Abstract
The phase III MPACT trial demonstrated the superiority of gemcitabine plus nab-paclitaxel (NABGEM) versus gemcitabine alone in previously untreated patients with metastatic pancreatic cancer (mPC). The aim of this study was to evaluate the responses in terms of efficacy and safety in patients treated with more than 6 cycles of chemotherapy. From January 2015 to December 2018, patients with mPC receiving first-line treatment with NABGEM were included in a multicentre retrospective observational study. Exploratory analyses of efficacy and safety were performed. The cohort included 153 patients with performance status of 1. The median overall survival and progression-free survival were 20 months (hazard ratio [HR] 0.28, 95% confidence interval [CI]: 0.17–0.44) and 10 months (HR 0.24 95% CI: 0.16–0.38) respectively, in patients who received >6 cycles compared to 9 and 5 months in those treated with ≤6 cycles (p < 0.001). The disease control rate was 100% versus 56% in patients receiving >6 and ≤6 cycles, respectively. No progression of disease was recorded in patients who received >6 cycles. Grade 1 neuropathy and grade 3 neutropenia were more frequent in patients treated with >6 cycles compared to patients receiving ≤6 cycles (p = 0.01; p = 0.03, respectively). Dose reduction was necessary for 70.1% and 53.4% of patients treated with >6 or ≤6 cycles, whereas treatment interruption occurred in 37.1% and 21.6%, respectively. Our results confirmed the efficacy and safety of NABGEM in untreated mPC. In particular, we highlighted significant clinical efficacy in patients who received >6 cycles of chemotherapy compared to those who received ≤6 cycles, with manageable toxicity profile.
Introduction
Pancreatic cancer (PC) is still considered one of the most lethal diseases in western countries [Citation1]. Most pancreatic tumours arise from the ductal epithelium with adenocarcinoma histology [Citation2]. Unfortunately, only 20% of cases are eligible for surgical resection [Citation3], with the vast majority of patients with metastatic disease at the time of diagnosis and consequently treated with systemic therapy. It has been reported that the five-year survival rate is less than 8% for metastatic pancreatic cancer (mPC) [Citation4]. The optimal regimen is not well established, albeit the gemcitabine plus nab-paclitaxel (NABGEM) is considered one of the main standard therapies for these patients. However, chemotherapy with fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) is administered in fit patients with a good performance status (PS) while gemcitabine monotherapy is used in patients with a poor PS [Citation5]. NABGEM has been introduced on the heels of a large randomized phase III trial (MPACT) in 2013 [Citation6]. In this study, a total of 861 patients were randomly assigned to receive either NABGEM (n = 431) or gemcitabine alone (n = 430). The median overall survival (OS) was 8.5 months in the NABGEM group (hazard ratio [HR] for death, 0.72). The survival rate was 35% in the NABGEM group at 1 year, and 9% versus 4% at 2 years. The median progression-free survival (PFS) was 5.5 months in the NABGEM group (HR for disease progression or death = 0.69). The most common adverse events (AEs) treatment-related were neutropenia, fatigue and neuropathy. In the MPACT trial, the median duration of treatment was 3.9 months (approximately four cycles of treatment); however, little data is available on the efficacy and safety of an extended time of therapy with NABGEM. Normally, according to local clinical guidelines, the treatment continues for 6–7 cycles in the presence of stable disease (SD) or objective response (OR), although some patients might continue the NABGEM regimen with additional chemotherapy cycles. Therefore, the aim of this study was to evaluate the responses in terms of efficacy and safety of patients with metastatic pancreatic cancer (mPC) treated with more than six cycles of NABGEM regimen.
Patients and methods
Study population
Patients matching the inclusion criteria were extrapolated from a retrospective registry (‘NAPA’) of patients administered with the NABGEM regimen from January 2015 to December 2018 in four Italian centres [Citation7]. The inclusion criteria were age ≥18 years, histopathological confirmed metastatic pancreatic cancer, Eastern Cooperative Oncology Group performance status (ECOG-PS) between 0 and 1 as well as adequate haematological, hepatic and renal functions. Exclusion criteria were localized disease, previous adjuvant chemotherapy and pancreatic cancer with different histology of adenocarcinoma. The treatment consisted of the administration of nab-paclitaxel 125 mg/m2, followed by intravenous gemcitabine 1000 mg/m2 administered on days 1, 8 and 15 every 4 weeks. Granulocyte-colony-stimulating factor (G-CSF) and erythropoietin (EPO) were allowed. Treatment was continued until disease progression or unacceptable toxicity. Tumour response evaluation was performed every 3 months by chest-abdomen computed tomography (CT) according to the Response Evaluation Criteria in Solid Tumour (RECIST) version 1.1 [Citation8]. Disease progression was assessed as either radiological or clinical progression. All patients signed informed consent before treatment onset and the study was approved by the Local Institutional Review Board for clinical experimentation of Tuscany (Italy) – ‘Area Vasta Centro’ section, with the number: 14565_oss.
Statistical analysis
The aim of this study was the identification of potential differences between the outcome and safety of patients according to the number of ≤6 and >6 cycles of the NABGEM regimen. In terms of statistical analysis, a preliminary data exploration analysis (DEA) was performed. Numerical variables were reported as median and range values, whereas qualitative data were reported as frequency and consequently summarised into contingency tables. Overall survival was defined as the time (months) from the diagnosis of advanced disease to either any cause of death or the last follow-up visit date. Progression-free survival was defined as the time from the radiotherapy onset at the cancer centre to the date of disease progression, as reported by the clinician. Disease Control Rate (DCR) was defined as the percentage of patients who have achieved complete response (CR), partial response (PR) and stable disease (SD). Time-dependent variables were calculated according to the Kaplan–Meier method. Delta time was defined as the difference between the OS and PFS. Cox proportional hazard regression linear model was used to compare the hazard ratio with a 95% confidence interval (95% CI). For the entire statistical analysis, the significance level was established at p < 0.05. All data were analysed with STATA software (StataCorp LP, Texas).
Results
Study population
From January 2015 to December 2018, a total number of 153 patients diagnosed with mPC and treated with NABGEM were recruited for our analyses on treatment safety and efficacy. The median age was 67 years (ranging from 50 to 84) with males the prevalent gender (57.5%). ECOG-PS = 1 was observed in approximately half of the patients (51.2%) with no patients reporting an ECOG-PS greater than 1. The median baseline CA19.9 was 547 U/ml (range 0.8–700,000 U/ml). Nearly 40% of patients (n = 61) reported three or more metastatic different sites, with the liver being the most frequent metastatic site. In terms of treatment, radiotherapy, surgery and the biliary stent were performed in 14 (9.9%), 37 (24.2%) and 46 (30.1%) patients, respectively. Fifty-nine (38.6%) patients reported symptoms correlated with the disease or cancer-related pain. No data on patients’ comorbidities are available. Differences in clinical features between patients who received <6 versus >6 cycles of therapy were not statistically significant. Patients’ characteristics are reported in detail in .
Table 1. Patient characteristics.
Treatment
During the study, patients were administered with a starting dose of nab-paclitaxel 125 mg/m2 plus gemcitabine 1,000 mg/m2 for a median of 5 cycles (range 1–17). Eighty-eight (57.5%) patients required dose reduction with a significant discrepancy in terms of numbers between the 62 patients (53.4%) who received six of fewer chemotherapy cycles, and the 26 patients (70.1%) who received more than 6 cycles. Treatment delay and interruption occurred in 51 (33.5%) patients, mostly due to asthenia, anaemia and neutropenia. Twenty-five patients (16.4%) required G-CSF prophylaxis: 19 (16.5%) from the groups administered with ≤6 cycles and 6 (16.2%) from the group administered with >6 cycles. Treatment data are summarised in .
Table 2. Dose reduction, treatment delay, treatment interruption and G-CSF prophylaxis according to cycles of therapy.
Efficacy
Efficacy data are summarised in . Overall, the median follow-up consisted of 10 months for all patients with a median PFS and OS of 6 and 11 months, respectively. Surprisingly, although the percentages for stable disease (SD) were similar among the two groups, a partial response (PR) was reported for the vast majority of those treated with >6 cycles (73%) and only approximately 30% of those treated with ≤6 cycles. Notably, in almost all patients (88.8%) partial response was observed within the 6th cycle of therapy. Additionally, 102 out of 153 patients (66.7%) observed a promising and significant DCR which was predominant in those treated with >6 cycles (100%) when compared to those treated with ≤6 cycles (56%). Of note, progression of disease (PD) was observed in 42 (36.2%) patients administered with ≤6 cycles whereas no patients treated with >6 cycles reported PD. Treatment response was not available for 9 out of 153 (5.9%) patients. In terms of PFS, a substantial difference was observed between the two groups under investigation: 10 months for those treated with >6 cycles, whereas only 5 months for those treated with ≤6 cycles (HR 0.24, 95%CI: 0.16–0.38, p < 0.001). Seemingly, a substantial difference was observed for the OS: patients treated with >6 cycles achieved an encouraging OS of 20 months (16–22 months) while only 9 months in those treated with ≤6 cycles (8–10 months) (HR 0.28, 95% CI: 0.17–0.44, p < 0.001) (). Indeed, the difference in months (delta) between OS and PFS was 4 versus 10 for patients treated with ≤6 cycles and >6 cycles, respectively.
Figure 1. Kaplan-Meier curves of progression-free survival and overall survival of patients according to the number of cycles (>6 or ≤6 cycles).
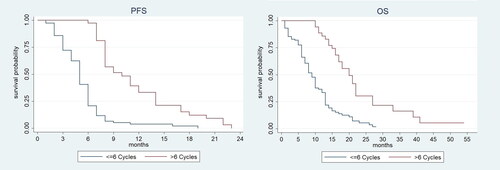
Table 3. Best response, PFS and OS according to cycles of therapy.
Safety
In agreement with the number of treatment cycles, the most relevant haematological and non-haematological AEs are summarised in and . Overall, treatment was well tolerated with the vast majority of non-haematological AEs reported as grade 1 or 2 (neuropathy and diarrhoea), and only grade 3 asthenia events were reported. No statistically significant difference was observed between the two groups except for G1 neuropathy which was more frequent in patients who received more than 6 cycles compared with those who received ≤6 cycles (p = 0.01). Concerning haematological AEs, patients experienced grade 3 or 4 anaemia and thrombocytopenia with no significant differences in terms of prevalence between the two groups. G3 neutropenia was more frequent in patients who received >6 cycles (27%) than in those who received ≤6 cycles (12.1%) (p = 0.03).
Figure 2. Most relevant haematological and non-haematological adverse events for all patients and patient subgroups according to the number of cycles (≤6 cycles or >6).
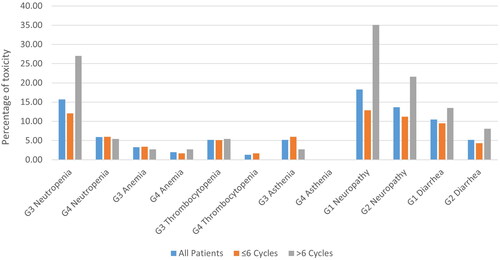
Table 4. Summary of most important adverse events according to cycles of therapy.
Discussion
Pancreatic cancer, albeit not one of the most common cancers, is certainly one of the most malignant, with less than 8% of the population surviving longer than 5 years and no standard second-line treatment following first-line failure. Our retrospective analysis, in agreement with the results from the randomized phase III MPACT trial [Citation6], seems to corroborate the data on treatment benefits for patients with mPC administered with NABGEM as the first-line setting. Overall, our data suggest that OS, PFS and DCR are increased in those administered with more than 6 cycles of the NABGEM regimen with no progression disease reported. Also, we could confirm that the NABGEM combination regimen was well-tolerated and effective in those administered with >6 cycles when compared to those administered with ≤6 cycles. However, we could not carry out a direct comparison between our real-world experience analyses and the pivotal trial, probably due to the small sample size and discrepancies with inclusion/exclusion criteria included. Indeed, once performed the aforementioned comparison, we observed some differences in terms of patient characteristics that we need to report. First, patients from MPACT trial were younger, with nearly 60% of patients less than 65 years old with a Karnofsky Performance Status of 100–90% [Citation6]. On the contrary, our cohort included older patients (1 out of three was aged 70 or more) than those from the MPACT study and approximately one out of two reported an ECOG-PS of 0. Secondly, a third of patients within our cohort were affected by concomitant comorbidities. In the last few years, several real-world studies in patients with locally advanced and metastatic patients treated with first-line NABGEM have been performed. Controversial and debatable results in terms of efficacy in pancreatic cancer patients with locally advanced and/or metastatic disease have been observed. For instance, Blomstrand et al. have observed a better prognosis trend in 22 patients diagnosed with locally advanced disease reporting a PFS and OS of 6.8 and 17.1 months, respectively; in contrast, patients with metastatic disease reported a PFS and OS of 4.5 and 9.4 months [Citation9]. Wang et al. reported a 10.5 months OS for the total cohort of patients and similar OS numbers for those with metastatic disease [Citation10]. In an additional multicentre retrospective analysis that recruited elderly patients, those with locally advanced disease observed PFS and OS of 12.1 and 21.8 months, respectively, whereas those with metastatic disease reported PF and OS of 5.9 and 13.3 months, respectively [Citation11].
Other studies have investigated the use of NABGEM in clinical practice for pancreatic cancer patients with either metastatic or locally advanced disease. While Montes et al. observed a PFS and OS of 9 and 15 months, respectively, in a cohort of 39 patients [Citation12], Papneja and colleagues reported a shorter PFS and OS of 4 and 9 months, respectively [Citation13]. Seemingly, Lo Re et al. registered a median PFS of 5.5 and an OS of 12.1 months among 37 patients [Citation14], while De Vita et al. observed a median PFS and OS of 6.7 and 10 months among 41 patients [Citation15].
Recently, a phase II study in patients with locally advanced pancreatic cancer treated with NABGEM as first-line was performed. The median PFS and OS were 11 and 21.2 months, respectively, with an ORR of 62.5%. Surgical resection was feasible in 4 patients; of these, 3 achieved complete resection [Citation16]. This study confirmed the result of a previous phase II trial, LAPACT [Citation17], proposing NABGEM as an option for first-line chemotherapy in patients with locally advanced pancreatic cancer, reporting significant tumour shrinkage, good toxicity profile, and allowing conversion to surgical resection in a subset of patients.
In agreement with the MPACT trial, we recruited only chemo-naïve patients with evident and clinically confirmed metastatic disease. Moreover, differently from the aforementioned studies, we assessed a greater number of patients (n = 153) from several institutions, confirming the evidence of a more efficacy of NABGEM for those patients treated with >6 cycles.
In our analyses, we have demonstrated that the PFS and OS were increased in patients administered with >6 cycles, and decreased in patients administered with ≤6 cycles. More in detail, we reported a median OS (HR 0.28, 95% CI: 0.17–0.44; p < 0.001) and PFS (HR 0.24, 95% CI: 0.16–0.38; p < 0.001) of 20 and 10 months, respectively, with a DCR of 100% in patients received >6 cycles whereas a median OS and PFS of 9 and 5 months, respectively, with a DCR of 56%, in those who received ≤6 cycles were recorded.
As far as safety is concerned, although dose reduction was required for 70% of patients administered with >6 cycles when compared to those treated with ≤6 cycles (53%), only 20% of patients within the >6 cycles group required treatment interruption and approximately 32% of patients from ≤6 cycles group. Non-haematological AEs such as neuropathy and diarrhoea were commonly reported as grade 1 or 2, except for asthenia, which was reported as grade 3 or 4 for both groups, although observed in a very limited number of patients. Haematological toxicity, primarily neutropenia, anaemia and thrombocytopenia, was severe (grade 3 and 4) with similar percentages within two groups and consistent with the data reported in the previous studies [Citation10,Citation11,Citation14,Citation15]. G1 neuropathy and G3 neutropenia were more frequent in patients who received >6 cycles than in those who received ≤6 cycles (p = 0.01 and p = 0.03). Overall, the regimen was well tolerated, in agreement with previously reported studies.
Recently, studies that evaluated different NABGEM administration regimens were performed. In Kokkali et al. study, a modified regimen with gemcitabine 1000 mg/m2 and nab-paclitaxel 125 mg/m2 administered every two weeks was associated with a median OS of 10 months and less severe (grade 3 or 4) neutropenia (19%) and peripheral neuropathy (2%) compared to the original regimen [Citation18]. However, a less favourable toxicity profile was noted for this biweekly regimen compared with standard nab-paclitaxel plus gemcitabine given weekly in a combined phase I/II trial conducted in patients with advanced pancreatic cancer and an ECOG PS of 2 [Citation19]. Moreover, a prospective randomised trial explored alternative scheduling of NABGEM (gemcitabine administered on days 2, 9 and 16, starting 24 h after nab-paclitaxel) compared to standard administration, showing a significantly improved PFS for the experimental arm without a corresponding benefit in terms of OS [Citation20]. However, these data represent only hypotheses that need furthermore in-depth evidence.
In our analyses, a few limitations must be reported. First and foremost, the retrospective nature of our data. Secondly, only confirmed metastatic patients were included. On the other hand, the large number of patients recruited (n = 153) substantially embodies the main study strength.
Conclusion
Over the last few years, clinical progress has been made regarding first and second-line treatments in locally advanced and mPC. The best treatment option for metastatic pancreatic cancer patients is primarily based on careful selection of patients, comorbidities and performance status. Our study corroborates the data on the efficacy and safety of gemcitabine plus nab-paclitaxel as a first-line regimen, in a large group of patients with exclusive metastatic pancreatic cancer. In particular, we observed a very manageable toxicity profile in patients who received more than 6 cycles of therapy, alongside good clinical outcomes. Considering the current few options available in the second-line setting, it is necessary to better identify the number of cycles of therapy for these patients. This study can be useful in providing clinicians with some indications. However, further confirmation from larger, randomized studies is needed.
Ethical statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All patients signed informed consent before treatment onset and the study was approved by the Local Institutional Review Board for clinical experimentation of Tuscany (Italy) – ‘Area Vasta Centro’ section, with the number: 14565_oss.
Data availability statement
The data used to support the findings of this study are available from the corresponding author upon request.
Disclosure statement
The authors know of no conflicts of interest associated with this publication.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249; [cited 2022 Apr 11]. Available from https://pubmed.ncbi.nlm.nih.gov/33538338/
- Haeberle L, Esposito I. Pathology of pancreatic cancer. Pathology of pancreatic cancer. Transl Gastroenterol Hepatol. 2019;4:50.
- Treatment for potentially resectable exocrine pancreatic cancer. Last accessed Feb 2023 . https://www.uptodate.com/contents/treatment-for-potentially-resectable-exocrine-pancreatic-cancer
- Bilimoria KY, Bentrem DJ, Ko CY, et al. Validation of the 6th edition AJCC pancreatic cancer staging system: report from the national cancer database. Cancer. 2007;110(4):738–744.
- Conroy T, Desseigne F, Ychou M, PRODIGE Intergroup, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825.
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-Paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703.
- Catalano M, Roviello G, Conca R, et al. Clinical outcomes and safety of patients treated with NAb-Paclitaxel Plus gemcitabine in metastatic pancreatic cancer: the NAPA study. Curr Cancer Drug Targets. 2020;20(11):887–895.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247.
- Blomstrand H, Scheibling U, Bratthäll C, et al. Real world evidence on gemcitabine and nab-paclitaxel combination chemotherapy in advanced pancreatic cancer. BMC Cancer. 2019;19(1):40.
- Wang Y, Camateros P, Cheung WY. A Real-World comparison of FOLFIRINOX, gemcitabine Plus nab-Paclitaxel, and gemcitabine in advanced pancreatic cancers. J Gastrointest Canc. 2019;50(1):62–68.
- Kobayashi S, Ueno M, Ikeda M, et al. A multicenter retrospective study of gemcitabine Plus Nab-Paclitaxel for elderly patients with advanced pancreatic cancer. Pancreas. 2020;49(2):187–192.
- Montes AF, Villarroel PG, Ayerbes M, et al. Prognostic and predictive markers of response to treatment in patients with locally advanced unresectable and metastatic pancreatic adenocarcinoma treated with gemcitabine/nab-paclitaxel: results of a retrospective analysis. J Cancer Res Ther. 2017;13(2):240–245.
- Papneja N, Zaidi A, Chalchal H, et al. Comparisons of outcomes of real-world patients with advanced pancreatic cancer treated with FOLFIRINOX versus gemcitabine and nab-paclitaxel: a population-based cohort study. Pancreas. 2019;48(7):920–926.
- Lo Re G, Santeufemia DA, Foltran L, et al. Prognostic factors of survival in patients treated with nab-paclitaxel plus gemcitabine regimen for advanced or metastatic pancreatic cancer: a single institutional experience. Oncotarget. 2015;6(10):8255–8260.
- De Vita F, Ventriglia J, Febbraro A, et al. NAB-paclitaxel and gemcitabine in metastatic pancreatic ductal adenocarcinoma (PDAC): from clinical trials to clinical practice. BMC Cancer. 2016;16(1):709.
- Fukahori M, Miwa K, Murotani K, et al. A phase II study of gemcitabine plus nab-paclitaxel as first-line therapy for locally advanced pancreatic cancer. Medicine (Baltimore). 2021;100(20):e26052.
- Philip PA, Lacy J, Portales F, et al. Nab-paclitaxel plus gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): a multicentre, open-label phase 2 study. Lancet Gastroenterol Hepatol. 2020;5(3):285–294.
- Kokkali S. Biweekly gemcitabine/Nab-Paclitaxel as first-line treatment for advanced pancreatic cancer. In Vivo (Brooklyn). 2018;32(3):653–657.
- Macarulla T, Pazo-Cid R, Guillén-Ponce C, et al. Phase I/II trial to evaluate the efficacy and safety of nanoparticle albumin-bound paclitaxel in combination with gemcitabine in patients with pancreatic cancer and an ECOG performance status of 2. J Clin Oncol. 2019;37(3):230–238.
- Corrie PG, Qian W, Basu B, et al. Scheduling nab-paclitaxel combined with gemcitabine as first-line treatment for metastatic pancreatic adenocarcinoma. Br J Cancer. 2020;122(12):1760–1768.
