?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
We analyzed the efficacy and safety of aminoglycosides in a retrospective study of 415 patients with acute appendicitis and 277 patients with acute cholecystitis. The following variables increased the incidence of postoperative complications, defined as surgical site infection, recurrent intraabdominal infection, non-infectious post-operative complication, or death: age (p = 0.016 and 0.011), kidney disease (p = 0.019 and <0.001), and ASA Score (p < 0.001). The type of antibiotic therapy did not have a statistically significant effect on the incidence of postoperative complications in patients with acute appendicitis and cholecystitis (p = 0.561 and 0.547, respectively). A linear regression model showed a higher complication rate in patients with kidney disease (p = 0.014) and neoplasms (p = 0.013); the type of antibiotic therapy did not have a significant effect on the outcome (p = 0.765). There was no statistically significant difference in the post-treatment levels of creatinine in patients treated with aminoglycosides (gentamicin 3 mg/kg once daily) and in those who received other antibiotics (p = 0.75).
Introduction
Intra-abdominal infections are an important cause of morbidity and mortality. Successful management of intra-abdominal infections includes an appropriate antibiotic therapy, treatment of systemic inflammatory response syndrome as well as adequate source control [Citation1,Citation2]. Recently published international guidelines for the treatment of patients with intra-abdominal infections by Sartelli et al. recommend an empiric therapy with a combination of beta-lactam and aminoglycoside antibiotics, or alternatively a combination of aminoglycosides and metronidazole in complicated appendicitis and complicated diverticulitis [Citation3]. Other treatment options include combinations of cephalosporins with metronidazole as well as a fluoroquinolone-based regimen [Citation3].
As aminoglycoside antibiotics are nephro- and ototoxic and because many safer and effective treatment options are available, the use of aminoglycosides has often been discouraged in the past decades [Citation4]. Recent EUCAST guidelines discourage the use of aminoglycoside antibiotics without other effective treatment while also highlighting the importance of correct dosing of treatment with aminoglycosides based on PK/PD modelling [Citation5]. For gentamicin and tobramycin, a daily dose of 7–7.5 mg/kg/day and for amikacin of 20–30 mg/kg/day is considered appropriate [Citation5,Citation6]. Clinical data on the relevance of PK/PD dosing are lacking. A 2007 systematic review and meta-analysis of aminoglycoside monotherapy concluded that ‘the present data support the use of aminoglycosides for urinary tract infections. The paucity of trials precludes firm recommendations for patients with infections other than of the urinary tract’ [Citation7]. According to recent EUCAST recommendations aminoglycosides could be used in infections outside urinary tract only in addition to another effective therapy, e.g. surgery [Citation5]. Dosage regimens were not formally examined in the review by Vidal et al. The EUCAST review of the references for urinary tract infection particularly complicated UTI (cUTI) showed satisfactory clinical responses to doses of ∼3 mg/kg/day for gentamicin and tobramycin [Citation6]. The prevalence of antimicrobial resistance to fluoroquinolones, cephalosporins and carbapenems has increased steadily in the past decades; studies confirm that these classes of antibiotics induce antimicrobial resistance significantly more often than aminoglycosides [Citation8–10]. The susceptibility to aminoglycosides at the same time remains high [Citation11,Citation12].
Aminoglycosides, especially gentamicin in lower dose (3 mg/kg body weight) in combination with antibiotics effective against anaerobes have been widely used for intra-abdominal infections in addition to surgery. In spite of the recent EUCAST guidelines the question remains if short courses of gentamicin treatment could still be effectively and safely used for surgically controlled intra-abdominal infections sparing other antimicrobial agents [Citation13–15].
We have conducted a retrospective clinical study aiming to re-evaluate the efficacy and safety of gentamicin in surgically treated common intra-abdominal infections.
Methods
Study population
In our study we included patients who were treated for acute cholecystitis or acute appendicitis in the University Medical Centre in Ljubljana, Slovenia during the time period from August 2012 to December 2018. Patients were eligible for enrolment in the study if they were 15 years of age or older; if they received surgical treatment; and if the diagnosis of acute appendicitis or cholecystitis was confirmed intraoperatively.
We excluded all patients who did not fulfil the diagnostic criteria for acute appendicitis or cholecystitis upon physical examination and intraoperatively; who were not treated surgically; who had a severe comorbidity (NYHA IV class of heart failure, chronic dialysis-dependent renal failure); who had an immunodeficiency disorder; and who were receiving immunosuppressive therapy at the time of diagnosis or in the year before.
Study design and statistical analysis
The data were collected from the hospital information system. In the first part of the study, we aimed to determine the factors related to post-operative complications. For the classification of post-operative complications, we used the system implemented by Sawyer et al. in their 2015 prospective study on treatment of intraabdominal infection, in which they divided different types of complications into (i) surgical site infection, (ii) recurrent intraabdominal infection and (iii) death [Citation2]. Additionally, we recorded all cases of non-infectious post-operative complications. We documented complications that occurred during the initial hospital stay as well as complications and subsequent check-ups or readmission within one month after surgery. To determine the factors that increased the occurrence of postoperative complications, we recorded the following variables: sex and age, presence and type of comorbidities, antibiotic treatment, surgical procedure, and ASA score. In the second part of the study, we focused on the effects of aminoglycoside treatment on kidney function. We documented the level of creatinine and glomerular filtration rate at admission and after completed antibiotic treatment for patients who were treated with aminoglycosides and those who received other types of antibiotic treatment.
The study utilized various statistical methods to analyze patient data. T-tests were employed to compare the characteristics of 692 patients with acute appendicitis and cholecystitis, to assess risk factors for complications, and to compare creatinine levels on admission and discharge in patients treated with aminoglycoside antibiotics versus other antibiotic regimens. Additionally, a logistic regression model was fitted to predict the occurrence of complications based on the type of antibiotic therapy, with the model being adjusted for comorbidities. All analyses were conducted using R version 4.3.1, employing the tidyverse [Citation16], arsenal [Citation17], and ggstatsplot [Citation18] packages.
Study oversight
The study was controlled and approved by the National Medical Ethics Committee of the Ministry of Health of the Republic of Slovenia. The research was conducted at the Department of Abdominal Surgery of the Ljubljana University Medical Centre. No data on patients’ personal information was recorded and their identity was protected.
Results
Study population
We collected data of 974 patients admitted because of acute appendicitis or acute cholecystitis. 282 patients were excluded from the study. Out of 692 patients included in the study, 415 had an intraoperatively confirmed diagnosis of acute appendicitis and 277 of acute cholecystitis. 76.9% of patients with acute appendicitis and 20.6% of patients with acute cholecystitis were treated with aminoglycoside antibiotics.
270 (97.5%) Of patients with acute cholecystitis received surgical treatment with laparoscopic cholecystectomy, in 5 (1.8%) patients the initial laparoscopic cholecystectomy was converted to open and in two (0.7%) cases median laparotomy with cholecystectomy was used. In the group of patients with acute cholecystitis, 13.6% of patients were treated with a combination of gentamicin and amoxicillin/clavulanic acid and 5.4% of patients with a combination of gentamicin and metronidazole. 45% were treated with amoxicillin/clavulanic acid only and 10.8% of patients received a combination of ciprofloxacin and metronidazole. Other treatments included piperacillin/tazobactam (2.9%), ertapenem (1.0%), and various other antibiotic combinations without an aminoglycoside.
In the group of patients with acute appendicitis, 410 (98.8%) of patients received a laparoscopic appendectomy, in 5 (1.2%) a conversion to open appendectomy was necessary. 76.9% of patients were treated with an aminoglycoside, either in a combination with amoxicillin/clavulanic acid or with metronidazole. 22.3% of patients were treated with amoxicillin/clavulanic acid as monotherapy, others received various antibiotic combinations without an aminoglycoside.
In all cases of treatment with aminoglycosides a once-daily dosing regimen and a fixed dose of 240 mg (approximately 3 mg/kg of lean body mass) was used. The mean duration of aminoglycoside therapy was 3 days in patients with acute appendicitis and 5.3 days in patients with acute cholecystitis. The characteristics of the patients included in the study are presented in .
Table 1. Characteristics of 692 patients with acute appendicitis and cholecystitis.
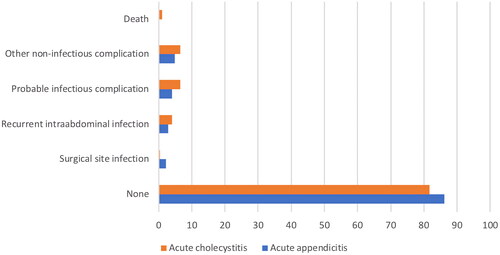
Postoperative complications occurred in 15% of patients with acute cholecystitis and appendicitis. Cumulatively we documented 10 cases of surgical site infection, 23 cases of recurrent intraabdominal infection, 35 cases of probable infectious complications (persistent increase of inflammatory parameters and probable or confirmed bacteraemia), 38 cases of other non-infectious complications (sonographically confirmed fluid collection or haematoma, bleeding, respiratory insufficiency, arrhythmia) and 3 deaths.
The postoperative complications are presented in .
Risk factors for postoperative complications
Of the 277 patients with acute cholecystitis 42 (15.2%) suffered postoperative complications, 7 patients (2.5%) underwent revision surgery. Among the analyzed variables, the presence of concomitant cardiovascular, lung and kidney disease as well as a high ASA-Score were shown to be statistically significant risk factors for postoperative complications (p-value <0.001). Treatment with aminoglycoside antibiotics did not influence the rate of postoperative complications (p-value 0.574) ().
Table 2. Risk factors for complications – acute cholecystitis.
Postoperative complications were documented in 64 (15.4%) cases of acute appendicitis. 6 patients (1.4%) underwent revision surgery. In this group as well the ASA-Score was a statistically significant factor influencing the number of postoperative complications. There was no correlation between the type of antibiotic therapy and postoperative complications (p-value 0.561) ().
Table 3. Risk factors for complications – acute appendicitis.
We fitted a logistic model to predict the occurrence of complications with the type of antibiotic therapy, the model was adjusted for comorbidities. The model’s explanatory power is weak (Tjur’s R2 = 0.05). The model’s intercept, corresponding to antibiotics = aminoglycoside, cardiovascular diseases = yes, kidney insufficiency = yes, immobility = yes, type 2 diabetes = yes, lung disease = yes, neurological disease = yes and neoplasm = yes, is at 0.68 (95% CI [−1.89, 3.03], p = 0.578).
Within this model, the effect of antibiotics [other antibiotics] is statistically non-significant and negative (beta = −0.10, 95% CI [−0.76, 0.52], p = 0.765); the effect of cardiovascular disease [no] is statistically non-significant and negative (beta = −0.02, 95% CI [−0.61, 0.59], p = 0.950); the effect of kidney insufficiency [no] is statistically significant and negative (beta = −1.34, 95% CI [−2.41, −0.26], p = 0.014); the effect of immobility [no] is statistically non-significant and positive (beta = 0.84, 95% CI [−0.63, 2.80], p = 0.316); the effect of diabetes type 2 [no] is statistically non-significant and positive (beta = 0.46, 95% CI [−0.45, 1.50], p = 0.348); the effect of lung disease [no] is statistically non-significant and negative (beta = −0.58, 95% CI [−1.34, 0.23], p = 0.146); the effect of neurological disease [no] is statistically non-significant and negative (beta = −0.22, 95% CI [−1.25, 0.97], p = 0.692); the effect of cancer [no] is statistically significant and negative (beta = −1.53, 95% CI [-2.77, −0.30], p = 0.013). Standardized parameters were obtained by fitting the model on a standardized version of the dataset. 95% Confidence Intervals (CIs) and p-values were computed using a Wald z-distribution approximation.
Influence of treatment with aminoglycoside antibiotics on kidney function
In the group of patients with acute cholecystitis, 5 patients presented with an acute prerenal kidney injury at admission. None of these patients were treated with aminoglycoside antibiotics. None of the patients who received aminoglycoside antibiotics developed an acute kidney injury in the course of treatment. In the group of patients with acute appendicitis, the GFR was initially lower in the patients who were afterwards treated with aminoglycoside antibiotics, the GFR at discharge did not change significantly overall. One patient with acute perforated appendicitis presented with an acute kidney failure; he received a laparoscopic appendectomy with conversion and antibiotic therapy with gentamicin and metronidazole. The antibiotic therapy was switched after two days to piperacillin/tazobactam, which was administered for another 7 days. During the subsequent course of treatment, the patient suffered a recurrent intraabdominal infection and underwent a revision laparoscopy and lavage.
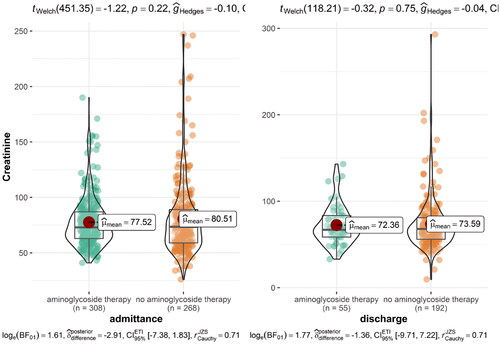
To evaluate the effect of aminoglycoside antibiotics on kidney function, we compared the glomerular filtration rate at admission and discharge in patients who were treated with aminoglycosides and in those who received other antibiotics. The results are presented in . There were no statistically significant differences in kidney function in patients treated with aminoglycoside antibiotics and other treatment regimens.
Discussion
Acute intraabdominal infection and treatment outcome
In our retrospective study of 692 patients treated for an acute intraabdominal infection, treatment with aminoglycoside antibiotics in combination with amoxicillin/clavulanic acid or metronidazole showed to be equally effective as other antibiotic regimens. The number of post-operative complications was affected by the patients’ age, comorbidities as well as the ASA score but was not influenced by the type of antibiotic therapy.
A large majority of patients in our study (98%) received laparoscopic surgical treatment. The complication rate after surgical treatment of acute cholecystitis in our study was 15.2%, which is comparable to the complication rate reported in literature. In a 2022 review of acute cholecystitis by Gallaher and Charles, the complication rate was 11.8% after early and 34.4% after late cholecystectomy [Citation19]. In a study published by the CholeS Study Group, the complication rate after laparoscopic cholecystectomy before and after 72 h after admission was 15.8 and 12.3%, respectively [Citation20]. In a study by Fugazzola et al. a 30-day mortality after cholecystectomy for acute calculous cholecystitis was found to be 1.1%, which corresponds exactly to the mortality rate in our study [Citation21]. For acute appendicitis, the complication rate in our study was 15.4%; in comparison, Patel et al. found that 25.2% in patients who received an appendectomy for non-perforated appendicitis and 35.5% of patients with perforated appendicitis had at least one complication [Citation22]. The difference could partly be explained by an inclusive definition of a postoperative complication in the study, as an emergency department visit after surgery was classified as a postoperative complication in Patel et al.’s study. Sartelli et al. report a 9.2% complication rate after 7 days and 3.3% complication rate after 30 days [Citation23].
The finding that cardiovascular and lung disease as well as renal insufficiency increase the number of complications is both unsurprising and confirmed by multicentre observational studies which found cardiovascular disease [Citation23–25] history of stroke and diabetes [Citation24], as well as chronic obstructive pulmonary disease [Citation25] to increase the post-operative complication rate.
In their prospective observational study on acute appendicitis, Sartelli and al. found that Charlson Comorbidity Index (CCI) Score over 5 and stages 3c and 4 of the WSES Sepsis Severity Score were independent predictors of mortality [Citation23] – a result comparable to our finding that classes III and IV of the ASA score were independent variables directly increasing the number of complications. In their study of risk factors for adverse outcomes after surgical treatment of appendicitis, Margenthaler and al. found the mortality was significantly higher in patients with the ASA score of III or IV [Citation25]. Studies also confirm the ASA score to be a strong predictor of complications after surgical treatment of cholecystitis [Citation26].
An important factor that has been proven by studies to influence the treatment outcome is infection with multi-drug resistant organisms [Citation27]. In our study, it was not possible to assess the significance of this as an independent variable since tissue samples were only collected in 1.4% of cases. Opinions of authors differ as to the necessity of intraoperative tissue sampling: in their guidelines for management of intra-abdominal infection, Mazuski et al. deem it to be largely futile, particularly in cases of expected short-term inpatient care [Citation28]; Montravers et al. recommend the collection of samples solely if the patient has received antibiotic treatment in the time span of three months before surgery or if the patient is in septic shock [Citation29]; Fuks et al. recommend regular tissue sampling in cases of acute cholecystitis, as empiric antibiotic treatment can be ineffective in as much as 23% of cases [Citation30]. In our opinion a prospective study with an equivalent objective, extensive intraoperative tissue sampling and subsequent bacterial isolation could be of significant value in explaining the treatment outcome.
Our finding that aminoglycoside treatment is not inferior to other antibiotics is in contradiction with results from some other related studies. In a 2004 meta-analysis by Bailey et al. that included 47 prospective studies, the authors concluded that aminoglycosides were less effective than other antibiotics in treatment of intraabdominal infections [Citation4]. We believe that the heterogeneity of the included studies should be taken into account when interpreting this result as bacteriological resistance profiles may vary significantly from region to region; it is essential to consider local epidemiology and bacterial resistance patterns when selecting an appropriate empirical antibiotic therapy. Additionally, the prevalence of MDR infections has increased since the time the studies included in the review were published. It is also worth noting that several studies excluded from their sample elderly patients and patients with comorbidities, who represented 41.5% of our sample. Furthermore, none of the studies employed once daily dosing of aminoglycosides – a treatment regimen which has been shown to be safer and equally or more effective than two- or three-times daily administration [Citation4,Citation31], and which was employed rigorously in all cases of our study.
Safety of aminoglycoside antibiotics
In our study, there were no statistically significant differences in kidney function in patients treated with aminoglycoside antibiotics and other treatment regimens. The mean duration of aminoglycoside treatment in our sample was 3.04 days in patients with acute appendicitis and 5.28 days in patients with acute cholecystitis. The fact that we could find no evidence of aminoglycoside-induced renal injury is understandable, as research suggests that their toxic action on distal tubules manifests predominantly in cases of prolonged treatment and more-than-once-daily dosing [Citation12,Citation32–34]. According to studies on aminoglycoside nephrotoxicity, there are numerous risk factors that can amplify aminoglycosides’ adverse effects on renal function, principally pre-existing kidney or liver injury, dehydration, and treatment with separate nephrotoxic drugs (most commonly NSAIDs) [Citation12,Citation32–36].
In our study, aminoglycosides were administered in a once-daily dosing regimen and a fixed dose of 240 mg (approximately 3 mg/kg of lean body mass) was used. This is a dosage that has been approved for combination therapy of streptococcal and enterococcal endocarditis [Citation37], but may be insufficient in intraabdominal infections. According to 2023 EUCAST guidelines, a once-daily administration of a higher dose (7–7.5 mg/kg/day for gentamicin and tobramycin) is recommended for optimal treatment effect [Citation6]. Under aminoglycoside therapy, renal function should be regularly monitored (e.g. monitoring of serum creatinine concentration and gentamicin level every two days) [Citation34,Citation35]. If these conditions are met, a short perioperative treatment course with aminoglycoside antibiotics can be a safe and effective alternative to avoid extensive use of other antibiotics with higher antimicrobial resistance potential.
Limitations to data interpretation and statistical bias
The limitations to our study are related primarily to its retrospective nature. As such, it faces difficulties in data acquisition as well as a possible selection bias. Despite the relatively large sample size the distribution of patients treated with aminoglycosides and those who were not was uneven. While conducting the study we also faced several limitations of the information system as data was often incomplete or missing, resulting in some variables (e.g. BMI) being excluded from further statistical analysis. The number of patients in our study who suffered an acute kidney injury was low and the analysis of the effects of antibiotic therapy on kidney function was limited.
Conclusions
In our study, a combination treatment with aminoglycosides (gentamicin 3 mg/kg/day) was equally effective and safe as other antibiotic regimens in treatment of acute surgically-managed cholecystitis and appendicitis. A higher incidence of post-surgical complications was observed in elderly patients with comorbidities, the type of antibiotic treatment did not have a statistically significant effect on the complication rate. There were no statistically significant differences in kidney function in patients treated with aminoglycosides and in those who received other non-aminoglycoside antibiotics. These results should be confirmed in a prospective, controlled study with a higher gentamicin dosage (7–7.5 mg/kg/day) in accordance with recent EUCAST guidelines.
Aminoglycoside antibiotics are classified by the World Health Organization as critically important antimicrobials in human medicine, particularly with regard to the rising prevalence of MDR Gram-negative infections. As aminoglycosides maintain high potency against multi-drug resistant Gram-negative bacteria and induce notably less bacterial resistance than cephalosporins, fluoroquinolones and carbapenems, we argue that they are a valuable treatment option in treatment of surgically-managed acute intra-abdominal infections.
Ethical approval
The study was conducted in accordance with the Declarations of Helsinki and was approved by the National Medical Ethics Committee of the Ministry of Health of the Republic of Slovenia on 5 December 2018 (Approval 0120-451/2018/7). All patient information, including names, screening IDs and patient IDs, was deidentified and patient consent was not required.
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, Professor Dr. Bojana Beović, upon request.
References
- Sartelli M, Chichom-Mefire A, Labricciosa FM, et al. The management of intra-abdominal infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2017;12(1):29. doi: 10.1186/s13017-017-0141-6.
- Sawyer RG, Claridge JA, Nathens AB, et al. Trial of short-course antimicrobial therapy for intraabdominal infection. N Engl J Med. 2015;72(21):1996–2005. doi: 10.1056/NEJMoa1411162.
- Sartelli M, Coccolini F, Kluger Y, et al. WSES/GAIS/SIS-E/WSIS/AAST global clinical pathways for patients with intra-abdominal infections. World J Emerg Surg. 2021;16(1):49. doi: 10.1186/s13017-021-00387-8.
- Bailey JA, Virgo KS, DiPiro JT, et al. Aminoglycosides for intra-abdominal infection: equal to the challenge? Surg Infect. 2002;3(4):315–335. doi: 10.1089/109629602762539544.
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters [Internet] ; 2023 [cited 2023 July 02]. Available from: http://www.eucast.org.
- The European Committee on Antimicrobial Susceptibility Testing. Gentamicin: rationale for the EUCAST clinical breakpoints diameters [Internet]; 2023 [cited 2023 July 02]. Available from: http://www.eucast.org.
- Vidal L, Gafter-Gvili A, Borok S, et al. Efficacy and safety of aminoglycoside monotherapy: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2007;60(2):247–257. doi: 10.1093/jac/dkm193.
- Hyle EP, Lipworth AD, Zaoutis TE, et al. Risk factors for increasing multidrug resistance among extended-spectrum betalactamase-producing Escherichia coli and Klebsiella species. Clin Infect Dis. 2005;40(9):1317–1324. doi: 10.1086/429239.
- Lee OS, Lee ES, Park Young S, et al. Reduced use of third-generation cephalosporins decreases the acquisition of extended-spectrum beta-lactamase producing Klebsiella pneumoniae. Infect Control Hosp Epidemiol. 2004;25(10):832–837. doi: 10.1086/502304.
- Martínez JA, Aguilar J, Almela M, et al. Prior use of carbapenems may be a significant risk factor for extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella spp. in patients with bacteraemia. J Antimicrob Chemother. 2006;58(5):1082–1085. doi: 10.1093/jac/dkl367.
- Štrumbelj I, Pirš M, Berce I, et al. Slovenska komisija za ugotavljanje občutljivosti za protimikrobna zdravila (SKUOPZ): pregled občutljivosti bakterij za antibiotike – Slovenija 2016 diameters [Internet]; 2016 [cited 2023 July 27]. Available from: http://www.imi.si/strokovnazdruzenja/skuopz/dokumenti/Pregledobutljivosti2016SKUOPZ97896194044231.pdf.
- Cronin RE. Aminoglycoside nephrotoxicity: pathogenesis and prevention. Clin Nephrol. 1979;11(5):251–256.
- Di Saverio S, Podda M, De Simone B, et al. Diagnosis and treatment of acute appendicitis: 2020 update of the WSES Jerusalem guidelines. World J Emerg Surg. 2020;15(1):27. doi: 10.1186/s13017-020-00306-3.
- Markotic F, Grgic S, Poropat G, et al. Antibiotics for adults with acute cholecystitis or acute cholangitis or both. Cochrane Database Syst Rev. 2020;2020(6):CD013646.
- Gomi H, Solomkin JS, Schlossberg D, et al. Tokyo Guidelines 2018: antimicrobial therapy for acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2018;25(1):3–16. doi: 10.1002/jhbp.518.
- Wickham H, Averick M, Bryan J, et al. Welcome to the tidyverse. JOSS. 2019;4(43):1686. doi: 10.21105/joss.01686.
- Heinzen E, Sinnwell J, Atkinson E, et al. An arsenal of 'R' functions for large-scale statistical summaries; R package version 3.6.3 [Internet]; 2021 [cited Sep 2]. Available from: https://CRAN.R-project.org/package=arsenal.
- Patil I. Visualizations with statistical details: the ‘ggstatsplot’ approach. JOSS. 2021;6(61):3167. doi: 10.21105/joss.03167.
- Gallaher JR, Charles A. Acute cholecystitis: a review. JAMA. 2022;327(10):965–975. doi: 10.1001/jama.2022.2350.
- CholeS Study Group, Griffiths E, Vohra R. Cholecystectomy for cholecystitis: delayed surgery minimises conversion and 30 day complication rate. HPB. 2016; 18(2):e673. doi: 10.1016/j.hpb.2016.01.028.
- Fugazzola P, Cobianchi L, Di Martino M, et al. Prediction of morbidity and mortality after early cholecystectomy for acute calculous cholecystitis: results of the S.P.Ri.M.A.C.C. Study. World J Emerg Surg. 2023; Mar 1818(1):20. doi: 10.1186/s13017-023-00488-6.
- Patel SV, Nanji S, Brogly SB, et al. High complication rate among patients undergoing appendectomy in Ontario: a population-based retrospective cohort study. Can J Surg. 2018;61(6):412–417. doi: 10.1503/cjs.011517.
- Sartelli M, Baiocchi GL, Di Saverio S, et al. Prospective observational study on acute appendicitis worldwide (POSAW). World J Emerg Surg. 2018;13(1):19. doi: 10.1186/s13017-018-0179-0.
- Cho JY, Ho-Seong H, Yoo-Seok Y, et al. Risk factors for acute cholecystitis and a complicated clinical course in patients with symptomatic cholelithiasis. Arch Surg. 2010;145(4):329–333. doi: 10.1001/archsurg.2010.35.
- Margenthaler JA, Longo WE, Virgo KS, et al. Risk factors for adverse outcomes after the surgical treatment of appendicitis in adults. Ann Surg. 2003;238(1):59–66. doi: 10.1097/01.SLA.0000074961.50020.f8.
- Yi NJ, Han HS, Min SK. The safety of a laparoscopic cholecystectomy in acute cholecystitis in high-risk patients older than sixty with stratification based on ASA score. Minim Invasive Ther Allied Technol. 2006;15(3):159–164. doi: 10.1080/13645700600760044.
- Herzog T, Chromik AM, Uhl W. Treatment of complicated intra-abdominal infections in the era of multi-drug resistant bacteria. Eur J Med Res. 2010;15(12):525–532. doi: 10.1186/2047-783x-15-12-525.
- Mazuski JE, Tessier JM, May AK, et al. The surgical infection society revised guidelines on the management of intra-abdominal infection. Surg Infect. 2017;18(1):1–76. doi: 10.1089/sur.2016.261.
- Montravers P, Dupont H, Leone M, et al. Guidelines for management of intra-abdominal infections. Anaesth Crit Care Pain Med. 2015;34(2):117–130. doi: 10.1016/j.accpm.2015.03.005.
- Fuks D, Cossé C, Régimbeau JM. Antibiotic therapy in acute calculous cholecystitis. J Visc Surg. 2013;150(1):3–8. doi: 10.1016/j.jviscsurg.2013.01.004.
- Stankowicz MS, Ibrahim J, Brown DL. Once-daily aminoglycoside dosing: an update on current literature. Am J Health Syst Pharm. 2015;72(16):1357–1364. doi: 10.2146/ajhp140564.
- Barclay ML, Kirkpatrick CM, Begg EJ. Once daily aminoglycoside therapy. Is it less toxic than multiple daily doses and how should it be monitored? Clin Pharmacokinet. 1999;36(2):89–98. doi: 10.2165/00003088-199936020-00001.
- Drusano GL, Ambrose PG, Bhavnani SM, et al. Back to the future: using aminoglycosides again and how to dose them optimally. Clin Infect Dis. 2007; 45(6):753–760. doi: 10.1086/520991.
- Rybak MJ, Abate BJ, Kang SL, et al. Prospective evaluation of the effect of an aminoglycoside dosing regimen on rates of observed nephrotoxicity and ototoxicity. Antimicrob Agents Chemother. 1999;43(7):1549–1555. doi: 10.1128/AAC.43.7.1549.
- Mingeot-Leclercq MP, Tulkens PM. Aminoglycosides: nephrotoxicity. Antimicrob Agents Chemother. 1999;43(5):1003–1012. doi: 10.1128/AAC.43.5.1003.
- Prins JM, Weverling GJ, van Ketel RJ, et al. Circadian variations in serum levels and the renal toxicity of aminoglycosides in patients. Clin Pharmacol Ther. 1997;62(1):106–111. doi: 10.1016/S0009-9236(97)90156-9.
- Grayson ML, Cosgrove S, Crowe S, et al. Kucers’ the use of antibiotics. Boca Raton: CRC Press; 2017.