Abstract
Recurring urinary tract infections (rUTIs) are frequently caused by Escherichia coli, which invades urothelial cells and forms quiescent bacterial reservoirs. D-mannose, an inert monosaccharide, represents a notable agent for rUTI prevention; however, there is no agreement on its dosage. To provide pharmacological basis for an effective dose, we evaluated its ability to inhibit adhesion of E. coli to urothelial cells. E. coli strains isolated from the urine of a woman with recurrent urinary tract infections were selected according to adhesion capacity. Anti-adhesive efficacy and invasion were tested using the TCC-5637 urothelial cell line. The IC50 for the anti-adhesive efficacy and anti-invasion activity of D-mannose were 0.51 mg/ml and 0.30 mg/ml, respectively, both with concentration-dependent inhibition. Lastly, the biofilm interference of D-mannose was evaluated to be 50 mg/ml. D-mannose inhibited the adhesion of E. coli to urothelial cells at high concentrations, whereas inhibition of invasion occurred at much lower concentrations.
Introduction
Recurrent urinary tract infections (rUTIs) are common in adult women. The epidemiology differs according to the various reports, but overall, rUTI is a relevant problem among women. According to some authors, approximately 25% of women with acute cystitis develop rUTI. The number of recurrence is different for each patient ranging between 0.3 and 7.6 episodes per year, and UTI episodes often recur with short intervals [Citation1]. In other reports, 11% of women reported at least one episode of UTI per year. Half of all women will have at least one episode of UTI during their lifetime, with 20–40% experiencing recurrent episodes UTI incidence is between 150 and 250 million cases worldwide per year [Citation2].
The current definition of rUTI in the European Association of Urology guidelines on urological infections is ‘recurrences of uncomplicated and/or complicated UTIs, with a frequency of at least three UTIs/year or two UTIs in the last six months’ [Citation3]. In addition to significant clinical discomfort, the economic burden is also not negligible, with data from the USA estimating an annual cost incurred by UTIs in all patients to be above $3.5 billion, including direct and indirect costs [Citation4, Citation5].
The majority of recurrent UTIs seem to be caused by uropathogenic E. coli, which is now known to invade urothelial cells and form quiescent bacterial reservoirs. These reservoirs may provide a source for bacterial persistence and UTI recurrence [Citation6].
UTIs are routinely treated with antibiotics; however, although antibiotics remain the cornerstone of UTI management, costs, adverse events, and the increase in antimicrobial resistance, in particular are becoming a real public health problem [Citation7, Citation8]. Thus, alternative prophylactic and therapeutic approaches should be explored.
In recent years, D-mannose, an inert monosaccharide that is metabolized and excreted in urine has been widely used in the prevention of UTI [Citation9–11].
D-mannose is a natural aldohexose sugar that differs from glucose by inversion of one of the four chiral centres of the molecule, precisely the one on the carbon atom in the 2nd position (). This sugar is normally present in human metabolism and plays an important biological role, especially because it is involved in the glycosylation of many proteins [Citation12].
The rationale for using D-mannose to prevent attachment of bacterial strains exposing type 1 fimbriae is that D-mannose competitively inhibits the bacterial adherence to urothelial cells, because of its structural similarity to the binding site of urothelial glycoprotein receptors adherence [Citation13]. It has been shown that this sugar binds and specifically blocks the FimH adhesin, which is positioned at the tip of the type 1 fimbria of enteric bacteria, and binds to carbohydrate-containing glycoprotein receptors on the epithelium of the urinary tract.
Some reviews and meta-analyses have reported positive data on the use of D-mannose in the prevention of UTI [Citation9–11]; however, there is no agreement on the dosage of D-mannose. The doses used in the various studies ranged from 250 mg to 3 g/day [Citation9, Citation10]. However, these dosages have been established empirically, and the threshold concentration of D-mannose necessary for triggering such purported pharmacological effects has not been defined in literature. There is no evidence that the concentration attainable in vivo in urine after administering the established active doses would exceed the minimum effective dose for achieving pharmacological effects.
Thus, the purpose of this study was to define the pharmacological basis for designing effective doses of orally administered D-mannose.
Materials and methods
Bacteria
E. coli strains were isolated from urine of a woman with acute exacerbation of recurrent urinary tract infections. Bacteria were cultured overnight at 37 °C in Luria − Bertani broth, and a bacterial suspension was prepared at a concentration of 1.5 × 108 bacteria/mL in phosphate buffer saline (PBS) for all assays.
Haemagglutination test
To select E. coli strains capable of adhering to cells, the adhesion capacity of 12 E. coli strains was preliminarily evaluated using the hemagglutination (HA) test as described earlier by Brument et al. [Citation13]. Briefly, the bacteria were collected and suspended in PBS at 540 nm optical density of 0.9. One millilitre of this suspension was centrifuged in a microcentrifuge and the pellet was suspended in 100 ml of PBS. The bacterial suspensions were serially diluted in ‘V’ bottom microtiter plates (Costar) containing PBS in each well. To each well, 25 mcL of Type A human blood (OD640 nm= 1.5, diluted in PBS) was added. After mixing, the plates were incubated at 4 °C for 2 h. The HA endpoint was defined as the final dilution before the formation of red cell buttons. Titres were expressed as the reciprocal of the dilution of the endpoint.
E. coli strain adhesion and invasion assays of urothelial epithelial cells
Bacteria were cultured overnight at 37 °C in broth and subsequently a bacterial suspension was prepared at a concentration of 1.5 × 108 bacteria/mL in PBS for adhesion assays. The ATCC-5637 urothelial cell line was used in these experiments. Cells were maintained in an atmosphere containing 5% CO2 at 37 °C in RPMI 1640 medium supplemented with 2 mM L-glutamine and 10% foetal bovine serum. Cells were seeded into 48-well tissue culture plates at a density of 1.5 × 105 cells/well and incubated at 37 °C for 48 h. Prior to infection, cells were washed with PBS and incubated for 1 hour with D-mannose at final concentrations of 0, 0.01, 0.10, 0.20, 0.50, 1.00, 2, 00, 4.00, 8.00, and 16.00 mg/ml. The effects of D-mannose treatment were compared to those of untreated cells. Epithelial cells were then infected in the presence of D-mannose with the EC14 strain (selected following hemagglutination tests) with 1.5 × 106 bacteria/well. The monolayers were washed three times with PBS and lysed with 1% Triton X-100 (Sigma) in deionized water. The samples were diluted and plated on agar plates to determine the number of colony-forming units. Adherence was calculated as the number of bacteria recovered after washing with PBS. To determine the invasion frequencies, after the first 2 h of incubation, an additional set of wells was washed twice with PBS (with Mg2 +/Ca2 +) and then incubated for another 2 h in a medium containing 100 µg/mL. of gentamicin (a bactericidal antibiotic impermeable to the membrane) to kill extracellular bacteria. The cells were then washed thrice with PBS, lysed in 1 mL of 0.1% Triton X-100 in ddH20 and plated on agar plates. Invasion was calculated as the number of bacteria that survived incubation with gentamicin.
Biofilm formation
To evaluate whether D-mannose may interfere with biofilm formation, experiments were conducted in which biofilms were grown in 96-well plates for 24 h using the EC 14 strain of E. coli. Bacteria were incubated for 24 h at 30 °C with or without D-mannose at different concentrations. Biofilm production was evaluated by measuring the absorbance of crystal violet, a dye specific for gram-positive colouring. The absorbance was measured at 570 nm using a microplate spectrophotometer. Viability was measured using phenoxazine, a blue dye used as a redox indicator. It turns pink and fluorescent when reduced to resorufin by aerobic respiration of the metabolic activity of cells. Resorufin fluorescence was measured at a wavelength of 590 nm with an excitation wavelength of 550 nm using a spectrofluorometer.
Estimation of urinary elimination of D-mannose
The urinary elimination of D-mannose has been extrapolated from previous studies in humans. Two studies have evaluated the kinetics of D-mannose but did not report urinary concentrations [Citation14, Citation15]. Wood et al. evaluated the elimination of D-mannose after venous administration of high doses of it (an average of 40 g). The data showed that 20% of the dose of D-mannose was eliminated in the first hour after administration. The calculation of the elimination constant may suggest that elimination occurs at a rate of 20% per hour. The data obtained from the study by Alton et al. confirmed the postulates posed in the previous study. In this study, D-Mannose was administered orally at a dosage of 0, 0.07, 0.14, or 0.21 g/kg of body weight. Administration resulted in a dose-dependent increase in plasma values, and therefore it was considered that the kinetics are linear. These previously obtained data that examined the plasma and urinary kinetics of d-mannose were sufficient to estimate the urinary concentration of D-mannose with a high approximation. This allowed us to extrapolate the kinetics for the different doses of D-mannose used in previous clinical studies, particularly for dosages of 0.7, 1, 2, and 3 g as a single administration.
Data and statistical analysis
Data and curve analyses were performed using GraphPad Prism software. The data were interpolated to fit mono-or biphasic sigmoidal regressions. This allowed us to calculate the maximum efficacy and relative power to define the IC50s. Statistical analyses were performed using GraphPad Instat.
Results
Haemagglutination test
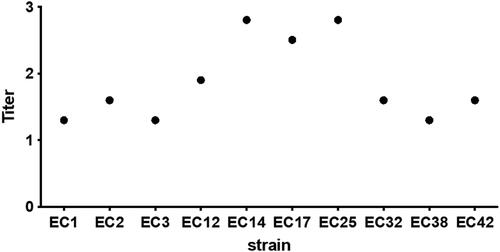
Hemagglutination titres are shown in . The 12 strains of E. coli expressed different abilities to express the pili capable of binding to the cell surface. Based on these experiments, we decided to use EC14 strain for subsequent experiments.
E. coli strain adhesion assay on urothelial epithelial cells
D-mannose was able to inhibit the adhesion of the E. coli EC14 strain to urothelial cells in a concentration-dependent manner (). The effect started at the concentration of 0.2 mg/mL and became maximum at the concentration of 4 mg/mL.
Figure 3. D-mannose dose-dependent inhibitory effects on the ability of the E. coli EC14 to adhere to ATCC-5637 cells. (A) total number of adherent bacteria. (B) non-linear regression of the adhesion of bacteria to urothelial cells.
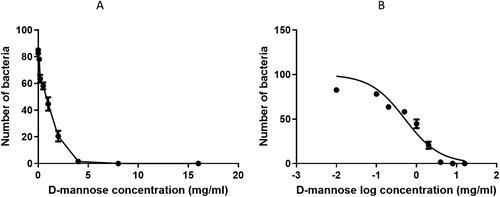
The regression curve relating to the adhesion data (Figure 1S supplementary materials) showed a good concentration-dependent linearity. The curve presented an excellent interpolation of the data, with an r2 value of 0.89. The anti-adhesive efficacy of D-mannose was equal to an IC50 of 0.51 mg/mL (Table 1S supplementary materials).
E. coli strain invasion assay in urothelial epithelial cells
D-mannose causes a reduction in bacterial invasion of urothelial cells. In addition, the inhibition was concentration-dependent in this case. Nonlinear regression shows good interpolation of the data (Supplementary material, Figure 2S) with an r2 of 0.94. The IC50 was equal to 0.30 mg/mL.
Biofilm formation
shows the concentration-dependent decrease in crystal violet absorbance (as a marker for biofilm matrix production), showing that D-mannose reduces biofilm formation (left). Inhibition of the biofilm occurs at high concentrations equal to 50 mg/mL which cannot be reached in the urine with the usual doses of D-mannose. However, at concentrations that can be reached in the urine, there is still an initial inhibition of the biofilm ().
Figure 4. Inhibition of the biofilm after incubation of the EC14 E. coli strain with various concentrations of D-Mannose. (A) Biofilm formation. The box shows the inhibition of the biofilm at the concentrations of D-mannose reachable in the urine, (B) viable bacteria.
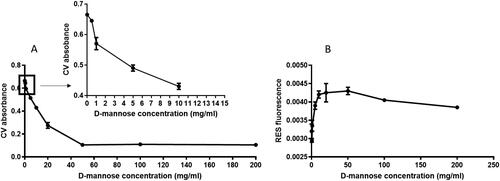
Figure 5. Urinary elimination estimation of D-mannose after oral administration of 0.7, 1, 2 or 3 grams. This estimate is made assuming an absorption of 90% and a urinary elimination of 80%. (calculated from ref. Citation12).
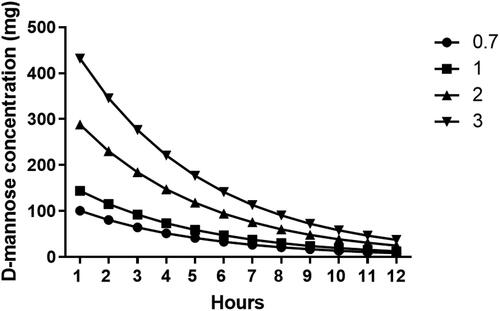
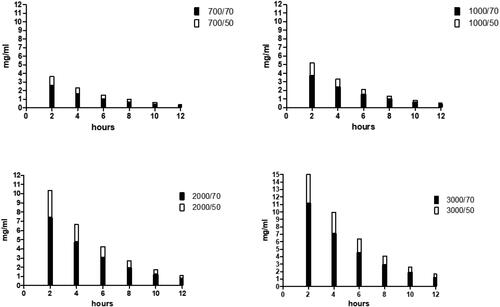
Figure 6. Estimation of the urinary concentration of D-mannose at the dosage of 0.7,1,2 and 3 grams administered orally. The estimate was made considering a diuresis of 50 ml / h (white column) or 70 ml / h (black column).
Resorufin fluorescence (as a marker for bacterial viability within the biofilm) increased at lower concentrations, with no further increase at higher concentrations, demonstrating that D-mannose has no direct antibacterial activity.
Estimation of urinary elimination of D-mannose
Urinary elimination was estimated assuming 90% absorption and 80% urinary elimination at a rate of 20% per hour. Urinary kinetic data were calculated for doses of 0.7, 1, 2, and 3 grams in a single administration (Figures 2S and 3S, supplementary materials).
Discussion
Bacteria entering the urinary tract may induce symptomatic infections involving the kidney, such as acute pyelonephritis, or infections limited to the bladder, such as acute cystitis. The type of UTI resulting in a susceptible host partly depends on the combination of virulence factors of the infecting strain [Citation16], including the ability to attach to human urinary tract epithelial cells. The urinary tract mucus membrane is coated with proteins that interfere with bacterial adhesion [Citation17]. This is a bacterial virulence factor that promotes UTI, especially E. coli [Citation18]. D-mannose is a monosaccharide that can be rapidly absorbed and excreted by the urinary tract and can prevent the adhesion of type 1 bacterial fimbria to the uroepithelium.
This study shows that D-mannose is capable of inhibiting the adhesion of E. coli to urothelial cells. The inhibition occurred at relatively high concentrations. The inhibition of approximately 100% occurs at a concentration of 4 mg/mL of D-mannose. However, the non-linear regression calculations showed an IC50 of 0.51 mg/mL.
Data relating to the inhibition of cellular invasion by D-mannose are very interesting. In this case, inhibition of invasion occurred at much lower concentrations. The IC 50 was 0.3 mg/mL. This indicates that lower concentrations of D-mannose are needed to inhibit the invasion of bacteria into urothelial cells. Alongside these data, inhibition of biofilm formation also appears to be interesting. Although the inhibitory activity of D-mannose appears to be less elevated at doses that are reached in the urine, it can help to promote the elimination of bacteria and improve the prevention of infections. Taken together, these data indicate that D-mannose could be a useful compound for the prevention of rUTIs.
The correct dosage of D- mannose is a limiting factor in its action. The urinary concentrations of D-mannose, as determined by us from the extrapolation studies, appears to be quite high; however, for the purpose of efficacy, the concentration of D-mannose in the urine is more important than total elimination.
The urinary concentration of D-mannose after oral administration depends on various factors, including dose, total elimination, urinary volume, and frequency of bladder emptying time.
The frequency of bladder emptying throughout the day varies among individuals; therefore, it is very difficult to precisely determine the urinary volume in which D-mannose is diluted. In healthy people, normal hourly diuresis fluctuates between 0.5% and 1 mL/h/kg, which means that in a normal weight adult, it generally ranges between 50 and 70 mL/h. From the previous urinary elimination data (), we estimated the urinary concentration of D-mannose with a diuresis of 50 or 70 mL/h. It appears evident that the dosage of one gram of D-mannose administered every 8 or 12 h reaches urinary concentrations sufficient to prevent the adhesion as well as the invasion of E. coli into the bladder epithelium. These data support the results of a recent study that showed that prophylaxis with 2 g of D-mannose daily for 6 days significantly reduced the risk of rUTI, and the results were equal to administration of 50 mg nitrofurantoin daily [Citation18].
A limitation of the study is the extrapolation of D-Mannose levels in urine from previous studies. However even if the levels are extrapolated, were estimated in a conservative way, so they shouldn't be not far from the levels obtainable with adequate doses of D-mannose rUTI remains a major problem in UTI management. Patients with rUTI are often treated with antibiotics associated with the occurrence of antibiotic resistance, as well as alterations in the normal microbiome of the vagina and gastrointestinal tract. Increasing antimicrobial resistance with its expenditure and health consequences has raised interest in using different non-antibiotic methods to prevent and treat uncomplicated lower UTIs. In this context, d-mannose may be useful for UTI prevention, although more clinically controlled trials are required.
Antibiotics remain the gold standard for UTI treatment. However, changing the therapeutic strategy by including non-antibiotic measures such as D-mannose in the management of UTIs could be successful in avoiding antimicrobial resistance.
Supplemental Material
Download MS Word (42 KB)Disclosure statement
Prof. Scaglione declares consulting fees and lecture fees from MSD, GSK, Pierre Fabre Pharma and Angelini Pharma. Other authors declare no COI.
Data availability statement
Data can be made available upon motivated request to the corresponding author.
Additional information
Funding
References
- Nosseir SB, Lind LR, Winkler HA. Recurrent uncomplicated urinary tract infections in women: a review. J Womens Health (Larchmt)). 2012;21(3):347–354.
- Hickling DR, Nitti VW. Management of recurrent urinary tract infections in healthy adult women. Rev Urol. 2013;15(2):41–48.
- Bonkat G, Bartoletti R, Bruyère F, et al. EAU guidelines on urological infections. Arnhem (The Netherlands): European Association of Urology; 2020.
- Foxman B, Frerichs RR. Epidemiology of urinary tract infection: II. Diet, clothing, and urination habits. Am J Public Health. 1985;75(11):1314–1317.
- Eells SJ, Bharadwa K, McKinnell JA, et al. Recurrent urinary tract infections among women: comparative effectiveness of 5 prevention and management strategies using a Markov chain Monte Carlo model. Clin Infect Dis. 2014;58(2):147–160.
- Berry RE, Klumpp DJ, Schaeffer AJ. Urothelial cultures support intracellular bacterial community formation by uropathogenic Escherichia coli. Infect Immun. 2009;77(7):2762–2772.
- Tamadonfar KO, Omattage NS, Spaulding CN, et al. Reaching the end of the line: urinary tract infections. Microbiol Spectr. 2019;7(3):BAI-0014-2019.
- Mazzariol A, Bazaj A, Cornaglia G. Multi-drug-resistant gram-negative bacteria causing urinary tract infections: a review. J Chemother. 2017;29(sup1):2–9.
- Kyriakides R, Jones P, Somani BK. Role of D-mannose in the prevention of recurrent urinary tract infections: evidence from a systematic review of the literature. Eur Urol Focus. 2021;7(5):1166–1169.
- Lenger SM, Bradley MS, Thomas DA, et al. D-mannose vs other agents for recurrent urinary tract infection prevention in adult women: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223(2):265.e1–265.e13.
- De Nunzio C, Bartoletti R, Tubaro A, et al. Role of D-mannose in the prevention of recurrent uncomplicated cystitis: state of the art and future perspectives. Antibiotics (Basel). 2021;10(4):373.
- Alton G, Hasilik M, Niehues R, et al. Direct utilization of mannose for mammalian glycoprotein biosynthesis. Glycobiology. 1998;8(3):285–295.
- Brument S, Sivignon A, Dumych TI, et al. Thiazolylaminomannosides as potent antiadhesives of type 1 piliated Escherichia coli isolated from Crohn's disease patients. J Med Chem. 2013;56(13):5395–5406.
- Wood FC, Jr, Cahill GF, Jr. Mannose utilization in man. J Clin Invest. 1963;42:1300–1312.
- Alton G, Kjaergaard S, Etchison JR, et al. Oral ingestion of mannose elevates blood mannose levels: a first step toward a potential therapy for carbohydrate-deficient glycoprotein syndrome type I. Biochem Mol Med. 1997;60(2):127–133.
- Gulsun S, Oguzoglu N, Inan A, et al. The virulence factors and antibiotic sensitivities of Escherichia coli isolated from recurrent urinary tract infections. Saudi Med J. 2005;26(11):1755–1758.
- Caretto M, Giannini A, Russo E, et al. Preventing urinary tract infections after menopause without antibiotics. Maturitas. 2017;99:43–46.
- Kranjčec B, Papeš D, Altarac S. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: a randomized clinical trial. World J Urol. 2014;32(1):79–84.