Abstract
The therapeutic management of uncomplicated bacterial urinary tract infections (UTIs) is based on short-term courses of oral antibiotics. The preferred drugs are nitrofurantoin trimethoprim-sulfamethoxazole, fosfomycin trometamol, fluoroquinolones and β-lactam agents. The choice of agent for treating uncomplicated UTIs should be based on the pharmacokinetic characteristics of the molecule so that clinical benefit is optimized and the risk of antibacterial resistance is minimized. This article discusses the general pharmacokinetic-pharmacodynamic (PK/PD) aspects of antimicrobial chemotherapy, the PK/PD characteristics of oral antimicrobial agents for the treatment of uncomplicated UTIs and the pharmacological and therapeutic strategies for limiting or preventing bacterial resistance.
Introduction
The therapeutic management of uncomplicated bacterial urinary tract infections (UTIs) is based on antibiotic therapy. Major international and national guidelines recommend the short-term use of oral antibiotics for uncomplicated UTIs; these are summarised in Table .Citation1–3 The preferred drugs are nitrofurantoin, trimethoprim-sulfamethoxazole, fosfomycin trometamol, fluoroquinolones and β-lactam agents.
Table 1 National and international guideline recommendation for the treatment of uncomplicated urinary tract infections
The choice of the most suitable agent to treat uncomplicated UTIs should be based not only on the spectrum of antimicrobial activity, but also on the pharmacokinetic (PK) characteristics of the molecule so that optimal clinical benefit can be achieved while avoiding antibacterial resistance. Other aspects to consider include patient characteristics (allergy, comorbidities, concomitant therapy and compliance), local practice patterns, local community resistance prevalence, product availability and costs.Citation4 Table shows the characteristics of an ideal drug for treatment of uncomplicated UTIs.Citation4
Table 2 Characteristics of an ideal drug for treatment of uncomplicated urinary tract infectionsCitation4
General pharmacokinetic-pharmacodynamic (PK/PD) aspects of antimicrobial chemotherapy
There has been much critical evaluation of the rules for selecting antibiotics and their ideal dosage regimen for the control of infections, with the goals of increasing treatment efficacy and reducing the risk of selecting multi-resistant pathogens.Citation5–9 PK and pharmacodynamic (PD) properties are fundamental to the choice of drug dose and regimen. PK parameters relate to drug absorption, tissue distribution, metabolism and elimination, while the antimicrobial activity is covered under PD parameters, the most important of which are the minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), post-antibiotic effect (PAE) and killing rate. On the basis of their different patterns of bactericidal activity (killing curves), antibiotics can be divided into two groups, having either time-dependent or concentration-dependent antimicrobial activity. Beta-lactams, nitrofurantoin, erythromycin, tigecycline and oxazolidinones are time-dependent drugs, whereas the aminoglycosides, quinolones, fosfomycin and semisynthetic macrolides tend to have concentration-dependent activity.Citation5–9
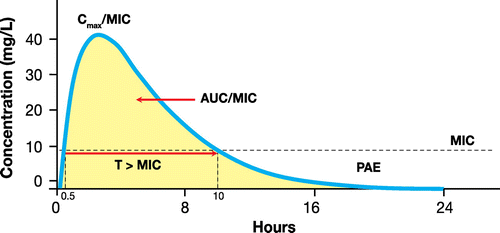
Some important PK-PD parameters have shown good correlation between in vitro and in vivo animal infection models and therapeutic efficacy of antibiotics.Citation9–11 The first of these is time between doses with antibiotic concentration exceeding the MIC (T > MIC), the second is the ratio between peak concentration (Cmax) and MIC (Cmax/MIC) and the third is the ratio between the area under the plasma concentration curve (AUC) and the MIC (AUC/MIC) (Fig. ).
The T > MIC parameter is recognized as being a predictor of clinical and microbiological success or failure for time-dependent antibiotics. Cmax/MIC and AUC/MIC are the PK-PD parameters that predict efficacy for concentration-dependent drugs, although there are some important clinical data demonstrating that the AUC/MIC ratio might also be useful for assessing the effectiveness of certain time-dependent antimicrobial agents.
PK-PD properties are useful for optimizing drug dosages and outcomes and for limiting or preventing bacterial resistance. However, it could be argued that PK-PD parameters are less useful for determining treatment for uncomplicated UTIs because most of the potential antibiotic agent choices are able to reach and maintain relatively high urinary concentrations, much higher than the MICs of the causative pathogens, thus theoretically reducing the need for a critical PK-PD analysis to ensure treatment success. Nevertheless, in our opinion, theoretical urinary PK-PD thresholds should be considered even in uncomplicated UTIs because the patient’s condition (i.e. urinary pH, different risk factors such as pre-existing diseases, reduced oral absorption and faster elimination) and bacterial strain characteristics (lower susceptibility, virulence factors etc.) may play an important role. Finally, because dose–effect relationships have now been quite well established for the majority of antibiotic classes, PK-PD data should be used to reappraise current clinical break points, giving the clinician more information on resistance and, therefore, on treatment choices.Citation9
PK/PD characteristics of oral antimicrobials in uncomplicated UTIs
Quinolones/fluoroquinolones
There are three generations of quinolone antibiotics. The first is represented by nalidixic acid and derivatives. In terms of second-generation quinolones, those still used for UTIs are broad-spectrum agents such as ciprofloxacin, ofloxacin, lomefloxacin, norfloxacin and prulifloxacin. Third-generation quinolones include newer agents that are especially active against Gram-positive bacterial species and anaerobes and are therefore not clearly indicated for UTIs,Citation10 with the exception of levofloxacin. Differences between the different generations are due to modifications in the structural formula, resulting in different activity.Citation11 The mechanism of action of these antibiotics is inhibition of DNA gyrase and partly of bacterial topoisomerase IV, resulting in cell death due to complex binary or tertiary formations among DNA, the enzyme and the antibiotic.Citation10–12 Quinolones have bactericidal activity, and the mechanisms of bacterial resistance are primarily linked to modifications at the binding site or active drug extrusion from the bacteria through efflux pumps.Citation10,11
Second- and third-generation molecules have favourable kinetic characteristics with high oral bioavailability (approximately 100% for levofloxacin), good tissue penetration and primarily renal elimination (Table ).Citation10,12–19 Among the fluoroquinolones indicated for the treatment of uncomplicated UTIs, both levofloxacin (85%) and ciprofloxacin (30–50%) are predominantly excreted via the renal route, reaching very high concentrations in urine (521–771 and 200 mg/L, respectively). The long half-life of some compounds, namely levofloxacin and prulifloxacin, allows for once-daily administration, which makes compliance with treatment easier. The combination of these PK characteristics together and bactericidal activity mean that second- and third-generation quinolones are indicated for the short-term treatment (3 days) of uncomplicated UTIs.
Table 3 Pharmacokinetic parameters of quinolones indicated for the treatment of uncomplicated UTIsCitation10,12–19
The post-antibiotic effect of fluoroquinolones contributes to their therapeutic efficacy.Citation20,21 Acidified urine (pH = 5–6) was found to impair the antimicrobial effects of fluoroquinolones in some in vitro studies. However, the impact of urine acidification on the prophylaxis and treatment of UTIs is controversial.Citation22–24
As mentioned, fluoroquinolones are concentration-dependent antibiotics, and a peak/MIC ratio of 10–12 and an AUC/MIC ratio of about 100–125 (at least against Gram-negative bacteria) must be obtained to avoid the increase of resistance and to guarantee potential efficacy. These values have been documented not only in animal models but also in serious infections in hospitalized patients.Citation25,26
Fluoroquinolones are generally well tolerated. Although the level of evidence for adverse events varies, these include gastrointestinal problems (e.g. nausea and vomiting), neurological disturbances (due to GABA inhibition) and cardiovascular effects (e.g. prolonged QT interval).Citation27 The risk of fluoroquinolone-induced tendinopathy and tendon rupture is well established and is higher in men than in women (2:1 ratio). The Achilles tendon is most commonly affected, and risk factors include age (>60 years), corticosteroid therapy, renal failure, diabetes mellitus and history of tendon rupture.Citation28
Administration of fluoroquinolones in combination with, or within a few hours of, antacids containing zinc (Zn++), aluminium (Al+++) or magnesium (Mg++) significantly reduces oral drug absorption and constitutes an important pharmacological interaction.Citation27 In patients with renal failure (creatinine clearance <50 mL/min), the dose or frequency of fluoroquinolones should be reduced.
Although fluoroquinolones are generally very active, their microbiological, pharmacological and toxicological properties mean that they should be used only for the most serious conditions and in special populations. Moreover, due to the fact that antimicrobial resistance in common urinary pathogens is increasing globally at an alarming rate due to the overuse and misuse of antibiotics, fluoroquinolones should no longer be used as first-line empiric therapy for uncomplicated UTIs.Citation29
Co-trimoxazole
Co-trimoxazole is a synergistic combination of trimethoprim and sulfamethoxazole (TMP/SMX) that has been used as first-line therapy for UTIs for over 30 years. After oral administration of SMX 800 mg and TMP 160 mg, the mean peak serum concentration is approximately 40/60 mg/L for SMX and 1–2 mg/L for TMP, with an elimination half-life of 10–12 h; in the first 24 h post-dose, 60–70% of SMX has been eliminated (20% as parent drug) and 50–60% of TMP has been eliminated (80% as parent drug), with peak urinary concentrations of 190 and 75 mg/L, respectively.Citation18,30,31
Table shows PK parameters after a single oral 800/160 mg dose of TMP/SMX. The main statistically significant difference in kinetic parameters between the two components is the volume of distribution, which is higher for TMP. However, the widespread use of TMP/SMX has resulted in a progressive emergence of resistance, and resistant strain rates of over 20% have been reported in several European countries and in the USA, as well as in developing countries, limiting the clinical usefulness of this therapy in the modern management of UTIs, as recognized in clinical guidelines. Several studies have shown that clinical outcomes in patients with UTIs due to TMP-SMX-resistant pathogens are worse than those in patients with UTIs due to susceptible isolates.Citation32
Table 4 Pharmacokinetic parameters after a single oral 800/160 mg dose of co-trimoxazoleCitation30,31
Common side effects associated with TMP/SMX, primarily occurring in elderly patients, are blood dyscrasias, drug fever, hyperkalemia and rash. In a nested case-control study, TMP/SMX-induced hyperkalemia was investigated in elderly patients receiving spironolactone. Compared with amoxicillin, TMP-SMX was associated with a more than 12-fold increase in the risk of hospital admission for hyperkalemia. It has been suggested that approximately 60% of all cases of hyperkalemia could be avoided if TMP/SMX was not prescribed.Citation33 It has also been suggested that acute kidney injury might be associated with TMP/SMX.Citation34 In patients with renal failure (creatinine clearance <80 mL/min), dosing frequency should be reduced (to every 18 h for those with creatinine clearance of 50–80 mg/mL and to every 24 h in those with creatinine clearance of 10–50 mg/mL); the administration of TMP/SMX should be avoided in patients with a creatinine clearance <10 mL/min. As a result of increasing resistance and these tolerability issues, TMP/SMX use is decreasing.Citation35
Beta-lactams
The beta-lactams, characterized by a mechanism of action targeting the bacterial cell wall, are generally active against both Gram-positive and Gram-negative bacteria. These antimicrobial agents have a time-dependent killing activity at therapeutically achievable concentrations. They have minimal to moderate persistent antibiotic effects, and their efficacy is optimized by maximising the duration of exposure. Therefore, time above a threshold amount of drug (e.g. MIC) is the dominant PD index.Citation36 Resistance in Enterobacteria is mainly due to inactivation by hydrolytic enzyme production (beta-lactamases). Therefore, there is a tendency to use aminopenicillins in combination with suicide inhibitors (e.g. amoxicillin-clavulanic acid) or oral cephalosporins, mainly third-generation agents, because of their higher potency against Gram-negative rods, even if they are not active against Enterococcus sp.
Table shows the main PK parameters of oral beta-lactams.Citation37–44 Many cephalosporins do not have high oral bioavailability and require administration as prodrugs (different esters). Beta-lactams generally have a relatively short elimination half-life, which means they should not be administered once-daily for the treatment of uncomplicated UTIs. Possible exceptions are cefixime and ceftibuten, although a twice-daily schedule would guarantee the maintenance of adequate antimicrobial levels for a longer time. Urinary recovery of oral beta-lactams ranges from 20 to 75%, amoxicillin/clavulanic acid and ceftibuten have the highest rates (75 and 70%, respectively). After an oral dose of ceftibuten 200 mg, the maximum urinary concentration is about 800 mg/L, while peak levels after a single oral dose of cefuroxime axetil 500 mg and amoxicillin/clavulanic acid 875 mg are about 400 mg/L and 200 mg/L, respectively. However, urinary levels fall rapidly within 2 to 4 h post-dose.Citation37–39
Table 5 Pharmacokinetic parameters of oral beta-lactamsCitation37–44
Pivmecillinam, a prodrug of mecillinam, has been widely used for the treatment of acute lower UTI, mainly in Northern Europe. Mecillinam is an amidine derivative of the penicillin group. This molecule has high activity against Gram-negative organisms such as Escherichia coli, with a low level of resistance (<2%). Pivmecillinam is well absorbed after oral administration, with a bioavailability of 60–75%, although the moderate reduction in Gram-negative Enterobacteriaceae in the intestinal flora may occur.Citation45 About half (45%) of the drug is excreted in the urine, and mean peak urinary concentrations might be as high as 300 mg/L, usually within the first 3-h period; these fell to almost 50 mg/L at 6 h after administration and were ≤5 mg/L after 12 h.Citation44,46
Beta-lactams are generally well tolerated by both paediatric and adult patients, and they can be used during pregnancy (with the partial exclusion of clavulanic acid). However, the rising prevalence of antimicrobial resistance in uropathogens, even in strains causing community-acquired uncomplicated infections, mainly for extended spectrum beta-lactamase (ESBL) production, and the increasing prevalence of invasive pan-resistant E. coli strains (resistant to aminoglycosides, fluoroquinolones and third-generation cephalosporins) suggest that a conservative approach to the use of these agents in community infections is warranted.Citation47,48
Nitrofurantoin
Nitrofurantoin, a synthetic nitrofuran derivative, has been available for the treatment of UTIs since 1952. It still has relatively high activity against E. coli and E. faecalis uropathogens. However, nitrofurantoin is less effective against Gram-negative pathogens other than E. coli, such as Klebsiella spp. (69.2%) or Enterobacter spp. (63%) and is almost inactive against Proteus mirabilis.Citation49 The nitro group coupled in the heterocyclic furan ring represents the specific active site of the drug and once activated by microbial nitro-reductases is able to interfere with protein and DNA synthesis, thus impairing energy metabolism, cell wall and carbohydrate synthesis.Citation50
From a PK point of view, the bioavailability of nitrofurantoin is about 90% but plasma concentrations are very low (<1 mg/L after an oral dose of 100 mg). The elimination half-life is short (around 1 h), and 27–50% of the drug is excreted unchanged in the urine. Even though peak urinary levels might be >100 mg/L (range 50–200 mg/L), these are maintained for a relatively short time.Citation18,51 Adkinson et al. confirmed the PK profile of nitrofurantoin in 3 groups of healthy subjects with different expression of the ATP-binding cassette transporter subfamily ABCG2 421 genotype, showing that the ABCG2 C421A polymorphism had no effect on nitrofurantoin plasma and urine pharmacokinetic parameters (Table ).Citation52
Table 6 Pharmacokinetic parameters of nitrofurantoin in healthy subjectsTable Footnote*,Citation52
Nitrofurantoin has a bactericidal effect against susceptible organisms. However, this antibiotic primarily has time-dependent activity, with T > MIC being the PK-PD parameter that best correlates with efficacy; maintenance of plasma concentrations higher than the MIC for a relatively long-time post-dose is required. Therefore, there might be a risk of treatment failure for pathogens with a higher MIC value, while the sensitivity threshold of 64 mg/L for urinary pathogens still seems too high.Citation53 Nitrofurantoin has been associated with neurotoxicity (including peripheral neuropathy, dizziness, vertigo, diplopia, and cerebellar dysfunction) and benign intracranial hypertension. These side effects are most prevalent in women and elderly patients, and their pathogenesis is hypothesized to be due to axon loss.Citation54 Other possible side effects, generally after long-term therapy, include sub-acute and chronic pulmonary reactions and chronic hepatitis. Over recent years, new insights into the immune capability of nitrofurantoin have emerged. Although nitrofurantoin rarely causes autoimmune hepatitis, prescribers should be aware of this adverse event and caution is warranted in the geriatric population because this agent is increasingly being used both for long-term prophylaxis and the treatment of acute and chronic UTIs.Citation55,56 Finally, nitrofurantoin should be avoided in patients with moderate-severe renal failure (creatinine clearance <50 mL/min).
Fosfomycin trometamol
Fosfomycin is a phosphonic acid derivative, isolated for the first time in Spain in 1969 from a Streptomyces fradiae strain, and has a broad spectrum of antibacterial activity against both Gram-positive and Gram -negative bacteria, including ESBL-producer strains. This antibiotic has good in vitro activity against more common multidrug-resistant uropathogens. In addition, in a recent microbiological study performed in Italy, fosfomycin inhibited all ESBL-positive E. coli, P. mirabilis and methicillin-resistant S. saprophyticus strains isolated from urine, as well as 82% of KPC-producing K. pneumoniae isolates.Citation57
The bactericidal activity of fosfomycin is a result of the epoxy ring, which inhibits a cytoplasmic enzyme (phosphoenolpyruvate synthetase) active during the first step of bacterial cell wall (peptidoglycan) synthesis.Citation58,59 Fosfomycin acts as a phosphoenolpyruvate analogue, irreversibly inhibiting enolpyruvil transferase (UDP-N-acetylglucosamine-3-O-enolpyruvil transferase), a cytoplasmic enzyme which catalyzes the first step of the biosynthesis of the heteropolymer peptidoglycan through the incorporation of phosphoenolpyruvate to uridine-diphosphate-N-acetyl-glucosamine forming uridine-diphosphate-N-acetylmuramic acid.Citation60 It has a relatively broad spectrum of activity and, notwithstanding the possible mechanisms of bacterial resistance, has maintained good activity against the main bacterial species responsible for UTIs.Citation60–63 Many different factors may play a role in this preserved activity. These include the use of a single dose for the treatment of uncomplicated UTIs and two consecutive doses for prophylaxis of urological procedures, the rapid concentration-dependent killing activity with reduced effect on selecting resistant mutants (and when present, these strains have reduced fitness due to the high biological cost of the genetic modification) and the limited use in veterinary medicine.Citation64 Fosfomycin has concentration-dependent activity against both Gram-positive and Gram-negative strains and has a long post-antibiotic effect (PAE) in vitro, even at sub-inhibitory concentrations and over periods of 3.2–4.7 h.Citation65–67
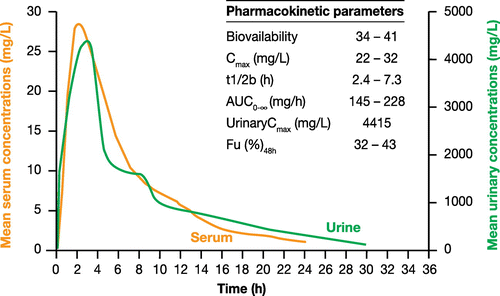
Fosfomycin trometamol is a monobasic anionic salt, with a molecular weight of 259.2 g/mol, metabolized from fosfomycin after absorption. Fosfomycin trometamol is more soluble and stable in the gastric acidic environment. After oral administration, it is rapidly absorbed and converted to the free acid, fosfomycin, with a good bioavailability (37–44%).Citation59 Co-administration of fosfomycin trometamol with food may reduce drug absorption (37% fasting versus 30% with food). After administration of a single 3 g oral dose of fosfomycin trometamol, peak plasma concentrations (Cmax) is 25–32 mg/L, occurring within 2–2.5 h (tmax), and trough concentrations at 24 h are about 3 mg/L. The corresponding AUC is approximately 145–228 mg∙h/L. There is a linear relationship between the main PK parameters, such as Cmax and AUC0-∞, and fosfomycin trometamol dosage.Citation58,65,67 The elimination half-life is 5–7 h, and the volume of distribution (after IV dosing) is 0.3–0.4 L/kg, with good distribution into the extravascular compartment (Table ).Citation58,60 Fosfomycin is widely distributed into tissues, achieving clinically relevant concentrations in serum, kidneys, bladder wall, prostate, lungs, inflamed tissues, bone, cerebrospinal fluid, abscess fluid and heart valves.Citation62 For example, at 3 and 12 h after an oral 3 g dose, prostatic fosfomycin concentrations are 25 and 7 mg/L, respectively.Citation68,69
Table 7 Pharmacokinetic parameters of fosfomycin trometamolCitation57,59
Fosfomycin is excreted unchanged in the urine via glomerular filtration. Urinary recovery is almost 40%, and high urinary concentrations (1000–5000 mg/L) are achieved and maintained for long periods after oral administration, with a mean urinary level of 1000 mg/L at 12 h and 500 mg/L at 24 h. Urinary concentrations remain >100 mg/L for at least 30–40 h post-dose. This means that drug levels capable of eliminating the majority of common uropathogens are maintained, providing good clinical efficacy in the treatment of uncomplicated UTIs even after a single dose (Fig. ).Citation58,60,65 Based on these data, both the urinary peak/MIC and AUC/MIC ratios are very high given that uropathogens have MIC values within the sensitivity threshold of 32 mg/L. In patients with chronic severe renal failure, the half-life of fosfomycin is increased significantly (up to 50 h), and fosfomycin recovery in urine is lower.Citation60 However, it is not necessary to reduce the dose if creatinine clearance is >10 mL/min.
Figure 2 Mean serum and urinary drug concentrations after a single oral 3 g dose of fosfomycin trometamol.Citation57
Fosfomycin trometamol is generally well tolerated, with a very low incidence of side effects. The most commonly reported events are gastrointestinal (e.g. diarrhoea and nausea), although these are usually mild in severity and occur at a low incidence (2–3%), lower than that observed with beta-lactamsCitation70. A single dose of fosfomycin trometamol has been used successfully to treat lower UTIs in pregnant women.Citation70,71
Overall, fosfomycin trometamol is an appealing oral outpatient alternative, even in older women and for resistant isolates.Citation48
Conclusions
An adequate therapeutic approach to bacterial infection must take into account drug safety, including the presence and incidence of side effects and the possibility of antimicrobial resistance (i.e. the selection of resistant organisms and colonization or infection with multidrug-resistant pathogens). Some pharmacological and therapeutic strategies for limiting or preventing bacterial resistance can be adopted in clinical practice. The treatment of uncomplicated UTIs is mostly empiric, based on the common aetiology of these infections (where E. coli represents 75–90% of causative pathogens), local epidemiology of resistance and local antimicrobial susceptibility patterns. It is well known that there is a considerable geographic variability in the susceptibility of uropathogens and therefore each physician should know their own local epidemiological pattern of resistance in uropathogens in order to avoid empiric treatment of uncomplicated UTIs with an ineffective antibiotic. Treatment guidelines recommend that a class of antibiotics should not be used when local resistance in E. coli exceeds 20% of strains. For example, in most countries in Southern Europe, the resistance of E. coli strains to quinolones and co-trimoxazole is >20%, automatically excluding these drugs from empiric use to treat uncomplicated UTIs. Given the trend for increasing resistance to most antimicrobials, ongoing monitoring is required to continually optimize empiric UTI therapy. One strategy for preventing or limiting bacterial resistance is to use antibiotics with adequate urinary kinetics (i.e. achievement of high urinary drug concentrations exceeding the MIC of uropathogens for a sustained period) at optimal dosage and for an appropriate duration. Based on this pharmacological and therapeutic strategy, fosfomycin trometamol appears to be a useful agent given its wide spectrum of activity (including ESBL-positive strains), a favourable PK profile (high urinary concentrations maintained over 3 days post-dose), proven clinical efficacy and safety in the management of uncomplicated UTIs and good compliance with the single-dose treatment regimen.
Conflict of interest
The authors state that they have no conflict of interest to declare.
Funding
No external funding was obtained for this article; all authors take full responsibility for the content.
Notes on contributors
Andrea Novelli MD is Professor of Pharmacology, Department of Health Sciences, Clinical Pharmacology and Oncology Section, University of Florence, President of Firenze University Press (FUP) 2016–2020, Former President of the Federation of European Societies of Chemotherapy and Infection (FESCI) 2013–2017, the former President of the Italian Society of Chemotherapy (SIC) 2013–2017, General Secretary of the Mediterranean Society of Chemotherapy and Infection (MEDSOCHEMI) 2015–2019 and Vice President of the Council of the Italian Federation of Human and Animal Mycology (FIMUA) 2015–2017. Professor Novelli is the author of more than 300 original papers published in international and national journals.
Elia Rosi, MD, is a visiting scientist at the Department of Health Sciences, Clinical Pharmacology and Oncology Section, University of Florence. He is member of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). His main interests are in the field of pharmacokinetic and pharmacodynamic properties of antimicrobial drugs. He works in a research group dealing with antimicrobial resistance, both in clinical and preclinical studies.
References
- Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonehritis in women: a 2010 update by the IDSA and European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103–20.10.1093/cid/ciq257
- Bonkat G, Pickard R, Bartoletti R, Bruyère F, Geerlings SE, Wagenlehner F, et al. Guidelines on urological infections. European Association of Urology; 2017.
- Battaglia M, Cai T, Concia E, De Nunzio C, Mazzei T, Pea F, et al. Raccomandazioni in tema di diagnosi, trattamento e profilassi delle infezioni delle vie urinarie, versione 1 anno 2015 SIU-UTI. Available from: https://www.siu.it/files/uploads/Linee_Guida_SIU_UTI_2015.pdf
- Neu HC. Optimal characteristics of agents to treat uncomplicated urinary tract infections. Infection. 1992;20(S4):S266–71.10.1007/BF01710012
- Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26(1):1–10.10.1086/cid.1998.26.issue-1
- Rodvold KA. Pharmacodynamics of antiinfective therapy: taking what we know to the patient’s bedside. Pharmacotherapy. 2001;21(11s):319s–30s.10.1592/phco.21.18.319S.33904
- McKinnon PS, Davis SL. Pharmacokinetic and pharmacodynamic issues in the treatment of bacterial infectious disease. Eur J Clin Microbiol Infect Dis. 2004;23(4):271–88.10.1007/s10096-004-1107-7
- Andes D, Craig WA. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int J Antimicrob Agents. 2002;19(4):261–8.10.1016/S0924-8579(02)00022-5
- Nicolau DP. Optimizing outcomes with antimicrobial therapy through pharmacodynamic profiling. J Infect Chemother. 2003;9(4):292–6.10.1007/s10156-003-0279-X
- Appelbaum PC, Hunter PA. The fluoroquinolone antibacterials: past, present and future perspectives. Int J Antimicrob Agents. 2000;16(1):5–15.10.1016/S0924-8579(00)00192-8
- Peterson LR. Quinolone molecular structure‐activity relationships: what we have learned about improving antimicrobial activity. Clin Infect Dis. 2001;33(Suppl 3):S180–6.10.1086/cid.2001.33.issue-s3
- von Rosenstiel N, Adam D. Quinolone antibacterials: an update of their pharmacology and therapeutic use. Drugs. 1994;47(6):872–901.10.2165/00003495-199447060-00003
- Novelli A, Cassetta MI, Fallani S, Periti P. Clinical pharmacokinetics and tissue penetration of ciprofloxacin after a single or multiple oral dose of 250 or 500 mg. Eur J Clin Microbiol Infect Dis. 1991;20:367–9.
- Schaeffer AJ. The expanding role of fluoroquinolones. Am J Med. 2002;113(1A):S45–54.10.1016/S0002-9343(02)01059-8
- Mazzei T, Novelli A, Reali EF, Balocchini E, Boni S, De Stasio A, et al. Farmacocinetica e penetrazione tissutale della oflossacina. Farmaci & Terapia. 1987;4(4):244–9.
- Novelli A. Antimicrobial prophylaxis in surgery: the role of pharmacokinetics. J Chemother. 1999 Dec;11(6):565–72.10.1179/joc.1999.11.6.565
- Pickerill KE, Paladino JA, Schentag JJ. Comparison of the fluoroquinolones based on pharmacokinetic and pharmacodynamic parameters. Pharmacotherapy. 2000;20(4):417–28.10.1592/phco.20.5.417.35062
- Gupta K, Hooton TM, Stamm WE. Increasing antimicrobial resistance and the management of uncomplicated community-acquired urinary tract infections. Ann Intern Med. 2001;135(1):41–50.10.7326/0003-4819-135-1-200107030-00012
- Picollo R, Brion N, Gualano V, Millérioux L, Marchetti M, Rosignoli MT, et al. Pharmacokinetics and tolerability of prulifloxacin after single oral administration. Arzneimittel-Forschung. 2003;53(3):201–5.
- Bolon MK. The newer fluoroquinolones. Infect Dis Clin North Am. 2009;23(4):1027–51.10.1016/j.idc.2009.06.003
- Frimodt-Møller N. Correlation between pharmacokinetic/pharmacodynamic parameters and efficacy for antibiotics in the treatment of urinary tract infection. Int J Antimicrob Agents. 2002;19(6):546–53.10.1016/S0924-8579(02)00105-X
- Erdogan-Yildirim Z, Burian A, Manafi M, Zeitlinger M. Impact of pH on bacterial growth and activity of recent fluoroquinolones in pooled urine. Res Microbiol. 2011;162(3):249–52.10.1016/j.resmic.2011.01.004
- L Yang, K Wang, H Li, JD Denstedt, PA Cadieux. The influence of urinary pH on antibiotic efficacy against bacterial uropathogens. Urology. 2014;84(3): 731 e1-7.
- Zhanel GG, Karlowsky JA, Davidson RJ, Hoban DJ. Influence of human urine on the in vitro activity and postantibiotic effect of ciprofloxacin against Escherichia coli. Chemotherapy. 1991;37(3):218–23.10.1159/000238857
- Preston SL, Drusano GL, Berman AL, Fowler CL, Chow AT, Dornseif B, et al. Pharmacodynamics of levofloxacin: a new paradigm for early clinical trials. JAMA. 1998;279(2):125–9.10.1001/jama.279.2.125
- Schentag JJ. Clinical pharmacology of the fluoroquinolones: studies in human dynamic/kinetic models. Clin Infect Dis. 2000;31(Suppl 2):S40–4.10.1086/314059
- Ball P. Future of the quinolones. Semin Respir Infect. 2001;16(3):215–24.10.1053/srin.2001.25628
- Kim GK. The risk of fluoroquinolone-induced tendinopathy and tendon rupture: what does the clinician need to know? J Clin Aesthet Dermatol. 2010;3(4):49–54.
- Shepherd AK, Pottinger PS. Management of urinary tract infections in the era of increasing antimicrobial resistance. Med Clin North Am. 2013;97(4):737–57.10.1016/j.mcna.2013.03.006
- Cockerill FR, Edson RS. Trimethoprim-sulfamethoxazole. Mayo Clin Proc. 1991;66(12):1260–9.10.1016/S0025-6196(12)62478-1
- Hutabarat RM, Unadkat JD, Sahajwalla C, McNamara S, Ramsey B, Smith AL. Disposition of drugs in cystic fibrosis. I. Sulfamethoxazole and trimethoprim. Clin Pharmacol Ther. 1991;49(4):402–9.10.1038/clpt.1991.47
- Raz R, Chazan B, Kennes Y, Colodner R, Rottensterich E, Dan M, et al. Empiric use of trimethoprim‐sulfamethoxazole (TMP‐SMX) in the treatment of women with uncomplicated urinary tract infections, in a geographical area with a high prevalence of TMP‐SMX–resistant uropathogens. Clin Infect Dis. 2002;34(9):1165–9.10.1086/cid.2002.34.issue-9
- Antoniou T, Gomes T, Mamdani MM, Yao Z, Hellings C, Garg AX, et al. Trimethoprim-sulfamethoxazole induced hyperkalaemia in elderly patients receiving spironolactone: nested case-control study. BMJ. 2011;343:d5228.10.1136/bmj.d5228
- Fraser TN, Avellaneda AA, Graviss EA, Musher DM. Acute kidney injury associated with trimethoprim/sulfamethoxazole. J Antimicrob Chemother. 2012;67(5):1271–7.10.1093/jac/dks030
- Nickel JC. Management of urinary tract infections: historical perspective and current strategies: part 2 – modern management. J Urol. 2005;173(1):27–32.10.1097/01.ju.0000141497.46841.7a
- MacGowan A. Revisiting Beta-lactams – PK/PD improves dosing of old antibiotics. Curr Opin Pharmacol. 2011;11(5):470–6.10.1016/j.coph.2011.07.006
- Klepser ME, Marangos MN, Patel KB, Nicolau DP, Quintiliani R, Nightingale CH. Clinical pharmacokinetics of newer cephalosporins. Clin Pharmacokinet. 1995;28(5):361–84.10.2165/00003088-199528050-00003
- Lode H, Fassbender M, Schaberg T, Borner K, Koeppe P. Comparative pharmacokinetics of the new oral cephalosporins. Drugs. 1994;47(Suppl 3):10–20.10.2165/00003495-199400473-00004
- Perry CM, Brogden RN. Cefuroxime axetil: a review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1996;52(1):125–58.10.2165/00003495-199652010-00009
- Periti P, Novelli A, Mini E, Mazzei T, Cassetta MI. Clinical pharmacokinetics of cefpodoxime proxetil in older patients. In: Dieter Adam, Hartmut Lode and Ethan Rubinstein. Recent Advances in Chemotherapy, Antimicrobial Section I. Proceedings of the 17th International Congress of Chemotherapy, Berlin, 1991. Monaco, Germania: Futuramed; 1992. p. 326–7.
- Heikkilä A, Pyykkö K, Erkkola R, Lisalo E. The pharmacokinetics of mecillinam and pivmecillinam in pregnant and non-pregnant women. Br J Clin Pharmacol. 1992;33(6):629–33.10.1111/bcp.1992.33.issue-6
- Roholt K, Nielsen B, Kristensen E. Pharmacokinetic studies with mecillinam and pivmecillinam. Chemotherapy. 1975;21(3–4):146–66.10.1159/000221856
- Bornemann LD, Castellano S, Lin AH, Enthoven D, Patel IH. Influence of food on bioavailability of amdinocillin pivoxil. Antimicrob Agents Chemother. 1988;32(4):592–4.10.1128/AAC.32.4.592
- Graninger W. Pivmecillinam–therapy of choice for lower urinary tract infection. Int J Antimicrob Agents. 2003;22(Suppl 2):73–8.10.1016/S0924-8579(03)00235-8
- Sullivan A, Edlund C, Svenungsson B, Emtestam L, Nord CE. Effect of perorally administered pivmecillinam on the normal oropharyngeal, intestinal and skin microflora. J Chemother. 2001;13(3):299–308.10.1179/joc.2001.13.3.299
- Kerrn MB, Frimodt-Møller N, Espersen F. Urinary concentrations and urine ex-vivo effect of mecillinam and sulphamethizole. Clin Microbiol Infect. 2004;10(1):54–61.10.1111/j.1469-0691.2004.00737.x
- Ricciardi W, Giubbini G, Laurenti P. Surveillance and control of antibiotic resistance in the mediterranean region. Mediterr J Hematol Infect Dis. 2016;8(1):e2016036.10.4084/mjhid.2016.036
- Mody L, Juthani-Mehta M. Urinary tract infections in older women: a clinical review. JAMA. 2014;311(8):844–54.10.1001/jama.2014.303
- Mazzulli T, Skulnick M, Small G, Marshall W, Hoban DJ, Zhanel GG, et al. Susceptibility of community gram-negative urinary tract isolates to mecillinam and other oral agents. Can J Infect Dis. 2001;12(5):289–92.10.1155/2001/601743
- Hof H. Antimicrobial therapy with nitroheterocyclic compounds, for example, metronidazole and nitrofurantoin. Immun Infekt. 1988;16(6):220–5.
- Ben-Noun L. Drug-induced respiratory disorders: incidence, prevention and management. Drug Saf. 2000;23(2):143–64.10.2165/00002018-200023020-00005
- Adkison KK, Vaidya SS, Lee DY, Koo SH, Li L, Mehta AA, et al. The ABCG2 C421A polymorphism does not affect oral nitrofurantoin pharmacokinetics in healthy Chinese male subjects. Br J Clin Pharmacol. 2008;66(2):233–9.10.1111/bcp.2008.66.issue-2
- Komp Lindgren P, Klockars O, Malmberg C, Cars O. Pharmacodynamic studies of nitrofurantoin against common uropathogens. J Antimicrob Chemother. 2015;70(4):1076–82.
- Mattappalil A, Mergenhagen KA. Neurotoxicity with antimicrobials in the elderly: a Review. Clin Ther. 2014;36(11):1489–511.10.1016/j.clinthera.2014.09.020
- Appleyard S, Saraswati R, Gorard DA. Autoimmune hepatitis triggered by nitrofurantoin: a case series. J Med Case Rep. 2010;4:311.10.1186/1752-1947-4-311
- Smyk DS, Bogdanos DP, Kriese S, Billinis C, Burroughs AK, Rigopoulou EI, et al. Urinary tract infection as a risk factor for autoimmune liver disease: from bench to bedside. Clin Res Hepatol Gastroenterol. 2012;36(2):110–21.10.1016/j.clinre.2011.07.013
- Mezzatesta ML, La Rosa G, Maugeri G, Zingali T, Caio C, Novelli A, et al. In vitro activity of fosfomycin trometamol and other oral antibiotics against multidrug-resistant uropathogens. Int J Antimicrob Agents. 2017;49(6):763–6.10.1016/j.ijantimicag.2017.01.020
- Patel SS, Balfour JA, Bryson HM. Fosfomycin tromethamine. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy as a single-dose oral treatment for acute uncomplicated lower urinary tract infections. Drugs. 1997;53(4):637–56.10.2165/00003495-199753040-00007
- Gobernado M. Fosfomycin. Rev Esp Quimioter. 2003;16(1):15–40.
- Michalopoulos AS, Livaditis IG, Gougoutas V. The revival of fosfomycin. Int J Infect Dis. 2011;15(11):e732–9.10.1016/j.ijid.2011.07.007
- Fuchs PC, Barry AL, Brown SD. Fosfomycin tromethamine susceptibility of outpatient urine isolates of Escherichia coli and Enterococcus faecalis from ten North American medical centres by three methods. J Antimicrob Chemother. 1999;43(1):137–40.10.1093/jac/43.1.137
- Garcia-Rodriguez JA, Trujillano Martin I, Baquero F, Cisterna R, Gobernado M, Linares F, et al. vitro activity of fosfomycin trometamol against pathogens from urinary tract infections: a Spanish multicenter study. J Chemother. 1997;9(6):394–402.10.1179/joc.1997.9.6.394
- Liao RZ, Thiel W. Determinants of regioselectivity and chemoselectivity in fosfomycin resistance protein FosA from QM/MM calculations. J Phys Chem B. 2013;117(5):1326–36.10.1021/jp4002719
- Zhanel GG, Walkty AJ, Karlowsky JA. Fosfomycin: a first-line oral therapy for acute uncomplicated cystitis. Can J Infect Dis Med Microbiol. 2016 May 10 [ epub ahead of print].
- Mazzei T, Cassetta MI, Fallani S, Arrigucci S, Novelli A. Pharmacokinetic and pharmacodynamic aspects of antimicrobial agents for the treatment of uncomplicated urinary tract infections. Int J Antimicrob Agents. 2006;28(Suppl 1):S35–41.10.1016/j.ijantimicag.2006.05.019
- Mei Q, Ye Y, Zhu YL, Cheng J, Chang X, Liu YY, et al. Testing the mutant selection window hypothesis in vitro and in vivo with Staphylococcus aureus exposed to fosfomycin. Eur J Clin Microbiol Infect Dis. 2015;34(4):737–44.10.1007/s10096-014-2285-6
- Roussos N, Karageorgopoulos DE, Samonis G, Falagas ME. Clinical significance of the pharmacokinetic and pharmacodynamic characteristics of fosfomycin for the treatment of patients with systemic infections. Int J Antimicrob Agents. 2009;34(6):506–15.10.1016/j.ijantimicag.2009.08.013
- Borghi CM, La Veneziana D, Riva A, Marca G, Zanninin G. Concentrazioni nel siero e nel tessuto prostatico di fosfomicina con fosfomicina-trometamolo, nuovo derivato della fosfomicina con elevata biodisponibilità per somministrazione orale. Farmaci & Terapia. 1986;3(3):216–20.
- Gardiner BJ, Mahony AA, Ellis AG, Lawrentschuk N, Bolton DM, Zeglinski PT, et al. Is fosfomycin a potential treatment alternative for multidrug-resistant gram-negative prostatitis? Clin Infect Dis. 2014;58(4):e101–5.10.1093/cid/cit704
- Periti P, Novelli A, Reali EF, Del Bono GP, Fontana P. Prophylactic chemotherapy with fosfomycin trometamol salt in transurethral prostatectomy. In: Neu HC, Williams JD. New trends in urinary tract infections. Basel: Karger; 1988. p. 207–23.
- Krcmery S, Hromec J, Demesova D. Treatment of lower urinary tract infection in pregnancy. Int J Antimicrob Agents. 2001;17(4):279–82.10.1016/S0924-8579(00)00351-4