Abstract
Antimicrobial resistance is continuously increasing among bacterial clinical isolates (especially methicillin resistance in Staphylococcus aureus, MRSA), negatively impacting on outcomes of patients with Surgical Site Infections (SSIs). A multi-disciplinary team work is essential for SSIs prevention and for the choice of antibiotic therapy of orthopaedic SSIs. In particular, an Antibiotic Stewardship (AS) approach is recommended for preserving the activity of old and new antimicrobials. Dalbavancin is a novel antimicrobial agent, belonging to the lipoglycopeptides family, recently approved by FDA for the treatment of ABSSSIs (Acute Bacterial Skin and Skin Structure Infections) and can be considered as a candidate for the treatment of orthopaedic superficial SSIs. An antimicrobial activity directed against MRSA and other multi-resistant Gram-positive pathogens, a bactericidal effect and an extremely extended half-life are among key features of this drug. Dalbavancin gives to clinicians the option to provide an intravenous antimicrobial agent shown to be as effective as conventional therapies, without requiring prolonged admission into the hospital, drastically reducing the length of hospital stay (without reducing the treatment compliance) and total cost per patient. In this paper, we analyze general, microbiological and pharmacological features of dalbavancin, aiming at supporting clinicians while positioning this drug in the context of orthopaedic SSIs.
Surgical Site Infections (SSIs) overview
SSIs, defined as infections that occur after surgery in the part of the body where the surgery took place, are among the most frequent Hospital Acquired Infections (HAI).Citation1 Superficial incisional (involving only skin or subcutaneous tissue of the incision) SSIs are considered, together with cellulitis and major cutaneous abscesses, a subgroup ABSSSIs (Acute Bacterial Skin and Skin Structure Infections)Citation2 and belong to the broader chapter of Skin and Soft Tissue Infections.Citation3,4
U.S. CDC estimates that patients with SSIs are at 2–11 times higher risk of death and 75% of deaths among these patients are directly attributable to SSI. In addition to the negative impact on patient outcomes, SSIs are associated with substantial economic costs. In fact, patients suffering from a SSI, compared with those who do not, remain in the hospital for longer periods, have a higher probability of intensive care unit (ICU) admission and higher rates of hospital readmissions. Costs are even higher for cases of infections caused by methicillin-resistant Staphylococcus aureus (MRSA) as opposed to other pathogens.Citation5–8
The last HAI Prevalence Survey, performed in 2011, estimated that about 150.000 SSIs occurred in U.S. acute care hospitals.Citation9
Accordingly, data of the last Point Prevalence Survey for HAI, promoted by the European Centre for Disease Prevention and Control (ECDC), in 2011–2012, showed that SSIs represent the 20% of HAI (16% in Italy) (http://ecdc.europa.eu/en/healthtopics/Healthcare-associated_infections/database/Pages/hai-pps-database-distribution-HAI-types.aspx).
From 2000–2003, in Europe, is active a surveillance programme (the ‘ECDC HAISSI protocol for the surveillance of SSIs’) which gathers data from 16 Countries, including Italy, and generates periodic reports. Last report, referring to years 2013–2014, documented a percentage of SSIs per 100 surgical procedures ranging from 0.6 to 9.5%, depending on the type of procedure.
Infectious complications are particularly relevant in orthopaedic surgery. In Europe, the reported percentages of SSIs were 1.1 and 0.6% for hip prosthesis and knee prosthesis, respectively http://ecdc.europa.eu/en/healthtopics/Healthcare-associated_infections/surgical-site-infections/Pages/Annual-epidemiological-report-2016.aspx.
Patients with orthopaedic SSIs have substantially greater physical limitations and significant reduction in their quality of life. In these patients, SSIs clinical manifestations can be subtle and infections are generally difficult to treat. In fact, especially when prosthetic material was implanted, or a multidrug-resistant (MDR) micro-organism is involved, the antibiotic treatment must be continued for long periods and the use of intravenous antibiotics, which can be administered in the hospital setting only, is often required (e.g. glycopeptides or daptomycin).
SSIs causative pathogens and antimicrobial resistance issues
The group of micro-organism most frequently isolated from orthopaedic SSIs is that of Gram-positive cocci (responsible of more than 65% of SSIs occurring after hip or knee surgery), with S. aureus being the most frequent micro-organism, followed by Coagulase-negative staphylococci and Enterococcus species. http://ecdc.europa.eu/en/healthtopics/Healthcare-associated_infections/surgical-site-infections/Pages/Annual-epidemiological-report-2016.aspx
For S. aureus and Enterococci (accounting together for almost one-third of orthopaedic SSIs), although with notable geographical variability, relevant antimicrobial resistance issues have been increasingly reported worldwide, mainly from high-income Countries.Citation10,11
In 2015, the EARS-Net surveillance programme reported for Italy a proportion of MRSA, over the total of S. aureus isolates from invasive infections, of 34.1% (European population-weighted mean: 16.8%) (data available at http://ecdc.europa.eu/en/publications/Publications/antimicrobial-resistance-europe-2015.pdf).
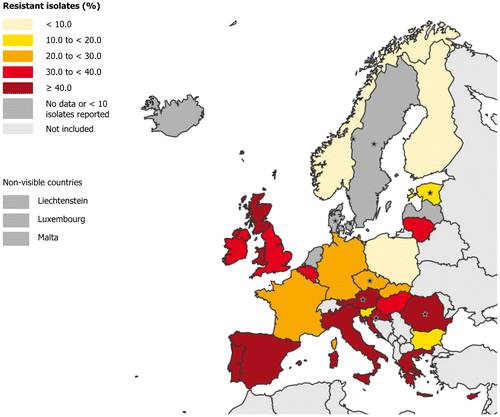
Data from the last ECDC point prevalence survey of health care-associated infections and antimicrobial use in acute care hospitals, performed in the period 2011–2012, showed that more than 40% of S. aureus isolates obtained from HAI, in Italy, were MRSA (therefore considered resistant to all ß-lactams with the exception of fifth generation cephalosporins) (Fig. ). Similarly, in a surveillance study promoted by AMCLI (Associazione Microbiologi Clinici Italiani), in 2012, that involved 52 Laboratories distributed over the Italian territory, an high prevalence of MRSA (35.5% of S. aureus) among isolates obtained from SSIs was reported.Citation12 For Enterococcus faecium, ECDC data relative to year 2015 (invasive infections) reported a percentage of strains resistant to ampicillin greater or equal to 75% in most of European Countries, including Italy (86.7%) (http://ecdc.europa.eu/en/data-tools/atlas/Pages/atlas.aspx).
Figure 1 Percentage of Staphylococcus aureus isolates resistant to meticillin (MRSA) in HAIs in acute care hospitals, ECDC PPS 2011–2012
Robust data on the dissemination, in Italy, of Enterococci resistant to vancomycin (VRE) are lacking. However, recent surveillance data showed a high variability with the presence of VRE endemic areas at a subregional level and in specific hospital settings (https://www.ars.toscana.it/files/pubblicazioni/Volumi/2015/Documento_ARS_84_2015_aggiornamento_16_XII_def.pdf).
Prevention of SSIs in orthopaedic surgery
In recent years, following the implementation of specific prevention guidelines, significant progresses have been done in preventing SSIs in orthopaedic surgery.Citation1,13
The most recent CDC’s annual National and State Healthcare-Associated Infections Progress Report (2014 data, published 2016) documented a 17% decrease in SSI related to the 10 selected procedures (including knee arthroplasty and hip arthroplasty) tracked in previous reports (www cdc gov/hai/progress report).
In Europe, for knee prosthesis surgery, a significantly decreasing trend for both the yearly percentage of SSIs and the incidence density of SSIs was observed in the period 2011–2014 (p value < 0.001).
As the aetiology of SSIs is multifaceted, existing guidelines recommend strategies based on ‘bundling’ activities, targeting a variety of risk factors together (Table ).
Table 1 Summary of CDC bundles strategy to prevent SSIs, from: www.cdc.gov/hai/pdfs/toolkits/SSI_toolkit021710SIBT_revised.pdf
Particular attention should be paid to the selection of the agent used for the antimicrobial prophylaxis, that can vary on the basis of surgical procedure, most common pathogens causing SSIs, and published recommendations.Citation1 The most recent Italian Society of Orthopaedics and Traumatology (SIOT) guidelines recommend the use of a first or second generation cephalosporin or, in selected cases, of a glycopeptide (patients with predisposing factors for MRSA infection).Citation14 However, due to the observed variability in the prevalence of MRSA and VRE, the selection of the most appropriate prophylactic regimen should be based on facility and ward-stratified antibiograms.
Despite implementation of the above-mentioned strategies, there are multiple non modifiable host- and procedure-related risk factors that make the complete elimination SSIs in orthopaedic surgery of virtually impossible (e.g. diabetes, rheumatoid arthritis, recent weight loss, dependent functional status, estimated blood loss of >1 l, longer procedure time, previous infection at site, low volume of procedures performed at hospital and low volume of procedures performed by surgeon).Citation13 Furthermore, the increasing number of patients with multiple underlying diseases undergoing surgery and the emergence of MDR pathogens are causes of concerns.
Therefore, there is increasing evidence that, in order to maximize the impact of preventative strategies and to promptly suspect and confirm SSIs, adopting a multi-disciplinary team approach is essential.Citation15
Core members of a SSIs multi-disciplinary team include:
| • | the orthopaedic surgeon | ||||
| • | an infectious diseases physician | ||||
| • | a clinical microbiologist | ||||
The optimum is to obtain the cooperation of other specialists also, including the anesthesiologist, the pharmacologist and the plastic surgeon.
Diagnosis of SSIs after orthopaedic surgery
In countries like Italy where a high prevalence of MDR pathogens has been observed, proceeding with an aetiologic diagnosis, based on microscopic and cultural exams of pathologic material obtained from SSIs, is recommended. In addition to conventional cultural methods, significant advancements, in terms of reduction of the time to personalized therapy for in-patients and out-patients with SSIs, can be achieved with the implementation of rapid, molecular test for bacterial identification and detection of selected antibiotic resistance markers (e.g. mecA and mecC) directly from clinical sample (including swabs and pus).Citation16
The choice of the sample type to be collected for laboratory diagnosis is of paramount importance.Citation17 Samples should be collected carefully to avoid touching non-involved surfaces of mucosae, colonized by contaminating bacteria.
For superficial SSIs, aspirations of pus or local irrigation fluid are preferable. The use of swabs should be avoided, however, swabs of purulence originating from beneath the dermis can be considered appropriate in selected cases. Swabs with transport system must be used. Storage at room temperature for less than 24 h is acceptable. In any case, transport and storage time should be kept to the minimum. For deep wounds, purulence, necrosis, or tissue from deep subcutaneous site is considered appropriate. The purulent fluid material should be aspirated with needle and syringe and aseptically transferred into an anaerobic transport system. The quantity of the aspirate must be greater than or equal to 1 mL. In deep infections, when implanted material is involved immediate inoculation of aspirated purulent material in blood culture vials is recommend to increase culture sensitivity.Citation18 Therefore, the selection of the more appropriate clinical sample should be done case by case depending also on the results of imaging studies used for differential diagnosis between SSIs and bone and joint infections (plain radiography, ultrasonography, CT scan or Magnetic Resonance).Citation19
Transport and storage conditions are identical to those indicated above for swabs. Swabs are not appropriate for sampling deep lesions. Tissue obtained during surgery or cutaneous biopsy can be used in case of insufficient fluid material. Tissue must be placed in an anaerobic transport system or sterile screw cap container. Several drops of sterile saline can be added to keep small pieces of tissue moist.Citation20
The selection of the SSIs antibiotic treatment; an antimicrobial stewardship approach
The choice of antibiotic therapy for orthopaedic SSIs, as well as in other bacterial infections, should respect the principles of a good Antibiotic Stewardship (AS).
In recent years, AS programmes have emerged as a powerful tool for fighting antimicrobial resistance. The AS concept is synonymous of a prescriptive approach that takes into account the conventional criteria of efficacy and tolerability and is also aimed at: (i) reducing the selective pressure on bacterial populations and; (ii) rationalizing health-care facilities resources.Citation21
Literature data suggest that a substantial percentage of the patients, ranging from 25 to 50%, receive antibiotics inappropriately, also in settings with a traditionally low use of antimicrobials.Citation22
The inappropriateness of antibiotics prescriptions is generally linked to the wrong choice of drug (typically quinolones), use of antibacterials with an excessively broad spectrum or to unnecessary continuation of the treatment.Citation23
The primary objective of AS programmes is to optimize clinical outcomes, minimizing the undesired consequences of antibiotics use, preventing the selection of other pathogens and the emergence of antibiotic-resistant strains. In a broader context, the AS can be considered an essential part of a patient safety approach and should be combined with surveillance and infection control programmes.
There is growing evidence that the implementation of AS programmes is effective in reducing the unnecessary prescription of antibiotic therapies and the incidence Clostridium difficile infections, while reducing the dissemination of MDR pathogens and realizing significant costs savings.Citation24,25
A multi-disciplinary team including an infectious disease physician, a clinical microbiologist, a clinical pharmacist, an information system specialist, an infection control professional and hospital epidemiologist is required to maximize the impact of an AS programme.Citation26
The empiric therapy for orthopaedic SSIs should include an agent that is effective against MRSA, especially in settings with a high prevalence of MRSA and in patients presenting known risk factors for MRSA infection (e.g. recent hospital admission, recent antibiotic therapy, history of MRSA infection or colonization). In fact MRSA infection is a risk factor for inappropriate initial antimicrobial treatment (IIAT).Citation27 Clinicians should be aware that IIAT of complicated skin and soft tissue infections is associated with significantly worse clinical and economic outcomes.Citation28
The most recent Infectious Diseases Society of America (IDSA) guidelines for diagnosis and management of skin and soft tissue infections, in the SSIs section, primarily underscore the importance of suture removal plus incision and drainage. Adjunctive systemic antimicrobial therapy is recommended for infections associated with a significant systemic response, such as erythema and induration extending >5 cm from the wound edge, temperature >38.5 °C, heart rate >110 beats/minute or white blood cell (WBC) count >12.000/μL. A first-generation cephalosporin or an antistaphylococcal penicillin for MSSA, or vancomycin, linezolid, daptomycin, telavancin, or ceftaroline where risk factors for MRSA are high are recommended.Citation29 Depending on the surgical site, it can be necessary to include an antibiotic active against Gram-negative bacteria and anaerobes.
Modification of empirical therapy and discontinuation of unnecessary antimicrobials (generally within 48–72 h), when warranted by preliminary culture results or clinical signs, is recommended to control antimicrobials overuse and resistance.
Once organism definitive identification and susceptibility testing are available, the possibility of a switch to a narrower-spectrum drug, to oral therapy or to a long-acting antibiotic can be considered
In particular, in the context of orthopaedic SSIs the use of antibiotics combining a long half-life with an anti-MRSA activity could be strategic.
The long-acting antibiotic dalbavancin
Dalbavancin is a novel antimicrobial agent, belonging to the lipoglycopeptides family (a subclass of glycopeptides), recently approved by FDA for the treatment of ABSSSI. The recommended dose is 1500 mg, given either as a single infusion or as 1000 mg in the first week followed by 500 mg one week later http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002840/human_med_001848.jsp.
An antimicrobial activity directed against Gram-positive pathogens, a bactericidal effect and an extremely extended half-life are among peculiar features of this drug.Citation30
Dalbavancin has an antimicrobial profile comparable to that of older glycopeptides (vancomycin and teicoplanin) with a spectrum targeting S. aureus (including MRSA), Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus anginosus group, Enterococcus faecalis e Enterococcus faecium (including ampicillin-resistant isolates). Furthermore, dalbavancin is active in vitro against S. aureus isolates with reduced susceptibility to vancomycin (vancomycin-intermediate S. aureus, VISA) and Enterococcus spp. isolates resistant to vancomycin due to vanB gene acquisition. VanA producing enterococci and S. aureus isolates resistant to vancomycin according to Clinical Laboratory Standard Institute (CLSI) criteria are resistant to dalbavancin. Retrospective data, obtained testing large collections of bacterial pathogens, showed that resistance to dalbavancin is very rare among staphylococci and streptococci.Citation31–34 The EUCAST committee states that in case of isolation of Staphylococcus spp. isolate resistant to dalbavancin the identification and antimicrobial susceptibility test must be confirmed and the isolate sent to a reference laboratory for further characterization. However, although infrequently, there is the possibility to find dalbavancin-resistant isolates in SSIs clinical samples (mainly VRE). For this reason, and also for epidemiological purpose, it is important to perform dalbavancin antimicrobial susceptibility testing (AST) for all clinically significant Gram-positive pathogens obtained from patients with SSIs.
Both the EUCAST committee and the FDA provide interpretative criteria for dalbavancin susceptibility testing. However, automated AST systems commonly used in the Clinical Microbiology Laboratory (Vitek2, Phoenix and MicroScan systems) are not yet updated for dalbavancin test and the updating process will require a relatively long period. For this reason, at least in the near future, alternative, less practicable techniques (e.g. agar gradient diffusion) will be adopted by most of Laboratories. Disc diffusion test is not recommended for this molecule (http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_7.0_Breakpoint_Tables.pdf).
If the Laboratory is unable to report dalbavancin AST, susceptibility to this drug can be deduced with sufficient accuracy from vancomycin test. In fact, a recent study, reported that for S. aureus, S. pyogenes, S. agalactiae and S. anginosus, a vancomycin Minimum Inhibitory Concentrations (MICs) below or equal to 1 μg/mL is highly predictive of susceptibility to dalbavancin.Citation35
Dalbavancin pharmacokinetics
Dalbavancin peculiar pharmacokinetics (PK) was evaluated in both animal models and clinical studies. Indeed, molecule PK, was assessed in healthy volunteers (either in single-dose or in multiple-dose studies), adolescent subjects, special populations (renal or hepatic dysfunction) and patients. Main pharmacokinetic parameters are shown in Table .
Table 2 Main pharmacokinetic parameters of dalbavancin
A three-compartment open model was used to describe the pharmacokinetic behaviour of this drug.Citation36
Pharmacokinetic parameters showed no significant inter-individual variability. In healthy subjects, dalbavancin exhibited linear dose-correlated pharmacokinetics: mean peak (or area under the plasma concentration-time curve, AUC) values increase proportionally with dose-escalation, whereas mean half-life, clearance and volume of distribution values do not change.Citation37
As shown in the table, the main pharmacokinetic property of this new lipoglycopeptide is represented by the long half-life, which reached an average value of about 7–8 days, thereby allowing mono-weekly administration.Citation36–39
The 1500 mg dose of dalbavancin recommended for Acute Bacterial Skin and Skin Structure Infections (ABSSSIs) may be administered either as a single or double infusion (1000 mg followed by 500 mg a week apart): a single intravenous (IV) administration demonstrated to be not inferior to the double regimen, both in terms of safety and clinical outcomes.Citation40
Dalbavancin demonstrated is highly bound to serum proteins (93%), in particular albumin.Citation41 Nevertheless, molecule penetration into animal tissues was thoroughly proved.Citation36
From a clinical point of view, drug concentrations in soft tissue interstitial fluid were investigated in healthy volunteers, showing values similar to those of plasma: tissue/plasma ratio reached a mean value of 0.60 (cantharidin-induced blister technique).Citation37,42
Dalbavancin distribution into bone and joint tissues was also studied: pharmacokinetic profile was analyzed in patients undergoing joint surgery, collecting bone and joint tissue samples at 0.5, 24, 72, 168, 240 and 336 h after the end of a single 1000 mg infusion. Mean drug concentrations in cortical bone and synovia at the end of infusion (12 h) and at 14 days (336 h) were 6.3, 25 μg/g and 4.1, 15.9 μg/g, respectively.Citation43 Furthermore, concerning bone drug penetration, dalbavancin effectiveness in the treatment of MRSA osteomyelitis was demonstrated in rats, showing a reduction in bone CFUs superior to placebo and similar to vancomycin.Citation44
No tissue accumulation after a multiple-dose regimen (1000 mg IV loading-dose on day 1 followed by 500 mg/week for 7 weeks) was observed.Citation43
Dalbavancin elimination follows two routes (renal/non-renal): after a 1000 mg single dose, in healthy volunteers, unchanged drug excretion into urine was 42% after 42 days, thus underlining the importance of non-renal elimination pathway.Citation41 Dose adjustment is required only in patients with severe renal impairment (<30 creatinine clearance <30 ml/min) not undergoing dialysis. Indeed, following a 1000 mg single dose, the AUC0-∞ of patients with mild and moderate renal dysfunction increased by about 10 and 50%, respectively, whereas, after a 500 mg single dose, patients with severe renal dysfunction showed a 100% increase in AUC0-∞.
No dose adjustment is necessary in hepatic dysfunction.Citation45
Dalbavancin PK was also described in hospitalized adolescents aged 12–17 years: average half-life and volume of distribution values were similar to those observed in adult patients affected by skin and skin structure infections, whereas mean AUC values appeared to be reduced by 30%.Citation46
As regards safety and tolerability, in healthy volunteers, dalbavancin was overall well tolerated, causing mild AEs such as pyrexia, headache and nausea.Citation37
A recent pooled analysis collected safety data from seven phase II and III studies: regarding this pre-marketing phase, this new antibiotic revealed a similar adverse effect profile to comparator molecules, such as cephalosporins, vancomycin, oxacillin, nafcillin and linezolid.Citation47
Additionally, dalbavancin did not affect cardiac electrophysiology, not prolonging the QTc interval.Citation48
Concerning PK-PD behaviour, this antibiotic is characterized by a concentration-dependent killing pattern. Indeed, Andes and Craig, demonstrated in vivo dose-dependent bactericidal activity: high doses and long intervals of administration guaranteed a faster bacterial killing, preventing pathogen regrowth. Peak/MIC and AUC/MIC ratios were the best PK/PD indices able to predict antimicrobial efficacy. Considering free-drug 24 h AUC to MIC ratio as the PK/PD index that most correlates with the efficacy in the animal model, target values between 100 and 300 should be reached in vivo.Citation49
In patients affected by Acute Bacterial Skin and Skin Structure Infections (ABSSSI), in comparison to conventional therapy (twice daily vancomycin infusion eventually switched with twice daily oral linezolid), dalbavancin showed similar results in terms of both early and late clinical outcomes.Citation50
Concluding remarks
Dalbavancin presents a promising alternative to conventional antibacterials for the treatment of ABSSSIs in adult patients.Citation51,52 The favourable pharmacokinetic profile, the broad antimicrobial spectrum (covering most frequently isolated Gram-positive MDR pathogens, e.g. MRSA and ampicillin-resistant Enterococci) and the safety features of dalbavancin make this drug suitable for the treatment of patients with orthopaedic superficial SSIs. In fact, dalbavancin gives to clinicians the option to provide an intravenous antimicrobial agent shown to be as effective as conventional therapies, without requiring prolonged admission into the hospital.Citation53 These features are expected to cause benefits for both the patient and the overall health care system.Citation54 Since dalbavancin has been introduced in the clinical practice only recently, robust evaluations of the economic impact of its use are not yet available. However, a recent HTA evaluation, conducted by a panel of Italian experts, concluded for a sustainable and potentially positive economic impact of dalbavancin use for the treatment of ABSSSIs, mainly related to the opportunity of earlier discharge of patients treated with dalbavancin in comparison with patients treated with other therapeutic regimens.Citation54
A setting where dalbavancin use could be significantly advantageous (for its safety profile and need of a single-shot intravenous administration) is that of outpatients with risk of low compliance to long-term antibiotic treatment such as drug addicted and old patients admitted to long-term care facilities.
Regarding future perspectives we should mention that dalbavancin has demonstrated, an in vitro anti-biofilm activity. In fact, a recent study showed that dalbavancin is able to successfully reduce MRSA and MRSE in biofilms, and therefore provides a promising option for the treatment of biofilm-associated infections, including those related to implant of prosthetic material.Citation55
Contributors
FA wrote the article in whole, and revised the article. ER wrote the article in part, and revised the article. ER wrote the article in part, and revised the article. CS wrote the article in part, and revised the article. GT wrote the article in part, and revised the article. CP wrote the article in part, and revised the article. MF wrote the article in part, and revised the article.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by Ethos s.r.l.
References
- Anderson DJ, Podgorny K, Berríos-Torres SI, Bratzler DW, Dellinger EP, Greene L, et al. Strategies to prevent Surgical Site Infections in acute care hospitals: 2014 update. Infect Control Hospital Epidemiol. 2014 Jun;35(06):605–27.10.1086/676022
- Food and Drug Administration. Acute bacterial skin and skin structure infections: developing drugs for treatment. 2013 Sep;24:1–18.
- Esposito S, Noviello S, Leone S. Epidemiology and microbiology of skin and soft tissue infections. Curr Opin Infect Dis. 2016 Apr;29(2):109–15.10.1097/QCO.0000000000000239
- Esposito S, Bassetti M, Bonnet E, Bouza E, Chan M, De Simone G, et al. Hot topics in the diagnosis and management of skin and soft-tissue infections. Int J Antimicrob Agents. 2016 May; 48(1):19–26.
- Patel H, Khoury H, Girgenti D, Welner S, Yu H. Burden of Surgical Site Infections Associated with arthroplasty and the contribution of Staphylococcus aureus. Surg Infect (Larchmt). 2016 Feb;17(1):78–88.10.1089/sur.2014.246
- Weigelt JA, Lipsky BA, Tabak YP, Derby KG, Kim M, Gupta V. Surgical Site Infections: causative pathogens and associated outcomes. Am J Infect Control. 2010 Mar 1;38(2):112–20.10.1016/j.ajic.2009.06.010
- Awad SS. Adherence to surgical care improvement project measures and post-operative Surgical Site Infections. Surg Infect. 2012 Aug;13(4):234–7.10.1089/sur.2012.131
- Sganga G, Tascini C, Sozio E, Carlini M, Chirletti P, Cortese F, et al. Focus on the prophylaxis, epidemiology and therapy of methicillin-resistant Staphylococcus aureus Surgical Site Infections and a position paper on associated risk factors: the perspective of an Italian group of surgeons. World J Emerg Surg. 2016 14; 11:26.10.1186/s13017-016-0086-1
- Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014 Mar 27;370(13):1198–208.10.1056/NEJMoa1306801
- Rossolini GM, Arena F, Pecile P, Pollini S. Update on the antibiotic resistance crisis. Curr Opin Pharmacol. 2014 Oct;18:56–60.10.1016/j.coph.2014.09.006
- Rossolini GM, Arena F, Pollini S. Novel infectious diseases and emerging gram-positive multi-resistant pathogens in hospital and community acquired infections. In: Antimicrobials. Berlin Heidelberg: Springer; 2014. p. 11–28.10.1007/978-3-662-45786-3
- Campanile F, Bongiorno D, Perez M, Mongelli G, Sessa L, Benvenuto S, et al. Epidemiology of Staphylococcus aureus in Italy: first nationwide survey, 2012. J Glob Antimicrob Resist. 2015 Dec 1;3(4):247–54.10.1016/j.jgar.2015.06.006
- Guide to the Elimination of Orthopedic Surgical Site Infections. AFPIIC 2011; 1–80.
- Tucci G, Romanini E, Zanoli G. Profilassi antibiotica perioperatoria nella chirurgia protesica dell’anca e del ginocchio. Giornale Italiano di Ortopedia. 2011 Jun 21;37:4–17.
- Stuyts B, Van den Eeden E, Fennema P. The role of other stakeholders than the surgeon in relation to Surgical Site Infections following total joint replacement. Acta Orthop Belg. 2016 Dec;81(4):578–86.
- Dubouix-Bourandy A, de Ladoucette A, Pietri V, Mehdi N, Benzaquen D, Guinand R, et al. Direct detection of staphylococcus osteoarticular infections by use of Xpert MRSA/SA SSTI Real-Time PCR. J Clin Microbiol. 2011 Nov 28;49(12):4225–30.10.1128/JCM.00334-11
- Esposito S, De Simone G, Gioia R, Noviello S, Pagliara D, Campitiello N, et al. Deep tissue biopsy vs. superficial swab culture, including microbial loading determination, in the microbiological assessment of Skin and Soft Tissue Infections (SSTIs). J Chemother. 2017 Mar 24;29(3):154–8.10.1080/1120009X.2016.1205309
- Larsen LH, Lange J, Xu Y, Schønheyder HC. Optimizing culture methods for diagnosis of prosthetic joint infections: a summary of modifications and improvements reported since 1995. J Med Microbiol. 2012 Mar;61(Pt 3):309–16.10.1099/jmm.0.035303-0
- Esposito S, Bassetti M, Borre S, Bouza E, Dryden M, Fantoni M, et al. Diagnosis and management of Skin and Soft-tissue infections (SSTI): a literature review and consensus statement on behalf of the Italian Society of infectious diseases and international Society of Chemotherapy. J Chemother. 2009 Mar;53(3):1260–3.
- Jorgensen JH, Pfaller MA, Carroll KC, Funke G, Landry ML, Richter SS, et al. Manual of clinical microbiology. 11th ed. 2015 Dec 5. p. 1–10.
- Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, et al. Implementing an Antibiotic Stewardship Program: guidelines by the infectious diseases society of America and the society for healthcare epidemiology of America. Clin Infect Dis. 2016 May 15;62(10):e51–77.10.1093/cid/ciw118
- Willemsen I, Groenhuijzen A, Bogaers D, Stuurman A, van Keulen P, Kluytmans J. Appropriateness of antimicrobial therapy measured by repeated prevalence surveys. Antimicrob Agents Chemother. 2007 Feb 21;51(3):864–7.10.1128/AAC.00994-06
- Mettler J, Simcock M, Sendi P, Widmer AF, Bingisser R, Battegay M, et al. Empirical use of antibiotics and adjustment of empirical antibiotic therapies in a university hospital: a prospective observational study. BMC Infect Dis. 2007 Mar 26;7(1):21.10.1186/1471-2334-7-21
- Tamma PD, Holmes A, Ashley ED. Antimicrobial stewardship: another focus for patient safety? Curr Opin Infect Dis. 2014 Aug;27(4):348–55.10.1097/QCO.0000000000000077
- Dellit TH, Owens RC, McGowan JE, Gerding DN, Weinstein RA, Burke JP, et al. Infectious diseases society of America and the society for healthcare epidemiology of America guidelines for developing an institutional program to enhance Antimicrobial Stewardship. Clin Infect Dis. 2007 Jan 15;44(2):159–77.10.1086/510393
- Lesprit P, Brun-Buisson C. Hospital Antibiotic Stewardship. Curr Opin Infect Dis. 2008 Aug;21(4):344–9.10.1097/QCO.0b013e3283013959
- Zervos MJ, Freeman K, Vo L, Haque N, Pokharna H, Raut M, et al. Epidemiology and outcomes of complicated skin and soft tissue infections in hospitalized patients. J Clin Microbiol. 2012 Jan 20;50(2):238–45.10.1128/JCM.05817-11
- Edelsberg J, Berger A, Weber DJ, Mallick R, Kuznik A, Oster G. Clinical and economic consequences of failure of initial antibiotic therapy for hospitalized patients with complicated skin and skin-structure infections. Infect Control Hosp Epidemiol. 2008 Feb;29(2):160–9.10.1086/526444
- Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJC, Gorbach SL, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis. 2014 Jul 15;59(2):e10–52.10.1093/cid/ciu296
- Roberts KD, Sulaiman RM, Rybak MJ. Dalbavancin and oritavancin: an innovative approach to the treatment of gram-positive infections. Pharmacotherapy. 2015 Oct 1;35(10):935–48.10.1002/phar.2015.35.issue-10
- Biedenbach DJ, Bell JM, Turnidge JD, Jones RN. Activities of dalbavancin against a worldwide collection of 81,673 gram-positive bacterial isolates. Antimicrob Agents Chemother. 2009 Feb 12:1–4.
- Biedenbach DJ, Jones RN. Multicenter evaluation of the in vitro activity of dalbavancin tested against staphylococci and streptococci in 5 European countries: results from the DECIDE Surveillance Program (2007). Diagn Microbiol Infectious Dis. 2009 Jun 1;64(2):177–84.10.1016/j.diagmicrobio.2008.12.019
- Jones RN, Sader HS, Flamm RK. Update of dalbavancin spectrum and potency in the USA: report from the SENTRY Antimicrobial Surveillance Program (2011). Diagn Microbiol Infectious Dis. 2013 Mar 1;75(3):304–7.10.1016/j.diagmicrobio.2012.11.024
- McCurdy SP, Jones RN, Mendes RE, Puttagunta S, Dunne MW. In vitro activity of dalbavancin against drug-resistant Staphylococcus aureus isolates from a global surveillance program. Antimicrob Agents Chemother. 2015 Aug;59(8):5007–9.
- Jones RN, Farrell DJ, Flamm RK, Sader HS, Dunne MW, Mendes RE. Surrogate analysis of vancomycin to predict susceptible categorization of dalbavancin. Diagn Microbiol Infect Dis. 2015 May;82(1):73–7.
- Cavaleri M, Riva S, Valagussa A, Guanci M, Colombo L, Dowell J, et al. Pharmacokinetics and excretion of dalbavancin in the rat. J Antimicrob Chemother. 2005 Mar;55 Suppl 2(suppl_2):ii31–5.10.1093/jac/dki006
- Leighton A, Gottlieb AB, Dorr MB, Jabes D, Mosconi G, VanSaders C, et al. Tolerability, pharmacokinetics, and serum bactericidal activity of intravenous dalbavancin in healthy volunteers. Antimicrob Agents Chemother. 2004 Mar;48(3):940–5.10.1128/AAC.48.3.940-945.2004
- Buckwalter M, Dowell JA. Population pharmacokinetic analysis of dalbavancin, a novel lipoglycopeptide. J Clin Pharmacol. 2005 Nov;45(11):1279–87.10.1177/0091270005280378
- Scoble PJ, Owens RC, Puttagunta S, Yen M, Dunne MW. Pharmacokinetics, safety, and tolerability of a single 500-mg or 1000-mg intravenous dose of dalbavancin in healthy Japanese subjects. Clin Drug Investig. 2015 Dec;35(12):785–93.10.1007/s40261-015-0340-4
- Dunne MW, Puttagunta S, Giordano P, Krievins D, Zelasky M, Baldassarre J. A randomized clinical trial of single-dose versus weekly dalbavancin for treatment of acute bacterial skin and skin structure infection. Clin Infect Dis. 2016 Mar 1;62(5):545–51.10.1093/cid/civ982
- Dowell, J. A., Gottlieb, A. B., Van Saders, C., Dorr, M. B., Leighton, A., and Cavaleri, M.. The pharmacokinetics and renal excretion of dalbavancin in healthy subjects. In 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy; 2002 Sep. p. 26–30.
- Nicolau DP, Sun HK, Seltzer E, Buckwalter M, Dowell JA. Pharmacokinetics of dalbavancin in plasma and skin blister fluid. J Antimicrob Chemother. 2007 Sep;60(3):681–4.10.1093/jac/dkm263
- Dunne MW, Puttagunta S, Sprenger CR, Rubino C, Van Wart S, Baldassarre J. Extended-duration dosing and distribution of dalbavancin into bone and articular tissue. Antimicrob Agents Chemother. 2015 Apr;59(4):1849–55.10.1128/AAC.04550-14
- Barnea Y, Lerner A, Aizic A, Navon-Venezia S, Rachi E, Dunne MW, et al. Efficacy of dalbavancin in the treatment of MRSA rat sternal osteomyelitis with mediastinitis. J Antimicrob Chemother. 2016 Feb;71(2):460–3.10.1093/jac/dkv357
- Marbury T, Dowell JA, Seltzer E, Buckwalter M. Pharmacokinetics of dalbavancin in patients with renal or hepatic impairment. J Clin Pharmacol. 2009 Apr;49(4):465–76.10.1177/0091270008330162
- Bradley JS, Puttagunta S, Rubino CM, Blumer JL, Dunne M, Sullivan JE. Pharmacokinetics, safety and tolerability of single dose dalbavancin in children 12–17 years of age. Pediatr Infect Dis J. 2015 Jul;34(7):748–52.10.1097/INF.0000000000000646
- Dunne MW, Talbot GH, Boucher HW, Wilcox M, Puttagunta S. Safety of dalbavancin in the treatment of skin and skin structure infections: a pooled analysis of randomized, comparative studies. Drug Saf. 2016 Feb;39(2):147–57.10.1007/s40264-015-0374-9
- Dunne MW, Zhou M, Darpo B. A thorough QT study with dalbavancin: a novel lipoglycopeptide antibiotic for the treatment of acute bacterial skin and skin-structure infections. Int J Antimicrob Agents. 2015 Apr;45(4):393–8.10.1016/j.ijantimicag.2014.12.021
- Andes D, Craig WA. In vivo pharmacodynamic activity of the glycopeptide dalbavancin. Antimicrob Agents Chemother. 2007 May;51(5):1633–42.
- Boucher HW, Wilcox M, Talbot GH, Puttagunta S, Das AF, Dunne MW. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med. 2014 Jun 5;370(23):2169–79.10.1056/NEJMoa1310480
- Ramdeen S, Boucher HW. Dalbavancin for the treatment of acute bacterial skin and skin structure infections. Expert Opin Pharmacother. 2015 Oct 12;16(13):2073–81.10.1517/14656566.2015.1075508
- Esposito S, Noviello S, Leone S. Dalbavancin for the treatment of acute bacterial skin and skin structure infections. Infez Med. 2015 Dec;23(4):313–7.
- Palmieri F, Alberici F, Deales A, Furneri G, Menichetti F, Esposito S, et al. Early discharge of infectious disease patients: an opportunity or extra cost for the Italian Healthcare System? 2013 Dec;21(4):270–8.
- Favaretti C, Kheiraoui F, di Pietro ML, Ferriero AM, Iodice L, Marino M, Parente P, Posteraro P. Valutazione dell’impatto clinico, organizzativo, economico ed etico dell’introduzione di una nuova tecnologia sanitaria, Dalbavancina, per il trattamento delle infezioni batteriche acute di cute e struttura cutanea in Italia. Quaderni dell’Italian J Public Health. 2016;5(4):1–60.
- Knafl D, Tobudic S, Cheng SC, Bellamy DR, Thalhammer F. Dalbavancin reduces biofilms of methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-resistant Staphylococcus epidermidis (MRSE). Eur J Clin Microbiol Infect Dis. 2017 Apr;36(4)
