Abstract
We investigated the relationship, at local level, between fitness (measured as fish condition) of Pomastoschistus marmoratus and environmental variables of shallow areas in the Mar Menor lagoon (Spain, Mediterranean Sea): water temperature (°C), water salinity (‰), depth (cm), submerged vegetation cover (%), submerged vegetation volume, substrate size, substrate heterogeneity, fish species richness, potential competitor fish species abundance, potential competitor fish species biomass, P. marmoratus abundance and P. marmoratus biomass. The mass–length relationships were used to test differences in fish condition between nine sampling sites. The ecological variable that accounted for most of the variation in condition was the abundance of potential competitor fish species, wich was related to interspecific fish interactions. The condition of P. marmoratus populations may be a good indicator of fish density interactions in coastal lagoons and could be considered when such populations are subjected to recovery plans or any other management programmes.
Introduction
Pomatoschistus marmoratus (Risso, 1810) is a resident benthic fish in the Mar Menor coastal lagoon. The marbled goby inhabits sandy and shallow bottoms in inshore, coastal lagoons and estuarine areas. Its geographical distribution ranges from the southwest Atlantic coast of Iberian Peninsula to Mediterranean and Black Seas (Miller Citation1986). Reproduction occurs in spring and summer, males build nests under mussel shells and defend and take care of the eggs deposited by the females (Mazzoldi & Rasotto Citation2001). Their food prey consists of small crustaceans, polychaetes and chironomid larvae (Miller Citation1986).
Gobies are an important component of fish assemblages in temperate estuaries and coastal lagoons (Arruda et al. Citation1993; Salgado et al. Citation2004; Malavasi et al. Citation2005). P. marmoratus is the most abundant benthic fish in sandy shallow habitats of some estuaries and costal lagoons of the Mediterranean Sea (Koutrakis et al. Citation2000, Citation2005; Malavasi et al. Citation2004; Berrebi et al. Citation2005). In terms of abundance and biomass, both larval and adult fish are dominant in fish assemblages in the Mar Menor (Pérez‐Ruzafa et al. Citation2004).
Abundant literature concerning other Pomatoschistus spp. species is available, although few studies exist on the biology and ecology of P. marmoratus. To date, two studies about biology of this fish were done from the Suez Canal (eastern Mediterranean), Egypt (Fouda et al. Citation1993; Fouda Citation1995). More recently, new data have been published on the genetic, reproductive biology and distribution of this species in Italian and French coastal lagoons (Arculeo et al. Citation1999; Mazzoldi & Rasotto Citation2001; Berrebi et al. Citation2005). However, no data about southwest Mediterranean populations of P. marmoratus have been published.
In the management of fish populations it is common to measure individual, cohort (e.g. age or size groups) and population fitness (Jakob et al. Citation1996). Such measurements are indicators of tissue energy reserves and may characterize components of the environment in which the fish live (e.g. food and habitat availability, competition, predation, physical factors and pollution) (Jackson et al. Citation2002; Copp Citation2003; Lloret & Planes Citation2003; Oliva‐Paterna et al. Citation2003). These measurements reflect what is generally known as fish condition. A low fish condition can negatively affect growth, survival, first maturity and reproductive effort in subsequent phases of fish life history (Rätz & Lloret Citation2003; Hoey & McCormick Citation2004; Morgan Citation2004; Lloret et al. Citation2005).
Fish assemblages of coastal lagoons can be classified by reference to functional guilds, defined as a group of species that exploit the same class of environmental resources in a similar way (Root Citation1967). In this sense, fish species could show an overlap in resource utilization in time and space and, at certain times and/or sites, this could produce inter‐ and intraspecific competition events (Rice Citation2005).
The study of fish condition together with the investigations concerning the habitat characteristics allow for a better understanding of the biology and ecology of fish populations. Therefore, the aim of this work was to examine spatial variations in condition, estimated from the mass–length relationship, of P. marmoratus. More specifically, our aim was to study the relationship between fish condition and several environmental variables such as: water temperature (°C), water salinity (‰), depth (cm), submerged vegetation cover (%), submerged vegetation volume, substrate size, substrate heterogeneity, fish species richness, potential competitor fish species abundance, potential competitor fish species biomass, P. marmoratus abundance and P. marmoratus biomass.
Materials and methods
Study area
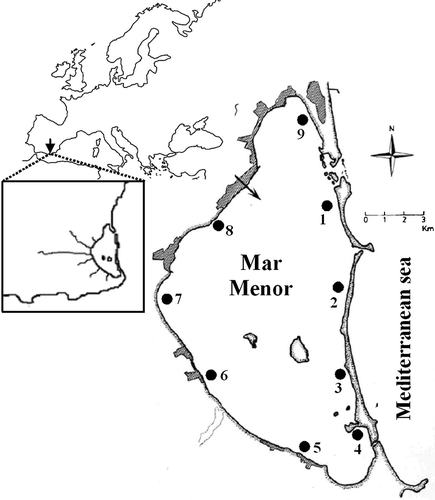
The Mar Menor is a hypersaline coastal lagoon located in a semiarid region in the south‐east of the Iberian Peninsula (Figure ). It is one of the largest coastal lagoons in the Mediterranean region and Europe, with a surface area of 135 km2 and an average depth of 3–4 m. It is separated from the Mediterranean Sea by a 22 km‐long sand bar with three narrow channels connecting it with the sea. The lagoon shows a salinity range of 39–45‰ and the temperature varies from 10°C in winter to 32°C in summer. Its bottom is principally covered by dense meadows of the invasive macroalga Caulerpa prolifera, although shallow areas are covered by meadows of Cymodocea nodosa (Pérez‐Ruzafa et al. Citation2005).
Since the 1970s, the Mar Menor has suffered strong environmental changes following widening of the connecting channels, which has caused a decrease in salinity from 50–52‰ to the present levels. Moreover, regular and intermittent watercourses flow into the lagoon, draining a large intensive agricultural area and leading to an important input of agrochemicals. Finally, the Mar Menor coastal lagoon supports important commercial fisheries and is subject to intensive tourist development (Pérez‐Ruzafa et al. Citation2005).
Sampling methods
The catches were carried out during July 2002 as part of a wider study to examine the effects of human activities on fish communities of the coastal lagoon. A total of nine sampling sites were selected in the perimeter shallow coastal areas. Samples were collected using a 10 m‐long bag seine net and 0.5 mm mesh size, which allowed the collection of juvenile fish and adults of small‐sized species. We collected three quantitative replicates at each sampling site by nearby (separated by approximately 50 m) 20 m reaches of shoreline at each site. In each reach, the bag seine was hauled offshore parallel to the shoreline in water <1.0 m for the whole length of the reach. The area covered by each haul was approximately 160 m2 (quantitative sampling).
Additionally, one bag seine haul and five quadrangular (40×40 cm) hand net sweeps (non‐quantitative samplings) were made along the shoreline in each sampling area. Our goal was to sample all shoreline habitats to detect species richness in a given sampling site.
Thus, three replicate samples were obtained at each of the nine sampling sites, enabling us to assess variance within sites and the efficiency of seining.
Fish from each of the three reaches (quantitative samplings) and non‐quantitative samplings were preserved in separate jars in 7% formaldehyde, before being removed and identified at species level in the laboratory (Whitehead et al. Citation1986; Arias & Drake Citation1990).
Relative abundance was expressed as catch per unit effort (CPUEs) and biomass per unit effort (BPUEs):
Each sampling site was characterized by 12 environmental variables and indices (quantified in each reach of every sampling site) related to water quality (weekly mean values), habitat structure (local level), possible resources exploited by fish populations and inter and intraspecific fish interactions: water temperature (°C), water salinity (‰), depth (cm), submerged vegetation cover (%), submerged vegetation volume, substrate size, substrate heterogeneity, fish species richness, potential competitor fish species abundance, potential competitor fish species biomass, P. marmoratus abundance and P. marmoratus biomass (Table ).
Table I. Mean values±SD of variables in each sampling site: water temperature; water salinity; depth; submerged vegetation richness; submerged vegetation cover; submerged vegetation volume: 0 (low density) to 5 (high density); substrate size (SS; mean value); substrate heterogeneity (SH; mean value); fish species richness; potential competitor fish species abundance; potential competitor fish species biomass.
Water temperature (±0.1°C) and salinity (±0.1) were measured three times using an universal pocket meter WTW® Multi340i. The assessment of submerged vegetation cover and submerged vegetation volume was made visually, the first recorded as the area percentage covered by submerged vegetation in each reach and the second as an ordinate categorical variable from 0 (low density of meadows) to 5 (high density of meadows). We classified substrate sensu Bain (Citation1999) [mud (1), sand (2), gravel (3), pebble (4) and boulder (5)] and assessed the substrate size (SS; average in each sampling site) and substrate heterogeneity (SH, standard deviation in each sampling site) by making at least 10 visual designations in each reach. The mean values of the above‐mentioned environmental variables were assessed at each sampling site. Fish species richness was evaluated as the total number of fish species at each sampling site.
Lipophrys dalmatinus, Salaria pavo, Gobius niger, Gobius paganellus, Callionymus pusillus, Diplodus sargus sargus (juveniles; total length ⩽7.0 cm) and Solea solea (juveniles; total length ⩽7.0 cm) were considered as potential competitor fish species due to their benthic habitat and dietary preferences (Dumay et al. Citation2004; Malavasi et al. Citation2004). In this way, potential competitor fish species and P. marmoratus relative abundance and biomass were assessed as mean CPUEs and BPUEs, respectively, at each sampling site.
A total of 325 individuals of P. marmoratus (with total lengths ranging from 27 to 53 mm) from nine sampling sites were measured for total length (TL, ±1 mm), standard length (SL, ±1 mm), total mass (TM, ±0.01 g) and eviscerated mass (EM, ±0.01 g). In addition, a subsample of potential competitor fish species were measured for total length (TL, ±1 mm): Lipophrys dalmatinus (n = 37), Salaria pavo (n = 19), Gobius niger (n = 15), Gobius paganellus (n = 21), Callionymus pusillus (n = 22), Diplodus sargus sargus (n = 89) and Solea solea (n = 20).
Statistical analyses
The statistical analysis used to compare fish condition was based on that used in previous studies (Vila‐Gisbert & Moreno‐Amich Citation2001; Oliva‐Paterna et al. Citation2003) and proposed by García‐Berthou & Moreno‐Amich (Citation1993). The method applies univariate analysis of covariance (ANCOVA) using eviscerated mass as the dependent variable and total length as the covariate. The relationship between eviscerated mass and length was clearly non‐linear, but was linear after log‐transformation. The homogeneity of the regression coefficients (slopes) was tested with an ANCOVA design that analysed the pooled covariate‐by‐factor interaction. If the covariate‐by‐factor interaction (homogeneity of slopes) was not significant (P>0.05), standard ANCOVA was applied to test differences in the parameter a (fish condition) between sampling sites.
A stepwise multiple regression analysis was performed to determine the amount of variation in parameter a (fish condition) associated with environmental variables. This regression procedure first selects the most correlated independent variable, and then removes the variance in the dependent variable. It then selects the second independent variable which most correlates with the remaining variance in the dependent variable, and so on until selection of an additional independent does not increase the r 2 by a significant amount (P⩽0.05).
Statistical analyses were performed with SPSS® software package and a significance level of P⩽0.05 was accepted.
To explore patterns of association among the environmental variables of the nine sampling sites, the principal component analysis (PCA) was applied to the correlation matrix. A Varimax rotation of the resulting matrix was performed (Visauta‐Vinacua Citation1997).
Results
Juvenile and adult individuals of 17 fish species were captured: Aphanius iberus, Atherina boyeri, Pomatoschistus marmoratus, Gobius niger, Gobius paganellus, Callionymus pusillus, Salaria pavo, Lypophrys dalmatinus, Solea solea, Diplodus sargus sargus, Sarpa salpa, Liza aurata, Liza saliens, Liza ramado, Hippocampus guttulatus, Syngnathus abaster and Belone belone.
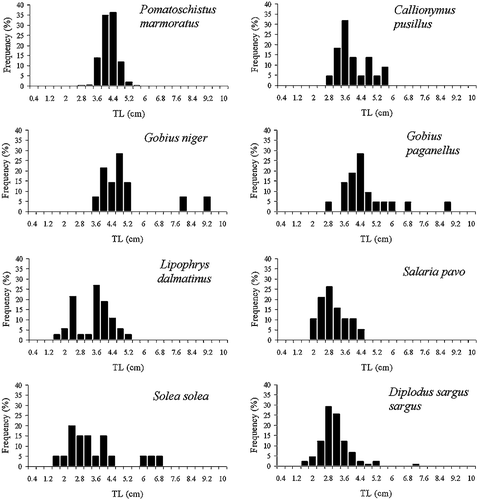
The size frequency distribution for the potential competitor fish species is shown in Figure .
Figure 2. Size frequency distributions forPomatoschistus marmoratus and potential competitor fish species in the Mar Menor coastal lagoon.
Coefficients of the mass–length relationship of sampling sites are presented in Table and the results of the ANCOVA are shown in Table . There was significant homogeneity between sampling sites on the slope (parameter b) of the EM–TL relationships (Preliminary design, Table ), although the y‐intercept (parameter a) varied significantly between sampling sites (Final design, Table ). As a result, sampling sites could be compared according to the values of parameter a of the EM–TL relationships. The La Chanta (1) sampling site showed the lowest fish condition value, and El Ciervo (4) showed the highest fish condition value.
Table II. Regression coefficients (a, b) ±SD and determination coefficients (r 2) of the log‐transformed mass–length relationship.
Table III. ANCOVA analyses of the mass–length relationship in Pomatoschistus marmoratus. All variables (dependent and covariate) were log‐transformed. Total length is the covariate.
A stepwise multiple regression model indicated that only potential competitor fish species abundance accounted for the variation between sampling sites (60.7%) of parameter a in the EM–TL relationships (Table ), pointing to a negative effect on the condition of P. marmoratus.
Table IV. Stepwise multiple regression model used to predict condition (a) of Pomatoschistus marmoratus from environmental variables.
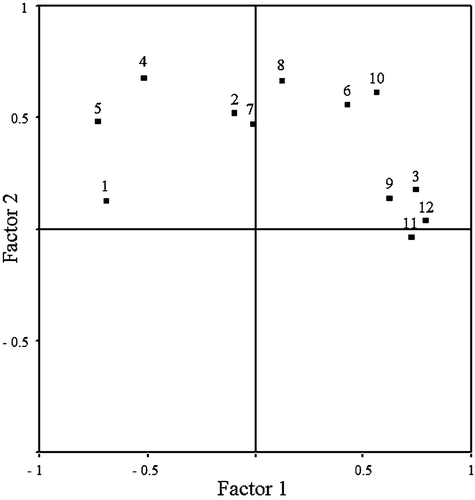
The first two factors extracted by PCA explained 52% of total variance (factor 1, 32.3%; factor 2, 19.7%). Inspection of Figure supports the following conclusions: there was a positive correlation of the first factor with P. marmoratus abundance and biomass, depth and potential competitor fish species abundance. Moreover, water temperature was negatively correlated with the first factor. Secondly, fish species richness was the variable associated most strongly with factor 2.
Figure 3. Result of the principal component analysis that includes the following variables: 1, water temperature; 2, salinity; 3, depth; 4, submerged vegetation cover; 5, submerged vegetation volume; 6, substrate size; 7, substrate heterogeneity; 8, fish species richness; 9, potential competitor fish species abundance; 10, potential competitor fish species biomass; 11, Pomatoschistus marmoratus abundance; 12, P. marmoratus biomass.
Discussion
In the investigation of intra‐ and interpopulation variations in fish condition, mass–length regressions provide a good alternative to relative mass indices (ratio‐related techniques), assuming that the slope does not vary between nearby sampling sites or that the slope is homogeneous for population on a local level (Sutton et al. Citation2000). Relative mass indices do not normally fulfil these underlying assumptions and have been criticized on statistical grounds (Jakob et al. Citation1996).
Our results showed that the condition of P. marmoratus differed between the sampling sites studied. Any differences in parameter a of the EM–TL relationship were probably caused by differences in ecological condition. Although fish size could be an important factor affecting fish condition (Pope & Willis Citation1996), the homogeneity of the slopes of the mass–length relationship obtained for the sampling sites studied indicated that condition was independent of fish body form (Winters & Wheeler Citation1994).
In our site level analysis of habitat‐fish condition relationships, potential competitor fish species abundance was the ecological variable that best correlated with P. marmoratus condition. Sampling sites with higher values of potential competitor fish abundance (La Chanta and Las Brisas) provided the lowest values for parameter a of the EM–TL relationship, demonstrating a poorer fish condition.
It is known that Mediterranean shallow coastal lagoons are environments that exhibit very high variability in biotic and abiotic parameters, at both temporal and spatial level (Malavasi et al. Citation2004; Koutrakis et al. Citation2005). In gobiid populations inhabiting these areas, environmental variability can lead to different degrees of competition, predation and mortality (Pampoulie et al. Citation2000, Citation2001; Pampoulie, Citation2001).
Fish population densities vary in time and space, and there may be strong seasonal variations in the effects of competitors (Steele & Forrester Citation2002).
In the warm season (June to September), the shallow areas of the Mar Menor are inhabited by high densities of juvenile fishes of different species (gobiidae and bleniidae principally), which use these areas as nursery sites. So, during the sampling period (July) the size structure of the fish community was very similar (see Figure ). Moreover, Pomatoschistus marmoratus abundance was positively correlated with the abundance of potential competitor fish species.
In such situation, competition for prey resources (due to trophic overlap) and/or for refuge from predators was probably higher. Competition for food and space, both within and between species, has been considered an important factor in the structuring of fish communities (Elliot & Hemingway Citation2002). Although no data about prey resources in the Mar Menor are available, it is certain that the sampling sites presented a relatively low substrate size (sand–gravel) and low submerged vegetation cover, so competition for refuge was likely higher.
Density‐dependent processes, such as competition and aggression among individuals of the same or different species, can be an influential factor in fitness, growth, reproduction and survival (Wootton Citation1998; Hensor et al. Citation2005). In addition, Jackson et al. (Citation2002) concluded that a reduction in prey availability for P. microps reduced their condition and, consequently, their fitness and nest quality.
In another scenario, the competitive superiority of larger individuals may reduce the availability of resources for smaller individuals, with the result that the dominant fish show a higher condition value than subordinate fish (Adams et al. Citation1998; Sloman et al. Citation2001). In sampling sites with a higher abundance of potential competitor fish species, it is very probable that the territorial and aggressive behaviour of larger individuals (see Figure ) of Gobius niger and G. paganellus denies P. marmoratus access to prey resources. Indeed, Malavasi et al. (Citation2005) suggested that the distribution of smaller gobies such as P. marmoratus and P. microps in the shallow areas of Venice lagoon could be related to the competitive and predation pressure due to the presence of larger gobies (e.g. G. niger).
Hence, a high abundance of potential competitor fish species produces an important level of stress in P. marmoratus individuals, which is reflected in a decrease of its somatic condition.
We found no relationships between the other variables of habitat structure and fish condition, probably due to the number of sampling sites or perhaps because any relationship was clouded by the very complexity of the ecological interactions (e.g. a nonlinear relationship between these variables). To a certain extent, this demonstrates the need for more investigation into the relationships between habitat characteristics, environmental variations and fish condition in the study area.
In conclusion, our results suggest that, at least in the study period, interspecific fish competition in the shallow areas of the Mar Menor can be an influential factor in the somatic condition of P. marmoratus individuals. This situation can affect the subsequent phases of its life history traits such growth and reproduction.
Acknowledgements
The authors are grateful to A. Bastida‐León, A. Andreu‐Soler, A. Egea‐Serrano, P.A. Miñano, and members of the Department of Zoology of the University of Murcia for help in field sampling; and P. Thomas for the English revision. Part of this research was supported by the Environmental Service of the Autonomous Government of Murcia, Spain.
References
- Adams , C. E. , Huntingford , F. A. , Turnbull , J. F. and Beattie , C. 1998 . Alternative competitive strategies and the cost of food adquisition in juvenile Atlantic salmon (Salmo salar). . Aquaculture , 167 : 17 – 26 .
- Arculeo , M. , Mauro , A. , Lo Brutto , S. , Mirto , S. , Cammarata , M. , Mazzola , A. and Parrinello , N. 1999 . Biochemical genetic differentiation between Pomatoschistus marmoratus and P. tortonesei . Journal of Fish Biology , 54 : 190 – 195 .
- Arias , A. M. and Drake , P. 1990 . Estados alevines y juveniles de la ictiofauna en los caños de las salinas de la bahía de Cádiz , Cádiz : Instituto de Ciencias Marinas de Andalucía .
- Arruda , L. M. , Azevedo , J. N. and Neto , A. I. 1993 . Abundance, age‐structure and growth, and reproduction of Gobies (Pisces; Gobiidae) in the Ria de Aveiro Lagoon (Portugal). . Estuarine, Coastal and Shelf Science , 37 : 509 – 523 .
- Bain , M. B. 1999 . “ Substrate. ” . In Aquatic habitat assesment: common methods , Edited by: Bain , M. B and Stevenson , N. J . 95 – 100 . Bethesda, Maryland : American Fisheries Society .
- Berrebi , P. , Rodriguez , P. , Tomasini , J. A. , Cattaneo‐Berrebi , G. and Crivelli , A. J. 2005 . Differential distribution of the two cryptic species, Pomatoschistus microps and P. marmoratus, in the lagoons of southern France, with an emphasis on the genetic organisation of P. microps. . Estuarine, Coastal and Shelf Science , 65 : 708 – 716 .
- Copp , G. H. 2003 . Is fish condition correlated with water conductivity? . Journal of Fish Biology , 63 : 263 – 266 .
- Dumay , O. , Tari , P. S. , Tomasini , J. A. and Mouillot , D. 2004 . Functional groups of lagoon fish species in Languedoc Roussillon, southern France. . Journal of Fish Biology , 64 : 970 – 983 .
- Elliott , M. and Hemingway , K. L. 2002 . Fishes in estuaries. , Oxford : Blackwell Science .
- Fouda , M. M. 1995 . Life history strategies of four small‐size fishes in the Suez Canal, Egypt. . Journal of Fish Biology , 46 : 687 – 702 .
- Fouda , M. M. , Hanna , M. Y. and Fouda , F. M. 1993 . Reproductive biology of a Red Sea goby, Silhouettea aegyptia, and a Mediterranean goby, Pomatoschistus marmoratus, in Lake Tisah, Suez Canal. . Journal of Fish Biology , 43 : 139 – 151 .
- García‐Berthou , E. and Moreno‐Amich , R. 1993 . Multivariate analysis of covariance in morphometric studies of the reproductive cycle. . Canadian Journal of Fish and Aquatic Science , 50 : 1394 – 1399 .
- Hensor , E. , Couzin , I. D. , James , R. and Krause , J. 2005 . Modelling density‐dependent fish shoal distributions in the laboratory and field. . Oikos , 110 : 344 – 352 .
- Hoey , A. S. and McCormick , M. I. 2004 . Selective predation for low body condition at the larval–juvenile transition of a coral reef fish. . Oecologia , 139 : 23 – 29 .
- Jackson , A. C. , Rundle , S. D. and Attrill , M. J. 2002 . Fitness consequences of prey depletion for the common goby Pomatoschistus microps. . Marine Ecology Progress Series , 242 : 229 – 235 .
- Jakob , E. M. , Marshall , S. D. and Uetz , G. W. 1996 . Estimating fitness: a comparison of body condition indices. . Oikos , 77 : 61 – 67 .
- Koutrakis , E. T. , Kokkinakis , A. K. , Eleftheriadis , E. A. and Argyropoulou , M. D. 2000 . Seasonal changes in distribution and abundance of the fish fauna in the two estuarine systems of Strymonikos gulf (Macedonia, Greece). . Belgian Journal of Zoology , 130 (Supplement 1) : 41 – 48 .
- Koutrakis , E. T. , Tsikliras , A. C. and Sinis , A. I. 2005 . Temporal variability of the ichthyofauna in a Northern Aegean coastal lagoon (Greece). Influence of environmental factors. . Hydrobiologia , 543 : 245 – 257 .
- Lloret , J. and Planes , S. 2003 . Condition, feeding and reproductive potential of white seabream Diplodus sargus as indicators of habitat quality and the effect of reserve protection in the northwestern Mediterranean. . Marine Ecology Progress Series , 248 : 197 – 208 .
- Lloret , J. , Galzin , R. , Gil de Sola , L. , Souplet , A. and Demestre , M. 2005 . Habitat related differences in lipid reserves of some exploited fish species in the north‐western Mediterranean continental shelf. . Journal of Fish Biology , 67 : 51 – 65 .
- Malavasi , S. , Fiorin , R. , Franco , A. , Franzoi , P. , Granzotto , A. , Riccato , F. and Mainardi , D. 2004 . Fish assemblages of Venice lagoon shallow waters: an analysis based on species, families and functional guilds. . Journal of Marine Systems , 51 : 19 – 31 .
- Malavasi , S. , Franco , A. , Fiorin , R. , Franzoi , P. , Torricelli , P. and Minardi , D. 2005 . The shallow water gobiid assemblage of the Venice Lagoon: Abundance, seasonal variation and habitat partitioning. . Journal of Fish Biology , 67 (Supplement B) : 146 – 165 .
- Mazzoldi , C. and Rassotto , M. 2001 . Extended breeding season in the marbled goby, Pomatoschistus marmoratus (Teleostei: Gobiidae), in the Venetian Lagoon. . Environmental Biology of Fishes , 61 : 175 – 183 .
- Miller , P. J. 1986 . “ Gobiidae. ” . In Fishes of the North‐eastern Atlantic and the Mediterranean, vol. III. , Edited by: Whitehead , P. J. P , Bauchot , M. L , Hureau , J. C , Nielsen , J and Tortonese , E . 1019 – 1085 . Paris : UNESCO .
- Morgan , M. J. 2004 . The relatioship between fish condition and the probability of being mature in American plaice (Hippoglossoides platessoides). . ICES Journal of Marine Science , 61 : 64 – 70 .
- Oliva‐Paterna , F. J. , Vila‐Gisbert , A. and Torralva , M. 2003 . Condition of Barbus sclateri from semiarid aquatic systems: Effects of habitat quality disturbances. . Journal of Fish Biology , 63 : 1 – 11 .
- Pampoulie , C. 2001 . Demographic structure and life history traits of the common goby Pomatoschistus microps (Teleostei, Gobiidae) in a Mediterranean coastal lagoon (Rhône River delta, France). . Acta Oecologica , 22 : 1 – 5 .
- Pampoulie , C. , Bouchereau , J. L. , Rosecchi , E. , Poizat , G. and Crivelli , A. J. 2000 . Annual variations in reproductive traits of Pomatoschistus microps in a Mediterranean lagoon undergoing environmental changes: Evidence of phenotypic plasticity. . Journal of Fish Biology , 57 : 1441 – 1452 .
- Pampoulie , C. , Chauvelon , P. , Rosecchi , E. , Bouchereau , J. L. and Crivelli , A. J. 2001 . Environmental factors influencing the gobiid assemblage of a Mediterranean Lagoon: Empirical evidence from a long‐term study. . Hydrobiologia , 445 : 175 – 181 .
- Pérez‐Ruzafa , A. , Quispe‐Becerra , J. I. , García‐Charton , J. A. and Marcos , C. 2004 . Composition, structure and distribution of the ichthyoplankton in a Mediterranean coastal lagoon. . Journal of Fish Biology , 64 : 202 – 218 .
- Pérez‐Ruzafa , A. , Marcos , C. and Gilabert , J. 2005 . “ The ecology of the Mar Menor coastal lagoon: a fast changing ecosystem under human pressure. ” . In Coastal Lagoons. Ecosystem Processes and Modelling for Sustainable Use and Development. , Edited by: Gönenç , I. E and Wolflin , J. P . 392 – 422 . Boca Raton : CRC Press .
- Pope , K. L. and Willis , D. W. 1996 . Seasonal influences on freshwater fisheries sampling data. . Reviews in Fisheries Science , 4 : 57 – 73 .
- Rätz , H. J. and Lloret , J. 2003 . Variation in fish condition between Atlantic cod (Gadus morhua) stocks, the effect on their productivity and management implications. . Fisheries Research , 60 : 369 – 380 .
- Rice , J. C. 2005 . Understanding fish habitat ecology to achieve conservation. . Journal of Fish Biology , 67 (Supplement B) : 1 – 22 .
- Root , R. B. 1967 . The niche exploitation pattern of the blue‐gray gnatcatcher. . Ecological Monographs , 37 : 317 – 350 .
- Salgado , J. P. , Nogueira‐Cabral , H. and Costa , M. J. 2004 . Feeding ecology of the gobies Pomatoschistus minutus (Pallas, 1770) and Pomatoschistus microps (Kroyer, 1838) in the upper Tagus stuary, Portugal. . Scientia Marina , 68 : 425 – 434 .
- Sloman , K. A. , Taylor , A. C. , Metcalfe , N. B. and Gilmour , K. M. 2001 . Effects of an environmental perturbation on the social behaviour and physiological function of brown trout. . Animal Behaviour , 61 : 325 – 333 .
- Steele , M. A. and Forrester , G. E. 2002 . Variation in the relative importance of sublethal effects of predators and competitors on growth of a temperate reef fish. . Marine Ecology Progress Series , 237 : 233 – 245 .
- Sutton , S. G. , Bult , T. P. and Haedrich , R. L. 2000 . Relationships among fat weight, body weight, water weight and condition factors in wild salmon parr. . Transactions of the American Fisheries Society , 129 : 527 – 538 .
- Vila‐Gisbert , A. and Moreno‐Amich , R. 2001 . Mass–length relationship of Mediterranean barbel as an indicator of environmental status in South‐west European stream ecosystems. . Journal of Fish Biology , 59 : 824 – 832 .
- Visauta‐Vinacua , B. 1997 . Análisis estadístico con SPSS para Windows , Madrid : McGraw‐Hill .
- Whitehead , P. J. P. , Bauchot , M. L. , Hureau , J. C. , Nielsen , J. and Tortonese , E. 1986 . Fishes of the North‐eastern Atlantic and the Mediterranean vol. III , Bungay : Unesco .
- Winters , G. H. and Wheeler , J. P. 1994 . Length‐specific weight as a measure of growth success of adult Atlantic herring (Clupea harengus). . Canadian Journal of Fisheries and Aquatic Science , 51 : 1169 – 1179 .
- Wootton , R. J. 1998 . Ecology of Teleost Fishes , Dordrecht : Kluwer Academic Publishers .