Abstract
Osimertinib, a third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI), has been recommended as a first-line treatment of EGFR-positive non-small cell lung cancer (NSCLC). Skin rash is one of the most common side effects of osimertinib, and can have an impact on patients’ quality of life and follow-up. However, there are few reports on the safety and efficacy of switching therapy with osimertinib and the other three generations of TKIs. In this paper, we present a case of NSCLC with an EGFR exon 19 deletion (19del) and MET gene amplification who developed a severe rash after 2 months of treatment with osimertinib that did not recur after switching to replacement therapy with aumonertinib. Our findings indicate that aumonertinib is as effective as osimertinib in treating EGFR19del, while also exhibiting a lower occurrence of adverse skin reactions. This may result in an improved quality of life for patients.
Introduction
Lung cancer is one of the most malignant tumors with the highest morbidity and mortality worldwide, with NSCLC accounting for approximately 85% [Citation1]. Mutation in the EGFR gene is a common driver of NSCLC progression.
Targeted therapy is currently recognized as one of the most effective treatment approaches in the field. In comparison to conventional chemotherapy, the first and second-generation tyrosine kinase inhibitors (TKIs) of epidermal growth factor receptors (EGFR-TKIs) have demonstrated remarkable efficacy in patients with non-small cell lung cancer (NSCLC) harboring positive EGFR mutations [Citation2–4]. To address this issue, third-generation EGFR TKIs, such as osimertinib and aumonertinib, have been developed and recommended as first-line treatment options [Citation5]. These novel agents exhibit potent inhibitory effects on both the T790M mutation and EGFR-sensitized mutations. Despite their efficacy, it is important to acknowledge that osimertinib treatment has been associated with several side effects, including rash and diarrhea, which can substantially impact patient follow-up [Citation6,Citation7]. Interestingly, there have been limited reports on the successful switch to alternative drugs following the occurrence of severe rash induced by osimertinib. In this context, we present a case report of a patient diagnosed with EGFR-positive lung adenocarcinoma who initially received osimertinib and subsequently developed a severe rash. However, the patient was switched to aumonertinib, which was better tolerated and resulted in improved prognosis.
Case presentation
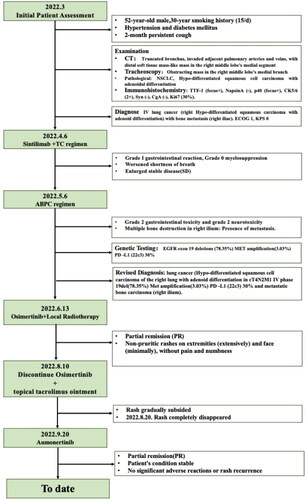
In March 2022, a 52-year-old male patient was hospitalized for a persistent cough that lasted for two months. The patient had a 30-year smoking history, consuming 15 cigarettes daily. Additionally, the patient had a medical history of hypertension and diabetes mellitus. No other specific comorbidities were reported. A comprehensive thoracic and abdominal CT scan () revealed the amputation of the medial segmental bronchus in the right middle lobe, along with the presence of a sizable soft tissue mass invading the adjacent pulmonary artery and vein. Additionally, there was evidence of enlarged mediastinal lymph nodes and destruction of the right ilium. Tracheoscopy revealed a mass partially obstructing the medial branch of the right middle lobe. The pathological findings indicated non-small cell lung cancer, Hypo-differentiated squamous cell carcinoma with adenoid differentiation. Immunohistochemical results were as follows: TTF-1 (focus +), NapsinA (–), p40 (focus+), CK5/6 (2 +), Syn (–), CgA (–), and Ki67 (30%). A whole-body bone scan and skull MRI did not reveal any evident metastatic lesions. An abdominal CT scan () demonstrated osteolytic destruction of the right iliac bone. The patient was diagnosed with lung cancer (right lung Hypo-differentiated squamous cell carcinoma with adenoid differentiation cT4N2M1 IV stage) with bone metastases in the right ilium. The patient’s condition was assessed as ECOG 1 NRS 0. However, the patient and his family declined genetic testing.
Figure 1. The clinical course according to CT scan findings: (A) (B) March 2022: a truncation observed in the medial segmental bronchus of the right middle lobe, accompanied by a distal mass-like soft tissue lesion; (C) March 2022: Osteolysis of the right iliac bone; (D) (E) April 2022: the constriction in the medial segmental bronchus of the right middle lobe has intensified. Therapeutic assessments indicate a progressive SD; (F) June 2022 chest CT: a reduction in the size of the pulmonary focus in the lung; (G): August 2022 chest CT, two months after starting osimertinib, showed a reduced right middle lobe tumor, evaluated as PR; (H) November 2022: PR observed two months after initiating aumornertinib; (I) PR on aumornertinib in february 2023.
On 6 April 2023, two weeks after the diagnosis, we initiated the PD-1 combined chemotherapy regimen. (sintilimab 200 mg intravenous drip d1+ albumin-paclitaxel 400 mg intravenous drip d1+ carboplatin 400 mg intravenous drip d1 + zoledronic acid 4 mg intravenous drip d1). After one month of treatment, the patient experienced a grade I gastrointestinal reaction and grade 0 myelosuppression. Furthermore, the patient reported an increased severity of shortness of breath. A chest CT scan on 5 May 2022 (), showed significant stenosis of the medial segmental bronchus in the right middle lobe, suggesting progressive Stable Disease (SD) compared to previous images. Given these findings, we decided to adjust the treatment approach. On 6 May 2022, the ABPC regimen was administered(Sintilimab 200 mg intravenous drip d1; Bevacizumab 900 mg intravenous drip d1; Albumin-Paclitaxel 400 mg intravenous drip d1; Carboplatin 400 mg intravenous drip d1; After one month of treatment, the main adverse events (AEs) were grade 2 gastrointestinal toxicity and grade 2 neurotoxicity. Additionally, upon re-examination of the chest and abdomen CT, a reduction in the size of the pulmonary focus in the lung was observed compared to the previous scan (). Multiple areas of bone damage in the right ilium were detected, indicative of metastasis. Subsequently, the patient was referred to the oncology department for further evaluation and treatment where genetic testing was conducted. Genetic testing indicated an EGFR 19 exon deletion (78.35%) and MET amplification (3.03%). Additionally, the expression of PD-L1 was determined to be 30% based on Tumor Proportion Score (TPS) analysis. Consequently, the modified diagnosis was lung cancer (Hypo-differentiated squamous cell carcinoma of the right lung with adenoid differentiation in cT4N2M1 IV phase 19del (78.35%) Met amplification (3.03%) PD-L1 (22c3) 30% and metastatic bone carcinoma (right ilium). No abnormalities were detected on cranial MRI, while whole-body bone scintigraphy revealed multiple bone metastases in the right iliac bone, left sixth rib, and vertebral body.
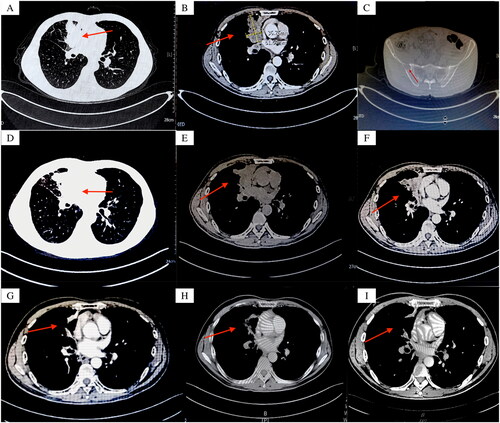
On 13 June 2022, based on genetic testing results, the patient received targeted therapy with osimertinib (osimertinib 80 mg once a day PO), local radiotherapy to a vertebral body, and a low-dose corticosteroid to relieve edema. The patient was evaluated as partial response (PR) on 10 August 2022, two months after initiating osimertinib treatment. Chest CT imaging () showed a reduction in the size of the tumor located in the middle lobe of the right lung. However, multiple rashes were observed on the patient’s extremities, with a small amount also present on the face (). Notably, these rashes were characterized by the absence of itching, pain, or numbness. A multidisciplinary treatment discussion (MDT) was organized involving dermatologists to investigate the underlying causes by evaluating the specific characteristics of the rash and determining the optimal course of action. Taking into account the rash’s characteristics and other relevant factors, it was concluded that the rash was due to targeted therapy. As a result, the decision was made to discontinue osimertinib and initiate topical tacrolimus ointment application on 10 August 2022.
Figure 2. (A) (B) Two months after osimertinib treatment: multiple rashes in the extremities, no itching, pain, and hypoesthesia; (C) (D) (E) (F) Rash gradually subsided.
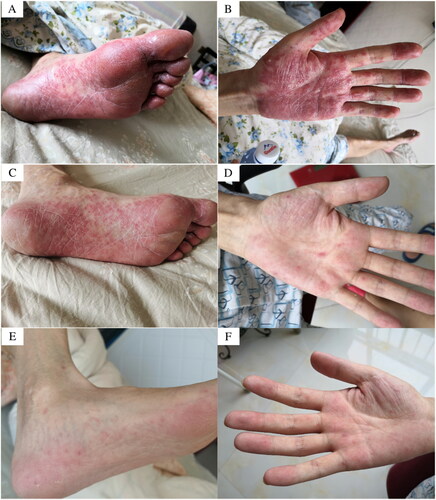
Over 10 days, the patient’s rash noticeably subsided (–F). Considering the limited benefits of immunotherapy and systemic chemotherapy, targeted therapy has demonstrated effectiveness in the current clinical scenario. In comparing the AENEAS study on aumonertinib with the FLAURA study on osimertinib, aumonertinib showed a lower incidence of rash. Therefore, on 20 September 2022, the patient was administered aumonertinib (aumonertinib 110 mg once a day) as targeted therapy. Two months later, the patient’s condition stabilized, and achieving a partial remission (PR) response (). Importantly, during this time, no significant adverse reactions or rash recurrence were observed
Discussion
In the past decade, there have been significant improvements in the treatment of non-small cell lung cancer (NSCLC). Several antineoplastic modalities are available, including surgery, chemotherapy, radiotherapy, immunotherapy, targeted therapy, and others.
For patients with advanced NSCLC, both targeted therapy and immunotherapy are of paramount importance. The incorporation of these therapeutic modalities has markedly enhanced the survival outcomes in patients with advanced NSCLC [Citation8]. Nevertheless, treatment can be hindered by the occurrence of serious adverse effects, leading to a decline in treatment adherence.
Currently, PD-1 and PD-L1 inhibitors are the primary immunotherapeutics in use. Common adverse reactions predominantly impact the skin (e.g. rash and itching), digestive tract (e.g. abdominal pain and diarrhea), liver (e.g. elevated transaminases and increased bilirubin), and hormonal balance (e.g. thyroid dysfunction, pituitary dysfunction), among others [Citation8–10]. Third-generation cohort models are being used for targeted therapeutics that specifically target the EGFR pathway. Compared with first- and second-generation EGFR tyrosine kinase inhibitors (TKIs), the adverse drug reactions of third-generation EGFR TKIs are significantly lower. However, common side effects such as diarrhea and rash may still occur [Citation11].
This patient experienced a severe skin rash following immunotherapy and targeted therapy. Since both treatments can lead to rashes as a side effect, it is essential to determine the specific cause of the rash. Specifically, around 30% to 40% of patients develop a rash 2 to 5 weeks after initiating immunotherapy [Citation12]. Most immunotherapy-related rashes are self-limiting, primarily occurring at grade 1 and 2 levels, and tend to appear on the trunk and limbs rather than the face. Itching or burning sensations often accompany these rashes; Conversely, rashes resulting from targeted therapy are dose-dependent and emerge within two weeks or 1–2 months after treatment. These rashes are typically accompanied by itching or tenderness and tend to occur more frequently in areas with a high density of sebaceous glands [Citation13].
In addition, steroids were primarily used to manage the severe rash caused by immunotherapy, gradually reducing its intensity until discontinuation [Citation14]. However, the rash resulting from targeted therapy was alleviated with the topical application of antibiotics, such as benzoic acid co, erythromycin ointment, etc. [Citation15]. The patient developed a non-pruritic rash accompanied by pain and hypoesthesia two months after initiating oral targeted drug treatment. The rash predominantly appeared in areas with a high concentration of sebaceous glands, such as the extremities (extensively) and the face (minimally). Considering the patient’s rash characteristics, medical history, concurrent vertebral radiotherapy, and ongoing steroid tapering during oral targeted drug therapy, as well as the inconsistency of the rash onset time caused by the interaction of two drugs, Osimertinib and Sintilimab, we infer that the rash is mainly caused by the third-generation targeted drug EGFR-TKI Osimertinib. The author conducted a literature search on rashes caused by immunotherapy and targeted therapy, summarizing their characteristics and treatment () [Citation16–25].
Table 1. Characteristics of targeted therapy and immunotherapy for skin.
Various targeted therapies exhibit a diverse spectrum of adverse effects. Osimertinib, the first third-generation EGFR-TKI to receive FDA approval for marketing, is indicated for the treatment of EGFR T790M-positive NSCLC patients who develop secondary drug resistance after prior first- or second-generation EGFR-TKI therapy. In the AURA3 trial [Citation26]: the osimertinib group exhibited a significantly longer median progression-free survival (mPFS) compared to the chemotherapy group (10.1 months vs. 4.4 months; hazard ratio [HR] = 0.30, 95% CI: 0.23–0.41, p < 0.0001). The FLAURA study further confirmed osimertinib’s significant prognostic benefits compared to standard EGFR-TKI treatment in the control group. Regarding safety, the most frequently reported treatment-related adverse events (incidence of ≥20%) were rash (54%), diarrhea (49%), dry skin (33%), onychomycosis (33%), and stomatitis (25%) [Citation27].
Aumonertinib, the first domestically developed third-generation EGFR-TKI in China, replaces the methyl group on the indole nitrogen with a cyclopropyl group, following the structural framework of the osimertinib molecule [Citation28]. This distinct structural characteristic enables it to display favorable efficacy and safety in advanced NSCLC patients with EGFR T790M mutation who have experienced disease progression following EGFR-TKI therapy, as evidenced in the APOLLO study [Citation29]; In the AENEAS study: the aumonertinib group exhibited significantly lower rates of rash and diarrhea compared to the gefitinib group (23.4% vs. 41.4%, 16.4% vs. 35.8%) [Citation30]. The primary adverse effect of aumonertinib was the increase in creatine kinase, and there was no report of severe rash caused by aumonertinib [Citation31]. Based on the results mentioned above, aumonertinib was approved in China for the treatment of non-small cell lung cancer (NSCLC) with an EGFR T790M mutation [Citation32]. In this case, after two months of Osimertinib treatment, a chest CT scan in August 2022 demonstrated a reduction in lung lesions compared to previous measurements, confirming the effectiveness of osimertinib. Given the limited efficacy of previous immunotherapy and systemic chemotherapy, targeted therapy proved to be an effective approach. It is noteworthy that aumonertinib exhibited a significantly lower incidence of skin rash compared to the outcomes observed in the AURA2 trial with osimertinib (13.9% vs. 40%) [Citation6,Citation29,Citation30]. In light of these findings, the decision was made to switch from osimertinib to aumonertinib while continuing targeted therapy to optimize the potential benefits. Subsequent regular follow-up assessments revealed a stable condition in the later phase, with no occurrence of skin rashes or significant adverse reactions.
Taking into account drug efficacy, safety, and previously successful treatments with EGFR-TKI alternative drugs after severe osimertinib side effects: Wu et al. [Citation33] successfully treated severe interstitial pneumonia caused by osimertinib using aumonertinib; Zhang et al. [Citation34] reported successful treatment of osimertinib-induced cardiotoxicity using aumonertinib; Ding et al. [Citation35] also achieved success with aumonertinib replacement therapy following failed osimertinib treatment. Consequently, aumonertinib was chosen as the replacement for osimertinib in this specific case to continue targeted therapy. Following the dressing change, the patient’s physical condition remained stable, and no adverse effects such as rash were observed.
Limitation
The Asian and Chinese guidelines for lung adenocarcinoma recommend simultaneous testing of PD-L1 expression and driver mutations (e.g. EGFR, ALK, ROS1) for all patients. It’s also advised to await these test results before initiating first-line treatments [Citation16,Citation36–38]. In this particular case, the absence of pre-treatment genetic testing led to suboptimal therapy and subsequent disease progression. This underscores the critical role of exhaustive biomarker testing for every non-small cell lung cancer patient, irrespective of clinical presentations. Such testing facilitates the identification of tumor-specific mutations, enabling tailored therapeutic approaches that potentially enhance patient outcomes.
Conclusions
In conclusion, we presented a case of EGFR 19 exon deletion NSCLC. While osimertinib’s targeted therapy was an effective alternative to immunotherapy, it resulted in a signifucant skin rash. Switching to aumonertinib proved benefical for the patient. Additionally, we provide an overview of the rash characteristics associated with both immunotherapy and targeted therapy, along with a comparison of the efficacy and side effects of osimertinib and aumonertinib. These findings have the potential to contribute to clinical decision-making regarding diagnosis and treatment for specific patient populations.
Study approval statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Consent to publish statement
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. We have made the best efforts to contact the relative and the article has been sufficiently anonymized to cause no harm to the patient or his or her family.
Author contributions
Conception and design: Qichen Zhang, Hui Qiao.
Administrative support: Hui Qiao.
Provision of study materials or patients: Hui Qiao.
Collection and assembly of data: Hui Qiao, Peng Xie, Xiaoming Hou, Chengpeng Zhao, Ling Duan.
Data analysis and interpretation: Qichen Zhang, Hui Qiao.
Manuscript writing: All authors.
Final approval of manuscript: All authors.
Acknowledgments
The authors greatly appreciate Yu Song of the University of Lanzhou of the First School of Clinical Medicine for her helpful contribution to language editing assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660.
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. doi:10.1056/NEJMoa0909530.
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128. doi:10.1016/S1470-2045(09)70364-X.
- Jonna S, Subramaniam DS. Molecular diagnostics and targeted therapies in non-small cell lung ca ncer (NSCLC): an update. Discov Med. 2019;27(148):167–170.
- Wang S, Cang S, Liu D. Third-generation inhibitors targeting EGFR T790M mutation in advanced non-small cell lung cancer. J Hematol Oncol. 2016;9(1):34. doi:10.1186/s13045-016-0268-z.
- Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2016;17(12):1643–1652. doi:10.1016/S1470-2045(16)30508-3.
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–640. doi:10.1056/NEJMoa1612674.
- Manz SM, Losa M, Fritsch R, et al. Efficacy and side effects of immune checkpoint inhibitors in the treatment of colorectal cancer. Therap Adv Gastroenterol. 2021;14:17562848211002018. doi:10.1177/17562848211002018.
- Braun GS, Kirschner M, Rubben A, et al. [Side effects of novel cancer immunotherapies]. Nephrologe. 2020;15(3):191–204. doi:10.1007/s11560-020-00424-8.
- Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94(8):1623–1640. doi:10.1016/j.mayocp.2019.01.013.
- 崔久嵬, 段建春, 任胜祥, 苏春霞, 王志杰, 杨帆, et al. 三代EGFR-TKI在EGFR突变NSCLC治疗中应用的专家共识(2022年版). 中国肺癌杂志. 2022;25(09):627–641.
- Haanen J, Obeid M, Spain L, et al. Management of toxicities from immunotherapy: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(12):1217–1238. doi:10.1016/j.annonc.2022.10.001.
- Chen KL, Lin CC, Cho YT, et al. Comparison of skin toxic effects associated with gefitinib, erlotinib, or afatinib treatment for Non-Small cell lung cancer. JAMA Dermatol. 2016;152(3):340–342. doi:10.1001/jamadermatol.2015.4448.
- Patel AB, Pacha O. Skin reactions to immune checkpoint inhibitors. Adv Exp Med Biol. 2020;1244:235–246.
- Annunziata MC, De Stefano A, Fabbrocini G, et al. Current recommendations and novel strategies for the management of skin toxicities related to anti-EGFR therapies in patients with metastatic colorectal cancer. Clin Drug Investig. 2019;39(9):825–834. doi:10.1007/s40261-019-00811-7.
- Mitsudomi T, Tan D, Yang JC-H, et al. Expert consensus recommendations on biomarker testing in metastatic and nonmetastatic NSCLC in asia. J Thorac Oncol. 2023;18(4):436–446. doi:10.1016/j.jtho.2022.10.021.
- Bhardwaj M, Chiu MN, Pilkhwal Sah S. Adverse cutaneous toxicities by PD-1/PD-L1 immune checkpoint inhibitors: pathogenesis, treatment, and surveillance. Cutan Ocul Toxicol. 2022;41(1):73–90. doi:10.1080/15569527.2022.2034842.
- Collins LK, Chapman MS, Carter JB, et al. Cutaneous adverse effects of the immune checkpoint inhibitors. Curr Probl Cancer. 2017;41(2):125–128. doi:10.1016/j.currproblcancer.2016.12.001.
- Belum V, Benhuri B, Postow M, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12–25. doi:10.1016/j.ejca.2016.02.010.
- Rapoport BL, van Eeden R, Sibaud V, et al. Supportive care for patients undergoing immunotherapy. Berlin: Springer; 2017.
- Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83(5):1255–1268. doi:10.1016/j.jaad.2020.03.132.
- Yalici-Armagan B, Ayanoglu BT, Demirdag HG. Targeted tumour therapy induced papulopustular rash and other dermatologic side effects: a retrospective study. Cutan Ocul Toxicol. 2019;38(3):261–266. doi:10.1080/15569527.2019.1594874.
- Macdonald JB, Macdonald B, Golitz LE, et al. Cutaneous adverse effects of targeted therapies: part I: inhibitors of the cellular membrane. J Am Acad Dermatol. 2015;72(2):203–218. quiz 19–20. doi:10.1016/j.jaad.2014.07.032.
- Morse L, Calarese P. EGFR-targeted therapy and related skin toxicity. Semin Oncol Nurs. 2006;22(3):152–162. doi:10.1016/j.soncn.2006.04.005.
- Lacouture ME, Anadkat MJ, Bensadoun RJ, et al. Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Support Care Cancer. 2011;19(8):1079–1095. doi:10.1007/s00520-011-1197-6.
- Carlisle JW, Ramalingam SS. Role of osimertinib in the treatment of EGFR-mutation positive non-small-cell lung cancer. Future Oncol. 2019;15(8):805–816. doi:10.2217/fon-2018-0626.
- Yi L, Fan J, Qian R, et al. Efficacy and safety of osimertinib in treating EGFR-mutated advanced NSCLC: a meta-analysis. Int J Cancer. 2019;145(1):284–294. doi:10.1002/ijc.32097.
- Nagasaka M, Zhu VW, Lim SM, et al. Beyond osimertinib: the development of third-generation EGFR tyrosine kinase inhibitors for advanced EGFR + NSCLC. J Thorac Oncol. 2021;16(5):740–763. doi:10.1016/j.jtho.2020.11.028.
- Lu S, Dong X, Jian H, et al. AENEAS: a randomized phase III trial of aumolertinib versus gefitinib as first-line therapy for locally advanced or metastatic non-small-cell lung cancer with EGFR exon 19 deletion or L858R mutations. J Clin Oncol. 2022;40(27):3162–3171. doi:10.1200/JCO.21.02641.
- Zhou C, Xie L, Liu W, et al. Absorption, metabolism, excretion, and safety of [(14)C]almonertinib in healthy Chinese subjects. Ann Transl Med. 2021;9(10):867. doi:10.21037/atm-21-1606.
- Lu S, Wang Q, Zhang G, et al. Efficacy of aumolertinib (HS-10296) in patients with advanced EGFR T790M + NSCLC: updated post-national medical products administration approval results from the APOLLO registrational trial. J Thorac Oncol. 2022;17(3):411–422. doi:10.1016/j.jtho.2021.10.024.
- Group JHP. Hansoh Pharma’s Ameile (Almonertinib) Receives Marketing Authorization in China for Second-Line Treatment for Patients With EGFR T790M-Mutation Non-Small Cell Lung Cancer March 19, 2020 Available from: http://www.hspharm.com.
- Wu L, Zhong W, Li A, et al. Successful treatment of EGFR T790M-mutant non-small cell lung cancer with almonertinib after osimertinib-induced interstitial lung disease: a case report and literature review. Ann Transl Med. 2021;9(11):950–950. doi:10.21037/atm-21-2823.
- Zhang Q, Liu H, Yang J. Aumolertinib effectively reduces clinical symptoms of an EGFR L858R-Mutant Non-Small cell lung cancer case coupled with Osimertinib-Induced cardiotoxicity: case report and review. Front Endocrinol. 2022;13:833929. doi:10.3389/fendo.2022.833929.
- Ding X, Ding J, Leng Z, et al. Aumolertinib challenge as an optional treatment in advanced non small-cell lung cancer after osimertinib failure with epidermal growth factor receptor-sensitive mutation: a case series. Oncol Lett. 2022;24(5):400. doi:10.3892/ol.2022.13520.
- Ettinger DS, Wood DE, Akerley W, et al. Non–small cell lung cancer, version 6.2015. J Natl Compr Canc Netw. 2015;13(5):515–524. doi:10.6004/jnccn.2015.0071.
- Kris M, Johnson B, Kwiatkowski D, et al. Identification of driver mutations in tumor specimens from 1,000 patients with lung adenocarcinoma: the NCI’s lung cancer mutation consortium (LCMC). J Clin Oncol. 2011;29(18_suppl):CRA7506–CRA7506. doi:10.1200/jco.2011.29.18_suppl.cra7506.
- 中国临床肿瘤学会指南工作委员会组织. 中国临床肿瘤学会(CSCO)小细胞肺癌诊疗指南-2021. 北京: 人民卫生出版社; 2021.