP, irrespective of the pathogen involved (93% agreement), and for patients with exacerbated COPD (90% agreement). An evident consensus (89%) was reached on oral cephalosporins as first-line treatment of mild-moderate AECB in elderly patients with risk factors, while the use of fluoroquinolones should be limited to more severe exacerbations, especially in patients with bronchiectasis.
Topic: pharmacoeconomics viewpoints on the prescribing practice of antibiotics (Table 7)
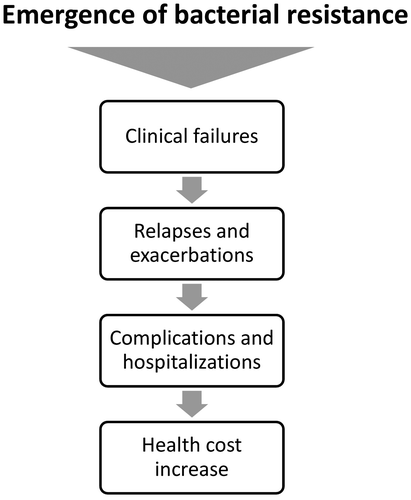
The emergence of bacterial resistance to antibiotics enhances the risk of therapeutic failure, increasing the health costs related to higher hospitalization rates and reducing the cost effectiveness of antibacterial therapy (Figure ).Citation52 However, costs associated with antibacterial resistance are rarely included in pricing decisions as they should be when choosing the most appropriate antibiotic for the treatment of LRTIs.Citation10 Inadequate antibiotic treatment may frequently lead to hospitalization due to worsening of patient’s conditions or to therapeutic failure, whose incidence was found up to 21% in case of AECBsCitation8 and that is accompanied to a many-fold increase of health costs. The analysis of the joint working group of the European Centre for Disease Prevention and Control/European Medicines (ECDC/EMA) revealed that in the European Union alone drug-resistant bacteria are estimated to cause 25,000 deaths and cost more than $ 1.5 billion every year in healthcare expenses and productivity losses.Citation53 Therefore, the choice of the most appropriate antibiotic is crucial also from the pharmacoeconomics point of view, aimed at obtaining a therapeutic success from the initial treatment. It has been estimated that for LRTIs, the influence on the total health care cost of the initial antibiotic treatment is 18% in absence of hospitalization and 10% in case of hospitalizationCitation9 and pharmacoeconomics evidence indicates that the first-line use of more expensive antibiotics may minimize treatment failure and associated high economic burden, leading to global cost effectivenessCitation8–10 Another important option for health cost avoidance is the switch-therapy, from parenteral to oral route of antibiotic administration.Citation54
Figure 3 Schematic representation portraying the consequences of the emergence of bacterial resistance to antibiotics. The risk of therapeutic failure favours relapses and exacerbation of LRTI symptoms, leading to increased incidence of hospitalization with consequent increase of health costs. This scenario abates the cost effectiveness of antibacterial therapy.
In view of the possible containment of health costs in LRTIs, this Delphi exercise reached a high level of consensus (88%) on the non-decisive role played by the price of the antibiotic used in first-line treatment of LRTIs. For the most appropriate choice of antimicrobial therapy almost global consensus (98%) was reached on the necessity of taking in consideration the therapeutic efficacy, as well as direct and indirect costs, including those related to the consequence of antibiotic resistance (Table ).
Table 7 Consensus on the management of acute LRTIs. Percentage of agreement to the statements of topic 4: Pharmacoeconomics viewpoints on the prescribing practice of antibiotics
Discussion
Most of LRTIs, like CAP and AECB, which account for high morbidity and mortality worldwide, are of bacterial origin and represent the indication for the treatment with antibiotics. However, the inappropriate choice of the antibiotics and their use may further aggravate the increasing incidence of resistant pathogens, with the consequent reduction of the antibiotic therapeutic potential.Citation7
The Delphi consensus exercise performed by this study reached consensus on many statements regarding debated issues on the appropriateness of prescribing antibiotics for the treatment of LRTIs. The following statement reached the highest level of consensus:
| • | In empiric therapy, antibiotics characterized by a resistance profile above the 10%-20% threshold and the non-protected betalactams should be avoided. | ||||
| • | For LRTI therapy, oral cephalosporins are often preferred among betalactams for their spectrum of action, resistance to betalactamase and good tolerability profile. Particularly, patients with LRTIs in poly-pharmacochemotherapy benefit from antibiotics with minimal drug interactions.Citation5 | ||||
| • | Amongst III-generation oral cephalosporins, the spectrum of cefditoren is particularly balanced, including both Gram-positive and Gram-negative species. The experts expressed the opinion that, due to its high intrinsic activity, cefditoren appears as an appropriate agent for either the treatment of LRTIs and for parenteral to oral switch therapy as well. Moreover, cefditoren at 400 mg dosage every 12 h ensures the coverage of penicillin-resistant Pneumococcal strains.Citation7,33,34 | ||||
| • | Oral cephalosporins can be included among the first-line treatments of mild-moderate AECB in elderly patients with risk factors, while the use of fluoroquinolones should be limited to more severe exacerbations, especially in patients with bronchiectasis. | ||||
| • | The price of the antibiotic used in first-line treatment has a minimum impact on the overall costs of LRTI management, while indirect costs related to the failures caused by bacterial resistance may have a considerable influence. | ||||
| • | The appropriateness of prescribing antibiotics should consider not only therapeutic efficacy and pharmacoeconomics considerations, but also a comprehensive process of disease management, from the diagnostic-therapeutic steps to the patient’s follow-up. | ||||
This study has some strengths and limitations as well. Strenghts: the study employed Delphi method for the first time in the field of antibiotics therapy to verify the consensus on a series of statements concerning issues about diagnostic and therapeutic approaches to LRTIs. This technique is a robust instrument used also for the development of guidelines based on the clinical experience and the viewpoints of the specialist experts involved. The methodology ensures the free expression of ideas in an anonymous fashion to avoid the influence of group dynamics. Limitations: the number of participants to the first and the second rounds were different. Moreover, this Delphi consensus exercise portraits a picture that is limited to the Italian clinical practice. Further similar studies and/or studies involving a broader panellist board will hopefully foster the discussion on these important issues with the medical community.
In conclusion, the empiric approach for the choice of the most appropriate antibiotic in LRTIs should be reconsidered in view of evidence-based medicine, as indicated by the results of this Delphi study.
Declaration of interest
FB has received research grants from Chiesi, Pfizer and Zambon, congress lecture fees from Astrazeneca, Boehringer Ingelheim, Chiesi, Dompe, Guidotti-Malesci, Grifols, InnovaPharma, Menarini, Novartis, GSK, Pfizer, Teva and Zambon, and consultancy fees from AstraZeneca, Menarini, Mundipharma, Novartis, GSK, Teva and Pfizer. SS has received research grants from Astra Zeneca, Basilea Pharma, Pfizer, Zambon, DMG; Consultancy and lecture fee from MSD. MG has received research grants from Thermo Fisher Diagnostics SPA. Advisor/Consultant and lecture fee from Angelini, Astellas, Basilea, MSD, Sanofi, Zambon, Guidotti, Malesci. All the other Authors report no declaration of interest.
Acknowledgements
Editorial assistance for the manuscript was provided by Dr. Luisa Granziero, on behalf of Health Publishing & Services srl and supported by Zambon. Dr. Granziero declares there are no potential conflicts of interest relating to her assistance.
References
- Mizgerd JP. Acute lower respiratory tract infection. N Engl J Med. 2008 Feb 14;358(7):716–27.10.1056/NEJMra074111
- Bartlett JG, Dowell SF, Mandell LA, File TM Jr, Musher DM, Fine MJ. Practice guidelines for the management of community-acquired pneumonia in adults. Clin Infect Dis. 2000 Aug;31(2):347–82.10.1086/313954
- Woodhead M, Blasi F, Ewig S, Garau J, Huchon G, Ieven M, et al. Guidelines for the management of adult lower respiratory tract infections – full version. Clin Microbiol Infect. 2011 Nov;17(Suppl 6):E1–59.10.1111/j.1469-0691.2011.03672.x
- Matkovic Z, Miravitlles M. Chronic bronchial infection in COPD. Is there an infective phenotype? Respir Med. 2013 Jan;107(1):10–22.10.1016/j.rmed.2012.10.024
- Di Marco F, Braido F, Santus P, Scichilone N, Blasi F. The role of cefditoren in the treatment of lower community-acquired respiratory tract infections (LRTIs): from bacterial eradication to reduced lung inflammation and epithelial damage. Eur Rev Med Pharmacol Sci. 2014;18(3):321–32.
- WHO. WHO global strategy for containment of antimicrobial resistance. Geneva: World Health Organization. 2001.
- Blasi F, Concia E, Mazzei T, Moretti AM, Nicoletti G, Novelli A, et al. Role of the oral Beta-lactams in the treatment of exacerbations of chronic bronchitis: critical analysis and therapeutics recommendations. J Chemother. 2010 Nov;22(Suppl 1):3–4.10.1179/joc.2010.22.Supplement-1.3
- Miravitlles M, Murio C, Guerrero T, Gisbert R. Pharmacoeconomic evaluation of acute exacerbations of chronic bronchitis and COPD. Chest. 2002 May;121(5):1449–55.
- Monte SV, Paolini NM, Slazak EM, Schentag JJ, Paladino JA. Costs of treating lower respiratory tract infections. Am J Manag Care. 2008 Apr;14(4):190–6.
- Morris S, Anderson P, Irwin DE. Acute exacerbations of chronic bronchitis: a pharmacoeconomic review of antibacterial use. PharmacoEconomics. 2002;20(3):153–68.10.2165/00019053-200220030-00002
- Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000 Oct;32(4):1008–15.
- Powell C. The Delphi technique: myths and realities. J Adv Nurs. 2003 Feb;41(4):376–82.10.1046/j.1365-2648.2003.02537.x
- Brown AK, O’Connor PJ, Roberts TE, Wakefield RJ, Karim Z, Emery P. Recommendations for musculoskeletal ultrasonography by rheumatologists: setting global standards for best practice by expert consensus. Arthritis Rheum. 2005 Feb 15;53(1):83–92.10.1002/(ISSN)1529-0131
- Mahler DA, Selecky PA, Harrod CG, Benditt JO, Carrieri-Kohlman V, Curtis JR, et al. American College of Chest Physicians consensus statement on the management of dyspnea in patients with advanced lung or heart disease. Chest. 2010 Mar;137(3):674–91.10.1378/chest.09-1543
- Zafar SY, Currow DC, Cherny N, Strasser F, Fowler R, Abernethy AP. Consensus-based standards for best supportive care in clinical trials in advanced cancer. Lancet Oncol. 2012 Feb;13(2):e77–82.10.1016/S1470-2045(11)70215-7
- Sanchini A, Campanile F, Monaco M, Cafiso V, Rasigade JP, Laurent F, et al. DNA microarray-based characterisation of Panton-Valentine leukocidin-positive community-acquired methicillin-resistant Staphylococcus aureus from Italy. Eur J Clin Microbiol Infect Dis. 2011 Nov;30(11):1399–408.10.1007/s10096-011-1234-x
- Johansson N, Kalin M, Tiveljung-Lindell A, Giske CG, Hedlund J. Etiology of community‐acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis. 2010 Jan 15;50(2):202–209.10.1086/648977
- Del Grosso M, Camilli R, D’Ambrosio F, Petrucci G, Melchiorre S, Moschioni M, et al. Increase of pneumococcal serotype 19A in Italy is due to expansion of the piliated clone ST416/CC199. J Med Microbiol. 2013 Aug;62(Pt 8):1220–5.10.1099/jmm.0.061242-0
- Zuccotti G, Mameli C, Daprai L, Garlaschi ML, Dilillo D, Bedogni G, et al. Serotype distribution and antimicrobial susceptibilities of nasopharyngeal isolates of Streptococcus pneumoniae from healthy children in the 13-valent pneumococcal conjugate vaccine era. Vaccine. 2014 Jan 23;32(5):527–34.
- ECDC. European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2014. Stockholm: ECDC, 2015 November. Report No.
- Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007 Mar;44(Suppl 2):S27–72.10.1086/511159
- Stefani S, Mezzatesta ML, Fadda G, Mattina R, Palù G, Rossano F, et al. Antibacterial activity of cefditoren against major community-acquired respiratory pathogens recently isolated in Italy. J Chemother. 2008 Oct;20(5):561–9.10.1179/joc.2008.20.5.561
- Yamada M, Watanabe T, Miyara T, Baba N, Saito J, Takeuchi Y, et al. Crystal structure of cefditoren complexed with streptococcus pneumoniae penicillin-binding protein 2X: structural basis for its high antimicrobial activity. Antimicrob Agents Chemother. 2007 Nov;51(11):3902–7.10.1128/AAC.00743-07
- Cherkaoui A, Diene SM, Emonet S, Renzi G, Francois P, Schrenzel J. Ampicillin-resistant Haemophilus influenzae isolates in Geneva: serotype, antimicrobial susceptibility, and β-lactam resistance mechanisms. Eur J Clin Microbiol Infect Dis. 2015 Oct;34(10):1937–45.10.1007/s10096-015-2435-5
- Giufrè M, Daprai L, Cardines R, Bernaschi P, Ravà L, Accogli M, et al. Carriage of Haemophilus influenzae in the oropharynx of young children and molecular epidemiology of the isolates after fifteen years of H. influenzae type b vaccination in Italy. Vaccine. 2015 Nov 17;33(46):6227–34.10.1016/j.vaccine.2015.09.082
- Lâm TT, Claus H, Elias J, Frosch M, Vogel U. Ampicillin resistance of invasive Haemophilus influenzae isolates in Germany 2009–2012. Int J Med Microbiol. 2015 Oct;305(7):748–55.10.1016/j.ijmm.2015.08.028
- Gonzalez N, Aguilar L, Alou L, Gimenez MJ, Sevillano D, Torrico M, et al. Influence of different resistance traits on the competitive growth of Haemophilus influenzae in antibiotic-free medium and selection of resistant populations by different {beta}-lactams: an in vitro pharmacodynamic approach. J Antimicrob Chemother. 2009 Jun;63(6):1215–22.10.1093/jac/dkp097
- Novelli A, Fallani S, Cassetta MI, Conti S. Pharmacokinetics and pharmacodynamics of oral cephalosporins as critical factors in choice of antibiotics. Int J Antimicrob Agents. 2000 Dec;16(4):501–5.10.1016/S0924-8579(00)00285-5
- MacGowan AP. Elements of design: the knowledge on which we build. Clin Microbiol Infect. 2004 Apr;10(Suppl 2):6–11.10.1111/j.1470-9465.2004.00863.x
- Levison ME, Levison JH. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect Dis Clin North Am. 2009 Dec;23(4):791–vii.10.1016/j.idc.2009.06.008
- Rinaldi S, Sesto A, Barsotti P, Faraggiana T, Sera F, Rizzoni G. Cyclosporine therapy monitored with abbreviated area under curve in nephrotic syndrome. Pediatr Nephrol (Berlin, Germany). 2005 Jan;20(1):25–9.10.1007/s00467-004-1618-6
- Saravolatz LD, Stein GE, Johnson LB. Ceftaroline: a novel cephalosporin with activity against methicillin-resistant staphylococcus aureus. Clin Infect Dis. 2011 May 1;52(9):1156–63.10.1093/cid/cir147
- Soriano F, Granizo JJ, Coronel P, Gimeno M, Rodenas E, Gracia M, et al. Antimicrobial susceptibility of Haemophilus influenzae, Haemophilus parainfluenzae and Moraxella catarrhalis isolated from adult patients with respiratory tract infections in four southern European countries. The ARISE project. Int J Antimicrob Agents. 2004 Mar;23(3):296–9.
- Wellington K, Curran MP. Cefditoren pivoxil: a review of its use in the treatment of bacterial infections. Drugs. 2004;64(22):2597–618.10.2165/00003495-200464220-00009
- Periti P, Mazzei T, Mini E, Novelli A. Adverse effects of macrolide antibacterials. Drug Saf. 1993 Nov;9(5):346–64.10.2165/00002018-199309050-00004
- Domagala JM. Structure-activity and structure-side-effect relationships for the quinolone antibacterials. J Antimicrob Chemother. 1994 Apr;33(4):685–706.10.1093/jac/33.4.685
- Ball P. Quinolone-induced QT interval prolongation: a not-so-unexpected class effect. J Antimicrob Chemother. 2000 May 1;45(5):557–9.10.1093/jac/45.5.557
- Kim GK. The risk of fluoroquinolone-induced tendinopathy and tendon rupture: what does the clinician need to know? J Clin Aesthet Dermatol. 2010 Apr;3(4):49–54.
- Thompson JW, Jacobs RF. Adverse effects of newer cephalosporins. An update. Drug Saf. 1993 Aug;9(2):132–42.10.2165/00002018-199309020-00005
- Mazzei T, Novelli A. Cefditoren Pivoxil. Una nuova cefalosporina orale per il trattamento delle infezioni respiratorie comunitarie. Farmaci & Terapia Int J Drugs Ther. 2004;XXV:1–20.
- Oosterheert JJ, Bonten MJ, Schneider MM, Buskens E, Lammers JW, Hustinx WM, et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomised trial. BMJ. 2006 Dec 9;333(7580):1193–1197. doi:10.1136/bmj.38993.560984.BE
- Ramirez JA, Srinath L, Ahkee S, Huang A, Raff MJ. Early switch from intravenous to oral cephalosporins in the treatment of hospitalized patients with community-acquired pneumonia. Arch Intern Med. 1995 Jun 26;155(12):1273–6.10.1001/archinte.1995.00430120050006
- Menendez R, Montull B, Mendez R. Antibiotic choice, route and durtion: minimising the harm associated with antibiotics. Eur Respir Monogr. 2014;63:155–67.
- O’Donnell DE, Aaron S, Bourbeau J, Hernandez P, Marciniuk DD, Balter M, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease – 2007 update. Can Respir J. 2007 Sep;14(Suppl B):5B–32B.10.1155/2007/830570
- Miravitlles M, Vogelmeier C, Roche N, Halpin D, Cardoso J, Chuchalin AG, et al. A review of national guidelines for management of COPD in Europe. Eur Respir J. 2016 Feb;47(2):625–37.10.1183/13993003.01170-2015
- Chalmers JD, Aliberti S, Blasi F. Management of bronchiectasis in adults. Eur Respir J. 2015 May;45(5):1446–62.10.1183/09031936.00119114
- Lim WS, Baudouin SV, George RC, Hill AT, Jamieson C, Le Jeune I, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009 Oct;64(Suppl 3):iii1–55.
- GOLD. Global Strategy for the Diagnosis, Management and Prevention of COPD. Full Report. Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2016; Available from: http://goldcopd.org/
- Nozzoli C, Fabbri LM, Gensini G, Consensus Conference. Diagnosi, valutazione di gravità e trattamento della broncopneumopatia cronica ostruttiva e delle malattie croniche concomitanti. Ital J Med. 2012;6 (Suppl): 1–33.
- NICE. Management of chronic obstructive pulmonary disease in adults in primary and secondary care. National Institute for Health and Care Excellence (NICE) clinical guideline 101. 2010; Available from: guidance.nice.org.uk/cg101
- Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004 Jun;23(6):932–46.10.1183/09031936.04.00014304
- Ball P, Baquero F, Cars O, File T, Garau J, Klugman K, et al. Antibiotic therapy of community respiratory tract infections: strategies for optimal outcomes and minimized resistance emergence. J Antimicrob Chemother. 2002 Jan;49(1):31–40.10.1093/jac/49.1.31
- ECDC/EMA. The bacterial challenge: time to react. Technical report. 2009;EMEA/576176/2009.
- Gonzalez-Castillo J, Martin-Sanchez FJ, Llinares P, Menendez R, Mujal A, Navas E, et al. Guidelines for the management of community-acquired pneumonia in the elderly patient. Rev Esp Quimioter. 2014 Mar;27(1):69–86.
- Hsueh PR, Huang WK, Shyr JM, Lau YJ, Liu YC, Luh KT. Multicenter surveillance of antimicrobial resistance of Streptococcus pyogenes, Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis to 14 oral antibiotics. J Formos Med Assoc. 2004 Sep;103(9):664–70.
