Abstract
Karyotypes of 13 species in the anuran family Hyperoliidae are described based on conventional staining, C‐banding, Ag‐NOR‐banding and staining with fluorochromes (CMA3 and DAPI). The nine studied species of the Malagasy genus Heterixalus, as well as African species of Acanthixalus, Hyperolius and Kassina, had a karyotype of 2n = 24 biarmed chromosomes with NORs on the ninth chromosome pair, whereas the sole species of Leptopelis studied had 2n = 24 with one telocentric pair and NORs on the fifth pair. These data confirm the isolated position of Leptopelis, which according to molecular data does not form a clade with other hyperoliids. Details of NOR location, relative chromosome size and heterochromatin distribution suggest a phylogenetic hypothesis, within Heterixalus, that is largely though not completely in agreement with bioacoustic and molecular data sets: ((betsileo, tricolor, variabilis, (andrakata, (alboguttatus, boettgeri))), (rutenbergi, (luteostriatus, punctatus))).
Introduction
Hyperoliid frogs are endemic to Africa, Madagascar, and the Seychelles. Their phylogeny and systematics have been quite intensively studied. Besides the most influential work of Drewes (Citation1984), recent contributions were published by Channing (Citation1989), Richards & Moore (Citation1998), Schiøtz (Citation1999), Vences et al. (Citation2003a), and Drewes & Wilkinson (Citation2004). Mitochondrial and nuclear DNA phylogenies have indicated that Leptopelis is the most divergent genus and does not even form a monophyletic group with the other hyperoliids (Emerson et al. Citation2000; Vences et al. Citation2003a; Van der Meijden et al. Citation2004). Consequently, this genus has recently been proposed to belong to a redefined family Arthroleptidae (Frost et al. Citation2006). Also, there is strong evidence that the Seychellean Tachycnemis is the sister group of the Madagascan genus Heterixalus (Richards & Moore Citation1998; Vences et al. Citation2003a), and the São Tomé and Principe endemic Nesionixalus is nested within the large genus Hyperolius and thus a junior synonym (Drewes & Wilkinson 2004). However, the limited molecular data available so far (Vences et al. Citation2003a; Drewes & Wilkinson Citation2004) were unable to clarify the phylogenetic affinities of a number of species‐poor enigmatic hyperoliid genera from tropical Africa, such as for example the Wax frogs, Cryptothylax, or the tree‐hole breeding African wart frogs, Acanthixalus.
Karyological data are known to bear potential for systematic studies in amphibians (King Citation1990), and they may also be relevant to understand mechanisms and rates of speciation in these animals (e.g. Bogart & Hedges Citation1995; Vences et al. Citation2002). Among the about 250 species of hyperoliids currently, chromosome information is only available for 42 species (summarized in Table ). All these karyotypes were described based on conventional staining, except for two species (one Semnodactylus, one Leptopelis) in which banding techniques to identify the nucleolus organizer regions (NOR) were also performed (reviewed in King Citation1990).
Table I. Summary of previous knowledge on chromosome numbers of hyperoliid frogs. For detailed references, see King (Citation1990). Semnodactylus weali was originally considered to belong to the genus Kassina and its karyotype described as Kassina weali.
Blommers‐Schlösser (Citation1978) described the karyotype of the endemic Malagasy hyperoliids Heterixalus betsileo and H. madagascariensis (as H. tricolor). These had 2n = 24 biarmed chromosomes, the first five pairs being distinctly larger than the remaining seven pairs. Pairs 2, 3 and 4 were submetacentric, the latter close to subtelocentric state, and the remaining pairs were all metacentric. These chromosomal characteristics were considered to be the basal state in hyperoliids (Bogart & Tandy Citation1981) and were observed in all genera and species examined to date, with the exception of Leptopelis. In this genus, the chromosome number is variable, with species having a larger (2n = 30) or smaller (2n = 22) number of chromosomes, and a variable number of telocentric elements. In Leptopelis bocagei, the position of the NORs was peritelomeric on the long arm of the fifth chromosome pair (Schmid Citation1980), whereas in Semnodactylus wealii they were telomeric on the long arm of the ninth pair (Schmid Citation1978).
The present paper aims at contributing to the knowledge of hyperoliid and leptopelid karyology, focusing mainly on the Malagasy genus Heterixalus for which we studied 9 out of 12 known species, as well as on 4 African hyperoliids: Acanthixalus spinosus, Hyperolius cf. viridiflavus, Kassina maculata and Leptopelis calcaratus. Hence, we provide previously undescribed karyotypes for seven species of Heterixalus, for Acanthixalus, and for one species of Leptopelis. We extend previous studies to include also banding analyses (Ag‐NOR, C‐banding, C‐ and Alu I banding, +CMA3+DAPI), and provide the first such data for the genera Acanthixalus, Heterixalus, Hyperolius and Kassina. We use the novel data to test the hypothesis of conserved karyotypic structure in hyperoliines vs. Leptopelis, and explore the utility of chromosome data to asses the intrageneric phylogeny of Heterixalus.
Materials and methods
We examined chromosome preparations of the following specimens: Heterixalus alboguttatus, one specimen from Ranomafana, Madagascar; H. andrakata, one male from Sambava, Madagascar; H. betsileo, one male from Manjakatompo, Madagascar (MRSN A4561); H. boettgeri, one specimen from near Tolagnaro, Madagascar; H. luteostriatus, two males from Betsimipoaka, Sahamalaza peninsula, Madagascar (MRSN A4552, A4550); H. punctatus, one male from Sambava, Madagascar; H. rutenbergi, four males and one female, from Madagascar (no precise locality; GA 01–05); H. tricolor, two males from the type locality Nosy Be, Madagascar; H. variabilis, one male and one female from Ambanja, Madagascar (MRSN A4556, A4555); Acanthixalus spinosus, one male from Cameroon; Hyperolius cf. viridiflavus, one female (from Kafue River, Zambia), Kassina maculata, one female from Natal, South Africa; Leptopelis calcaratus, two males from Cameroon (ZFMK uncatalogued) (collection acronyms are GA, Gennaro Aprea fieldnumbers, specimens to be deposited in the Museo Regionale di Scienze Naturali, Torino, Italy, MRSN, ZFMK, Zoologisches Forschungsmuseum A. Koenig, Bonn, Germany).
Heterixalus specimens were processed during fieldwork in Madagascar in 2000–2003. Specimens were injected with 0.1 ml/10 g body weight of a 0.5 mg/ml colchicine solution. One hour later specimens were euthanized by immersion in a MS 222 solution, and intestine, lungs, spleen and gonads removed. These organs were incubated for 30 min in a solution of sodium citrate (0.5%) and fixed in 3:1 methanol and acetic acid. The fixed material was preserved at 4°C and transferred to the laboratory in Naples. African hyperoliids were transferred alive to Naples and processed there.
Chromosomes were revealed using the air‐drying method. They were studied with conventional methods (5% Giemsa at pH 7) and subsequently with banding techniques: Ag‐NOR following Howell & Black (Citation1980); chromomycin A3 (CMA3)/methyl green according to Sahar & Latt (Citation1980) but reducing the time of exposure to the non‐fluorescent methyl green to a few seconds only; C‐banding according to Sumner (Citation1972) but incubating the preparations in barium hydroxide at 45°C for 5 min; in‐situ digestion with the Alu I endonuclease following Mezzanotte et al. (Citation1983). In addition we performed a sequential treatment of the preparations: after hydrolysis with Ba(OH)2 or Alu I digestion, chromosomes were stained with CMA3 and DAPI (Odierna et al. Citation1999).
Results
All Heterixalus species studied had a similar general chromosome morphology: 2n = 24 biarmed elements of decreasing size. Relative chromosome lengths and centromeric indices for all species studied are summarized in Table . In most species, the first chromosome pair was distinctly larger than pairs 2–5, and the second, third and fourth pairs were submetacentric (Figure ; Table ). In some species, also a variable number of the elements 7–12 was submetacentric (Table ). Heterixalus luteostriatus and H. punctatus differed in that their second pair was metacentric rather than submetacentric, and of similar length as the first pair (Figure ). Acanthixalus spinosus, Hyperolius cf. viridiflavus and Kassina maculata had karyotypes similar to those found in Heterixalus (Figure ). Leptopelis calcaratus had 2n = 24 chromosomes, all biarmed except the 12th pair that was telocentric (Figure ). In Kassina, the 12th chromsome pair was strongly submetacentric (centromeric index 24.2±2.8; Table ), but clearly biarmed in contrast to Leptopelis where it was fully telocentric.
Figure 1 Giemsa stained karyotypes of the 13 studied hyperoliids.A, Heterixalus alboguttatus; B, H. andrakata; C, H. betsileo; D, H. boettgeri; E, H. luteostriatus; F, H. punctatus; G, H. rutenbergi; H, H. tricolor; I, H. variabilis; J, Acanthixalus spinosus; K, Hyperolius cf. viridiflavus; L, Kassina maculata; M, Leptopelis calcaratus. The Ag‐NOR banded pairs are reported above the corresponding Giemsa stained pairs.

Table II. Relative lengths (±SD; upper value) and centromerix indices (±SD; lower value) of chromosomes 1–12 in hyperoliid karyotypes studied.
Loci of NORs, as individuated from Ag‐NOR‐ and CMA3‐staining, were located in pericentromeric position on the long arm of the fifth chromosome pair in Leptopelis calcaratus, whereas they were on the ninth chromosome pair in all other hyperoliids studied. However, the exact NOR position was variable: in telomeric position on the short arm in Acanthixalus; telomeric on the long arm in Kassina and Hyperolius; peritelomeric on the long arm in most Heterixalus, and interstitially on the short arm in Heterixalus luteostriatus, H. rutenbergi and H. punctatus.
The various banding techniques revealed further differences, also among those karyotypes of similar general morphology (Figures ). Heterixalus luteostriatus had mostly telomeric C‐bands, H. alboguttatus and H. boettgeri had paracentromeric bands on almost all chromosomes, H. andrakata had paracentromeric bands on only some chromosomes, and the other Heterixalus species had centromeric bands on all chromosomes. Further differences were observed after the various fluorochrome staining methods and are summarized in Table . Acanthixalus had solid centromeric and telomeric C‐bands on all chromosomes, and a strong band that made up most of the distal part of the long arm of the sixth chromosome pair. These bands were negative to both fluorochromes (CMA3 and DAPI). Hyperolius had largely telomeric heterochromatin that was CMA3‐ and DAPI‐negative as well. Kassina maculata had centromeric C‐bands on all chromosomes which were CMA3‐positive subsequent to C‐banding but negative to both fluorochromes after Alu I digestion. Leptopelis had centromeric and telomeric C‐bands on all chromosomes, the centromeric bands being negative to CMA3 and DAPI subsequent to C‐banding but positive to both stains after Alu I digestion.
Figure 2 C‐banded karyotypes of the 13 studied studied hyperoliids. A, Heterixalus alboguttatus; B, H. andrakata; C, H. betsileo D, H. boettgeri; E, H. luteostriatus; F, H. punctatus; G, H. rutenbergi; H, H. tricolor; I, H. variabilis; J, Acanthixalus spinosus; K, Hyperolius cf. viridiflavus; L, Kassina maculata; M, Leptopelis calcaratus.

Figure 3 Sequential C‐banding (rows I and II) or Alu I (rows III and IV)+CMA3 (rows I and III)+DAPI (rows II and IV) of: A, H. alboguttatus; B, H. andrakata; C, H. betsileo; D, H. boettgeri; E, H. luteostriatus; and F, H. punctatus. The colour version of this figure is available online.
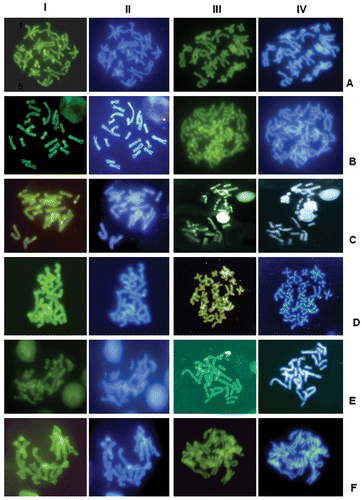
Figure 4 Sequential C‐banding ( rows I and II) or Alu I (rows III and IV)+CMA3 (rows I and III)+DAPI (rows II and IV) of A, H. rutenbergi; B, H. tricolor; C, H. variabilis; D, Acanthixalus spinosus; E, Hyperolius cf. viridiflavus; F, Kassina maculata; and G, Leptopelis calcaratus. The colour version of this figure is available online.
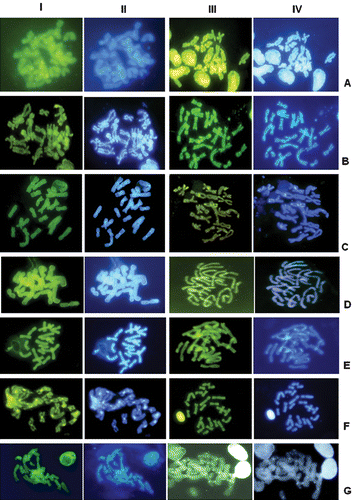
Table III. Summary of karyotype data resulting from the present study. Abbreviations used: Cen = centromeric; Tel = Telomeric; Pcen = Paracentromeric; m = metacentric; sm = submetacentric; p→int = NORs interstitial on short arm; q→stel = NORs peritelomeric on long arm; p→tel = NORs telomeric on short arm; q→tel = NORs telomeric on long arm; p→cen = pericentromeric on long arm; Neg = negative; pr/prs = pair(s).
Discussion
Tempo and pattern of chromosome evolution
Our study provides evidence that in hyperoliid frogs (excluding Leptopelis which we consider as not belonging to the hyperoliidae; Frost et al. Citation2006) some chromosomal rearrangements have taken place, although these did not result in changes in chromosome number or in NOR‐bearing chromosomes. Inversions are most likely responsible for the relocation of the NORs from being terminal on the long arm (Hyperolius, Kassina) to terminal on the short arm (Acanthixalus), or from peritelomeric to interstitial on the long arm, within Heterixalus. The larger size of the second chromosome pair in Heterixalus luteostriatus and H. punctatus is probably a result of a translocation of genomic material from the first to the second chromosome pair. Numerous events of deletion/amplification, minute insertions and amplification of specific families of satellite DNA may explain the large variability in the distribution of heterochromatin among the species; this genomic material, largely consisting of repetitive DNA and mainly localized in the centromeric or telomeric regions, is not subjected to meiotic constraints and therefore variable even within groups of closely related species (John Citation1988; King Citation1990).
Among anurans, several lineages are known to be characterized by a remarkable evolutionary stability of their karyotype (e.g. Boophis and Mantella, Blommers‐Schlösser Citation1978; Pintak et al. Citation1998; Odierna et al. Citation2001; Aprea et al. Citation2004; or Bufo, e.g. Baldissera et al. Citation1999) while others have a very high rate of chromosomal change (e.g. Eleutherodactylus and some lineages of Mantidactylus, Bogart & Hedges Citation1995; Andreone et al. Citation2003). Apparently, a low rate of chromosomal evolution also characterizes the Hyperoliidae. All species studied to date have a karyotype of 2n = 24 biarmed chromosomes (Morescalchi et al. Citation1970; Blommers‐Schlösser Citation1978; Schmid Citation1978, Citation1980; Bogart & Tandy Citation1981; this study), and those studied with Ag‐NOR banding techniques, belonging to the genera Acanthixalus, Heterixalus, Hyperolius, Kassina and Semnodactylus, have NORs located on the ninth chromosome pair (Schmid Citation1978, Citation1980; this study). Our study provides evidence that, nevertheless, events such as inversions and translocations do occur in hyperoliines, but apparently did not or rarely affect the stability of chromosome number and NOR location. Deciphering the factors that influence the frequency of chromosomal rearrangements, and their influences on the karyotype, in different lineages of frogs appears to be a fruitful field of study.
Cytosystematics
The low variability in general chromosome number and NOR location encountered among congeneric taxa of hyperoliids, and the absence of individual differences in the species of Heterixalus where more than one individual was studied (Heterixalus luteostriatus, H. rutenbergi, H. tricolor, H. variabilis), indicates that the obtained karyotypes are likely to represent the typical pattern for each species, despite the low number of individuals available to us for several species (in several cases a single specimen only). Since no sex chromosomes were detected in the species where males and females were observed (Heterixalus rutenbergi, H. variabilis), and sex chromosomes are in general rare in amphibians (King Citation1990), we are confident that no bias due to sexual dimorphism in karyotype is present in our data set, allowing for some systematic considerations.
The general karyotypes found in the hyperoliid species studied herein agree with those described for 16 species of Hyperolius (Bogart & Tandy Citation1981), and for one Semnodactylus and two Kassina (Morescalchi et al. Citation1970; Schmid Citation1978, Citation1980; Bogart & Tandy Citation1981). Also the karyotype of Leptopelis calcaratus agrees with the data of Bogart & Tandy (Citation1981) for this species. In conjunction with the published data, our results from general chromosome morphology, NOR location, and banding are informative to assess the systematics of hyperoliid frogs in various respects.
Available molecular phylogenies for the genus Heterixalus are inconclusive (Vences et al. Citation2003a). At present, we are in the process of analysing larger and more comprehensive data sets (ca. 2000 bp data set of two nuclear and three mitochondrial genes; Wollenberg, Glaw, Meyer and Vences, in preparation). However, also this data set does not resolve satisfyingly several basal relationships within the genus. It therefore seems reasonable to explore whether the karyological data may be informative to help reconstructing intrageneric Heterixalus phylogeny. Of the other hyperoliid genera studied here, Hyperolius is the one most closely related to the Malagasy Heterixalus (Frost et al. Citation2006), which are most probably a monophyletic group (Vences et al. Citation2003a). The fact that the NORs are in terminal position in Hyperolius as well as in the more distantly related Kassina lead us to consider this position as plesiomorphic in hyperoliines. A similar (subterminal) position is observed in Heterixalus alboguttatus, H. andrakata, H. betsileo, H. boettgeri, H. tricolor and H. variabilis, and we hypothesize that it represents the ancestral state for Heterixalus. A modification occurred by a pericentromeric inversion to the interstitial position on the short arm in H. luteostriatus, H. punctatus and H. rutenbergi, which would characterize these three species as monophyletic group. Of these species, H. luteostriatus and H. punctatus further share the metacentric state and larger size of the second chromosome pair.
The remaining Heterixalus can be divided in two subgroups in which the heterochromatin is distributed in (1) mainly pericentromeric bands (H. alboguttatus, H. andrakata, H. boettgeri) or (2) mainly centromeric bands (H. betsileo, H. tricolor, H. variabilis). In the first subgroup, H. alboguttatus and H. boettgeri further share more homologous chromosome pairs with paracentromeric bands than each of them with H. andrakata.
Summarizing, the phylogenetic relationships among Heterixalus suggested by our interpretation of the chromosomal data are ((betsileo, tricolor, variabilis, (andrakata, (alboguttatus, boettgeri))), (rutenbergi, (luteostriatus, punctatus))). This hypothesis is in relatively good agreement with bioacoustic data as summarized by Glaw & Vences (Citation1993, Citation1994), and molecular data (Vences et al. Citation2003a, and unpublished data). The close relationships of H. alboguttatus and H. boettgeri are obvious from all available data sets, and also supported by the karyological characters. The close relationships of H. tricolor and H. variabilis as suggested by the karyological characters are also well assessed by molecular and bioacoustic means, and indeed H. variabilis may be a junior synonym of H. tricolor (Glaw & Vences Citation1993). The similarity of advertisement call structure would predict a closer relationship of H. luteostriatus with H. boettgeri and H. alboguttatus, but molecular data so far failed to confirm this, in agreement with the distinctly different karyotypes of these species. Interestingly, the possibility of relationships between H. punctatus and H. rutenbergi, as inferred from the chromosomal characters, is also indicated by unpublished comprehensive molecular data. The divergent karyotype of H. andrakata appears to confirm its status as distinct taxon, despite its very obvious and close relationships (by bioacoustic and genetic data) to H. tricolor and H. variabilis.
As a second cytosystematic aspect, the isolated position of Leptopelis in a position unrelated to hyperoliids is supported by several lines of evidence. First, representatives of this genus have a variable chromosome number, although the species studied here, L. calcaratus, agrees with the other hyperoliids in its diploid complement of 2n = 24. Second, all Leptopelis studied so far had at least one telocentric chromosome pair, a state that so far is unknown in other hyperoliids (Kassina having a strongly subtelocentric but not telocentric twelfth pair). Third, the NOR position on the fifth chromosome pair differs from all other hyperoliids. These data confirm that Leptopelis is the most deviant of the genera studied here, and are in agreement with the hypothesis that this genus is more closely related to astylosternid and possibly arthroleptid frogs (Emerson et al. Citation2000; Vences et al. Citation2003a, Citation2003b; Frost et al. Citation2006). However, karyology seems to provide little information in this respect. Arthroleptids are characterized by a strong chromosome reduction (2n = 18, 16 and 14), whereas the two astylosternids studied have higher numbers (2n = 28 in Nyctibates, and 4× = 54 in Astylosternus diadematus) (references in King Citation1990), of which the presumably tetraploid numbers in Astylosternus are in need of confirmation. The location of NORs has proven to be of high significance for the assessment of phylogenetic relationships and systematics of amphibians (King Citation1990), including Malagasy frogs (Andreone et al. Citation2003; Aprea et al. Citation2004), but no banding has been performed so far to localize the NORs in representatives of the Astylosternidae or Arthroleptidae.
Acknowledgements
We are grateful to Marius Burger, Alan Channing, Oliver Euskirchen, Andreas Schmitz, Jasmin E. Randrianirina and Frank Glaw for assistance in the field. The authorities of Cameroon, Madagascar and South Africa kindly issued research and export permits.
References
- Andreone , F. , Aprea , G. , Vences , M. and Odierna , G. 2003 . A new Mantidactylus frog from the rainforests of north‐eastern Madagascar, and its karyological relationships. . Amphibia–Reptilia , 24 : 285 – 303 .
- Aprea , G. , Andreone , F. , Capriglione , T. , Odierna , G. and Vences , M. 2004 . Evidence for a remarkable stasis of chromosome evolution in Malagasy tree‐frogs (Boophis: Mantellidae). . Italian Journal of Zoology , 71 (Suppl. 2) : 237 – 243 .
- Baldissera , F. A. , Batistic , R. F. and Haddad , C. F. B. 1999 . Cytotaxonomic considerations with the description of two new NOR locations for South American toads, genus Bufo (Anura: Bufonidae). . Amphibia–Reptilia , 20 : 413 – 420 .
- Bogart , J. P. and Hedges , S. B. 1995 . Rapid chromosome evolution in Jamaican frogs of the genus Eleutherodactylus (Leptodactylidae). . Journal of Zoology, London , 235 : 9 – 31 .
- Bogart , J. P. and Tandy , M. 1981 . Chromosome lineages in African ranoid frogs. . Monitore Zoologico Italiano (Suppl.) , 15 : 55 – 91 .
- Blommers‐Schlösser , R. M. A. 1978 . Cytotaxonomy of the Ranidae, Rhacophoridae, Hyperoliidae (Anura) from Madagascar with a note on the karyotype of two Amphibians of the Seychelles. . Genetica , 48 : 23 – 40 .
- Channing , A. 1989 . A re‐evaluation of the phylogeny of Old‐World treefrogs. . South African Journal of Zoology , 24 : 116 – 131 .
- Drewes , R. C. 1984 . A phylogenetic analysis of the Hyperoliidae (Anura). Treefrogs of Africa, Madagascar, and the Seychelles Islands. . Occasional Papers of the California Academy of Sciences , 139 : 1 – 70 .
- Drewes , R. C. and Wilkinson , J. A. 2004 . The California Academy of Sciences Gulf of Guinea expedition (2001) I. The taxonomic status of the genus Nesionixalus Perret, 1976 (Anura: Hyperoliidae), treefrogs of São Tomé and Príncipe, with comments on the genus Hyperolius. . Proceedings of the California Academy of Sciences , 55 : 395 – 407 .
- Emerson , S. B. , Richards , C. , Drewes , R. C. and Kjer , K. M. 2000 . On the relationships among ranoid frogs: A review of the evidence. . Herpetologica , 56 : 209 – 230 .
- Frost , D. R. , Grant , T. , Faivovich , J. , Bain , R. H. , Haas , A. , Haddad , C. F. B. , de Sá , R. O. , Channing , A. , Wilkinson , M. Donnellan , S. C. 2006 . The amphibian tree of life. . Bulletin of the American Museum of Natural History , 297 : 1 – 370 .
- Glaw , F. and Vences , M. 1993 . Zur Bioakustik, Biologie und Systematik der Gattung Heterixalus aus Madagaskar (Anura: Hyperoliidae). . Salamandra , 29 : 212 – 230 .
- Glaw , F. and Vences , M. 1994 . A fieldguide to the amphibians and reptiles of Madagascar, 2nd edition , Köln : Vences and Glaw .
- Howell , W. M. and Black , D. A. 1980 . Controlled silver staining of nucleolus organizer regions with a protective colloidal developer: 1‐step method. . Experientia , 36 : 1014 – 1015 .
- John , B. 1988 . “ Biology of heterochromatin. ” . In Hetechromatin molecular and structural aspects , Edited by: Verma , R. S . 1 – 147 . Cambridge : Cambridge University Press .
- King , M. 1990 . “ Amphibia, Vol. 4 Chordata 2. ” . In Animal Cytogenetics. Stuttgart , Edited by: John , B . 1 – 241 . Berlin : Gebrüder Borntraeger .
- Mezzanotte , R. , Bianchi , U. , Vanni , R. and Ferrucci , L. 1983 . Chromatin organization, restriction nuclease activity on human metaphase chromosomes. . Cytogenetics Cell Genetics , 36 : 562 – 566 .
- Morescalchi , A. , Gargiulo , G. and Olmo , E. 1970 . Notes on the chromosomes of some Amphibia. . Journal of Herpetology , 4 : 77 – 79 .
- Odierna , G. , Aprea , G. and Capriglione , T. 1999 . Chromosomal and molecular analysis of some repeated families in Discoglossus Otth, 1837 (Anura, Discoglossidae): Taxonomic and phylogenetic implications. . Italian Journal of Zoology , 66 : 275 – 283 .
- Odierna , G. , Vences , M. , Aprea , G. , Lötters , S. and Andreone , F. 2001 . A karyological phylogeny of Malagasy poison frogs (Amphibia: Ranidae: Mantella). . Zoological Science , 18 : 505 – 514 .
- Pintak , T. , Vences , M. , Glaw , F. and Böhme , W. 1998 . Comparative chromosome morphology of Malagasy poison frogs (Amphibia:Ranidae: Mantella ). . Folia Zoologica , 47 : 197 – 204 .
- Richards , C. M. and Moore , W. S. 1998 . A molecular phylogenetic study of the old world treefrog family Rhacophoridae. . Herpetological Journal , 8 : 41 – 46 .
- Sahar , E. and Latt , S. A. 1980 . Energy Transfer and binding competition between dyes used to enhance staining differentation in metaphase chromosomes. . Chromosoma , 79 : 1 – 28 .
- Schiøtz , A. 1999 . Treefrogs of Africa , Frankfurt : Edition Chimaira .
- Schmid , M. 1978 . Chromosome banding in Amphibia. II. Constitutive heterochromatin and nucleolus organizer regions in Ranidae, Microhylidae and Rhacophoridae. . Chromosoma , 68 : 131 – 148 .
- Schmid , M. 1980 . Chromosome banding in Amphibia. IV. Differentiation of GC‐ and AT‐rich chromosome regions in Anura. . Chromosoma , 77 : 83 – 103 .
- Sumner , A. T. 1972 . A simple technique for demonstrating centromeric heterochromatin. . Experimental Cell Research , 75 : 304 – 306 .
- Van der Meijden , A. , Vences , M. and Meyer , A. 2004 . Novel phylogenetic relationships of the enigmatic brevicipitine and scaphiophrynine toads as revealed by sequences from the nuclear Rag‐1 gene. . Proceedings of the Royal Society of London B (Supplement) , 271 : S378 – S381 .
- Vences , M. , Aprea , G. , Capriglione , T. , Andreone , F. and Odierna , G. 2002 . Ancient tetraploidy and slow molecular evolution in Scaphiophryne: Ecological correlates of speciation mode in Malagasy relict amphibians. . Chromosome Research , 10 : 127 – 136 .
- Vences , M. , Kosuch , J. , Glaw , F. , Böhme , W. and Veith , M. 2003a . Molecular phylogeny of hyperoliid treefrogs: Biogeographic origin of Malagasy and Seychellean taxa and re‐analysis of familial paraphyly. . Journal of Zoological Systematics and Evolutionary Research , 41 : 205 – 215 .
- Vences , M. , Vieites , D. R. , Glaw , F. , Brinkmann , H. , Kosuch , J. , Veith , M. and Meyer , A. 2003b . Multiple overseas dispersal in amphibians. . Proceedings of the Royal Society of London B , 270 : 2435 – 2442 .