Abstract
The maturation process of starfish and Xenopus oocyte are described in detail. For the former, the role of 1‐methyl adenine is recalled, together with that the dissociation of a G protein into its alpha, beta and gamma subunits of and the role of the M‐phase promoting factor (MPF). The detailed roles of gonadostimule and of 1‐methyladenine are described, as it is that of the following steps, i.e. that of the product of the radial nerve, which stimulates 1‐methyladenine excretion. As for Xenopus, the mechanism of MPF activation by progesterone is described.. This brings to the translation of the messenger for the protein Pp39mos, which activates a MAPK pathway. The mechanism of the second arrest at metaphase II, because of the effect of the cytostatic factor involving the action of the proteins XErp1/Emi2, Plx1 and Cam II, is also described in detail.
Keywords:
Introduction
The process of oocyte maturation is a general one preceding fertilization. It is, however, known that fertilization can occur at different stages of oocyte meiosis in different species (Giudice Citation1991).
Oocyte maturation has been studied in several species. Among these, a marine invertebrate (a sea starfish) and a vertebrate (the amphibian Xenopus), have been the object of the most intensive studies. We therefore decided to review these two cases, with the object to obtain information on the evolutive conservation of such an important process. We will therefore describe in part one oocyte maturation in starfishes, and in part two the same process on Xenopus.
The aim of the present review is that of reporting and commenting on some data relevant to the problem of oocyte maturation, as studied in two of the main model systems, starfishes and frogs.
Starfishes
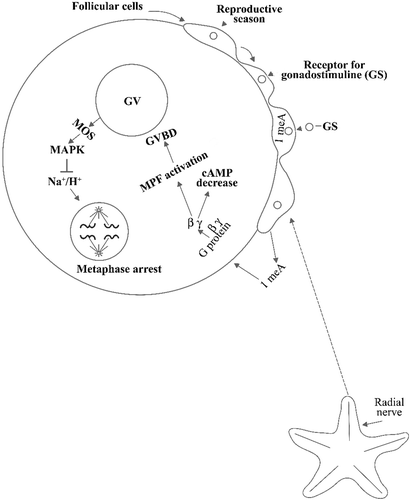
As starfish are concerned, it is known that the oocytes are blocked at the stage of the first meiotic division (Kishimoto Citation1998; Figure ). During the reproductive season a receptor appears on the surface of the follicle cells surrounding the oocytes (Mita et al. Citation1999). A gonadostimuline binds to this receptor, which causes the formation within the follicular cells of a small hormone molecule, the 1‐methyladenine (Kanatani et al. Citation1969). At this point a peptide hormone is produced in the radial nerves, within the starfish arms, which stimulates the export of the 1‐methyladenine from the follicular cells to the oocyte surfaces (Kanatani Citation1973). Here 1meA stimulates a G protein, producing the dissociation of the beta gamma subunits from the alpha. The receptor of the 1meA has not been identified yet. Another of the effects of 1meA is that of lowering cAMP concentration, therefore lowering its inhibitory activity on maturation. At meiosis reinitiation the disassembly of the nuclear envelope and breakdown (GVBD) start. It has been shown by Santella et al. (Citation2000) that there occurs a nuclear Ca2+ increase by adding 1meA to the seawater and a specific degradation of some proteins such as tubulin, lamin, and B actin, related to the cytoskeleton. The specific protein degradation was also obtained by calpain microinjection, thus indicating its possible involvement by Ca2+ activation. Calpain, activated by Ca2+ increase, can induce proteolysis of cytoskeletal proteins during GVBD. It has been proposed that the G‐protein mediates the activation of PI 3‐kinase (phosphatidylinositol‐3‐OH kinase) which generates PIP3 (3‐phosphatidylinositol 3, 4.5.triphosphate). This may signal the plasma membrane and activate PDK1 (3‐phosphoinositide‐dependent kinase1) where Atk/protein kinase B (PKB) becomes phosphorylated in the Thr 308 residue. PKB is believed to be an M‐phase initiator because it phosphorylates and inactivates Myt1 kinase, which normally inhibits the Cdc2 of the cyclin B complex or MPF (M‐phase promoting factor). For the resumption of meiosis, early experiments in frogs by Kishimoto (Citation2003, Citation2004) showed that MPF extracted from frog oocytes were able to induce the M phase when injected into oocytes of starfish. Kishimoto (Citation2003, Citation2004) was studying a cytoplasmic molecule as a mediator under the influence of progesterone.
Figure 1. Scheme showing a starfish oocyte with part of the follicular cells which surround it. A ligand(the gonadostimuline, GS), in the reproductive season stimulates the secretion of the hormone 1‐methyladenine within the follicular cells. A peptide hormone, produced by the radial nerves, elicits 1‐methyladenine excretion from the follicular cells allowing it to bind the oocyte surface where it stimulates a G protein whose βγ subunits cause on one side cAMP decrease and on the other MPF activation with consequent germinal vesicle breakdown. With this breakdown there is further progress toward maturation until metaphase occurs, mediated by MOS and MAPK, but with no activation of the Na+/K+ antiporter, which is activated after oocyte shedding into seawater.
In starfish the Cdc25 phosphatase acts on the cyclin B complex leading to its initial activation at the meitoic G2/M phase transition. The Cdc2/cyclin complex is stable for 60 min, after which cyclin B is degraded. GVBD also was obtained in cell‐free preparations, where MPF activity was stable for 2 h (Chiba et al. Citation1999). Harada et al. (Citation2003) found that after GVBD the oocytes undergo a metaphase arrest induced by Mos and the MAPK pathway.
By contrast, in Xenopus oocytes at meiosis reinitiation, MAP kinase is activated prior to cyclin B–Cdc2 activation, and it is essential for this process.
In starfish oocytes the MAPK (mitogen‐activated protein kinase) pathway at low pH blocks the cyclin B degradation. Resumption of meiosis is also due to the activity of PDK1 and of Akt kinase. Hiraoka et al. (Citation2004) provided evidence that PDK1 is required for meiosis resumption in starfish, and that PDK2 has a distinct role in this maturation process, which fully activates Akt only if PDK1 is active.
Oocytes can be released from this metaphase arrest by shedding into seawater with the consequent activation of the Na+/H+ antiporter and pH increase. The described pH increase causes polyubiquitinated cyclin B degradation and release from the metaphase arrest. At his point starfish oocyte maturation is completed.
Mori et al. (Citation2006) propose that whereas there is a CSF (cytostatic factor) for the II metaphase arrest of amphibian oocytes, one should add to this a G1–CSF downstream of which MAPK and p90Rsk sequentially act to cause the G1 arrest.
Xenopus
The main studies of frogs have been carried out in Xenopus laevis. In this case, oocytes are blocked at the stage of diplotene of the first meiosis for a number of reasons, among which we will mention here the possible effect of the Chk1 kinase on some Cdc phosphatases (Nakajo et al. Citation1999); the effect of the Gs protein (Kalinowski et al. Citation2004); the action of GSK3 (Fisher et al. Citation1999), and the action of a XPAK1 Kinase, a member of the Ste/PAK family (Faure et al. Citation1997). For the assembly of the first meiotic spindle the proteins XMAP215, XKCM1, NuMA and cytoplasmic dynein are required (Becker et al. Citation2003).
The oocytes are surrounded by follicular cells (Figure ) which have receptors for the hormone progesterone on their surfaces (Tian et al. Citation2000; Bayaa et al. Citation2000; Frank‐Vaillant et al. Citation2000). When in the reproductive season, this is produced by the hypophysis, which binds to its receptor and inhibits a G protein from releasing its beta and gamma subunits (Lutz et al. Citation2000; Sheng et al. Citation2001; Wang & Liu, Citation2003). This causes a decrease of the cAMP content and a subsequent inhibition of the Kinase PKA (Wang & Liu, Citation2004). Progesterone causes a series of reactions culminating in the translation of the RNA for the protein Pp39mos, laying till that point inactive in the cytoplasm. This happens because a protein called CEBP gets phosphorylated by the effect of the protein Xgef (Martinez et al. Citation2005) and binds to a sequence of this RNA (Howard et al. Citation1999; Dickson et al. Citation1999; Reverte et al. Citation2003), made by 6 U and 1 A, causing the RNA polyadenylation (Hake & Richter Citation1994; Sheets et al. Citation1994; Barkoff et al. Citation1998) in 3'. At the same time the cap at its 5' end becomes methylated (Kuge et al. Citation1998). The Pp39‐mos protein produced at this point activates a MAPKKK which activates a MAPKK, which in turn activates a MAPK (Muslin et al. Citation1993; Matten et al. Citation1996; Radziwill et al. Citation1996; Barkoff et al. Citation2000; Ranginwale et al. Citation2001; Walter et al. Citation2000; Frank‐Vaillant et al. Citation2001) that with the aid of Hsp70 and Hsp90 (Fisher et al. Citation2000) activates the Cdc‐25 phosphatase. This finally activates the MPF (De Smedt et al. Citation2002), thus inducing histone phosphorylation and germinal vesicle breakdown (GVBD) (Sagata et al. Citation1988). It has been indicated that Mos and Cdc2 activate the translation of the FGF receptor‐1 during progesterone‐induced maturation (Culp & Musci Citation1999). A protein necessary and sufficient to activate MPF has also been described (Ferby et al. Citation1999). Results obtained by the use of a P90rsk mutant that constitutively interacts with MAPK indicate that p42mpk1 activation normally precedes the activation of pre‐MPF but that nuclear migration to the oocyte cortex can occur also in the absence of pre‐MPF activation (Gavin et al. Citation1999).
Figure 2. (a) Scheme showing the pituitary gonadotropin acting on the follicular cells surrounding the Xenopus oocyte and stimulating progesterone production. Progesterone acts in three ways: one, liberating Cdc25 from the 14‐3‐3 protein and moving it to oocyte pronucleus; second, binding its receptor and therefore inhibiting a G protein from breaking into its subunits; and third, by stimulating binding of the protein CPEB to its site (CPR) in the Mos mRNA. As a consequence of this, Mos mRNA becomes polyadenylated at its 3'end and capped at its 5'end, and therefore translates into its Pp39‐mos protein. This stimulates a MapKK, which in turn activates a MapK and with the aid of Hsp 70 and 90 activates the phosphatase Cdc‐25, which finally activates MPF. Lastly, MPF induces germinal vesicle breakdown. This moves the oocyte to what is described in Figure . (b) Histone phosphorylation which precedes germinal vesicle breakdown makes the oocyte proceed until the II metaphase at which point it becomes arrested again because of the sequential effects of P42, P‐90 RsK, CSF (cytostatic factor), and Emi‐1, until fertilization.
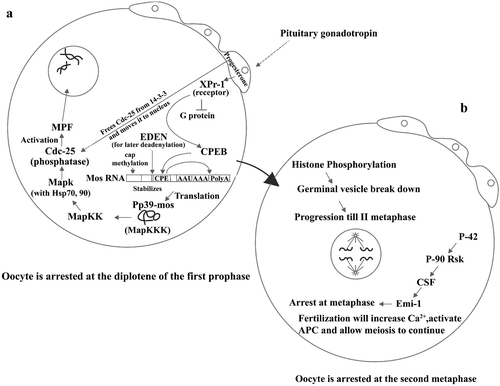
It has also been observed that Mos can activate germinal vesicle breakdown independently of the MAPK cascade, by triggering phosphorylation of Myt1 (a kinase which negatively regulates MPF) (Palmer et al. Citation1998; Peter et al. Citation2002 ).
After GVBD, however, the oocytes can progress until the II metaphase, where they become arrested again (Figure ) because of the action of the cytostatic factor (CSF) (Masui Citation2000; Kubiak & Ciemerych Citation2001; Duesbery & Vande Woude Citation2002; Tunquist & Maller Citation2003). It has been shown that DNA replication might occur at this point because of the presence of the mRNA for protein Cdc6 in the oocytes. If this were translated, however, CSF would contrast DNA synthesis by maintaining high levels of active Cdc2/Cyclin B (Lemaitre et al. Citation2002; Whitmire et al. Citation2002). The absence of an S phase between the two meiotic divisions is due to the absence of the protein Wee1, as recently demonstrated (Nakajo et al. Citation2000). See also the comment of reference Kubota & Takisawa (Citation2003).
The progression from meiosis I to meiosis II occurs through the synthesis of new B‐type cyclins (Frank‐Vaillant et al. Citation1999; Hochegger et al. Citation2001). For this progression to occur the protein Aurora kinase is necessary (Castro et al. Citation2003; Ma et al. Citation2003; Gadea & Ruderman Citation2005). The CSF is in turn activated by the MAPK P42 (Tunquist & Maller Citation2003) which activates the serine threonine kinase P‐90Rsk (Schwab et al. Citation2001). P90Rsk activates the protein Bub1, which is responsible of the kinetochore checkpoint (Schwab et al. Citation2001). The activated CSF stimulates the protein Emi (early mitotic inhibitor) which is ultimately responsible for oocyte arrest at metaphase (Reimann et al. Citation2001a,Citationb; Reimann & Jackson, Citation2002; Liu & Maller, Citation2005).
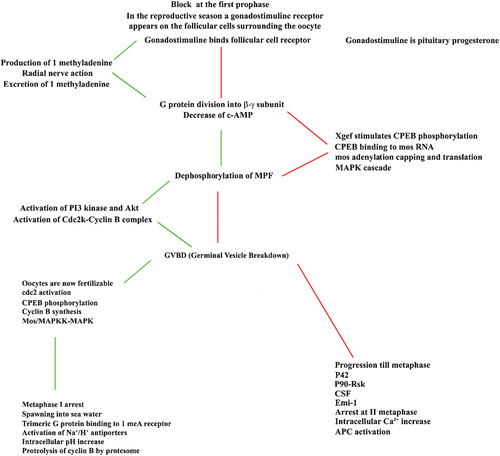
One of the proteins responsible for the II metaphase arrest is the CENP‐E, a kinetochore motor, which is probably masked during the arrest (Duesbery et al. Citation1997). A comparison between starfish and Xenopus oocyte maturation is reported in Figure .
Figure 3. Comparison between the events leading to the maturation of oocytes of starfish, indicated on the left column, and of amphibians indicated in the right column: in the centre column the events common to the system are reported. The temporal sequence of events is from top to bottom and oblique segments indicate which events precede the other ones.
The oocytes, however, can be fertilized at this point and fertilization will bring about a Ca2+ increase (El‐Jouni et al. Citation2005) by the involvement of an IP3 receptor within the endoplasmic reticulum (Boulware & Marchant Citation2005), which will activate the APC (anaphase promoting complex) therefore allowing meiosis to continue through the phosphorylation of the proteins XErp1/Emi2 by Plx1 and CaMK II (Liu & Maller Citation2005), and because of the action of the protein Fizzy (Lorca et al. Citation1998). A role of the protein XCdl‐1 for the release from the metaphase block, which may be independent of APC, has also been proposed (Papin et al. Citation2004). Mos proteolysis and consequent release of the metaphase arrest, has also been obtained, independently of Ca2+ increase, by oocyte treatment with 6‐DMAP (the kinase inhibitor 6‐dimetyl‐amino purine) (Bodart et al. Citation2001). Recent works on the role of kinases in progesterone stimulated Xenopus oocytes are resumed in Figure .
Figure 4. Scheme illustrating recent work on the role of kinase in progesterone‐dependent Xenopus oocyte maturation. The symbol ↑ indicates stimulation, and the symbol ⊥ indicates inhibition. G.V. = germinal vesicle.
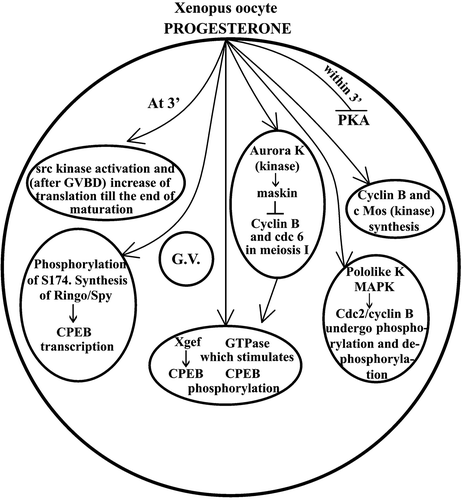
Progesterone acts through a protein kinase C in order to prepare the cytoplasm of the mature oocytes to contract for the first division (Johnson & Capco Citation1997). To this end, the actin–myosin cytoskeleton reorganization also collaborates (Noguchi & Mabuchi Citation2001). For chromosomal DNA replication, a DNA polymerase ε is also required (Waga et al. Citation2001). In order to initiate DNA replication the protein Cut5 also is required (Hashimoto & Takisawa Citation2003).
Among the rather recent reviews on the mechanisms of Xenopus oocyte maturation, we recommend those of references Ferrel (Citation1999), Garrington & Johnson (Citation1999), Nebreda & Ferby (Citation2000), Kishimoto (Citation2003) and Tunquist & Maller (Citation2003).
Discussion
Analogies and differences between these two systems are shown in Figure and it is surprising to see that so many similarities are conserved in two so distant phyla, whose evolution separated about 300 million years ago. See, for example, the similarities that of a G protein activation and division into the three subunits, which leads in both systems to the stimulation of MPF and to cyclin B translation, to stimulation of the CSF translation, followed by formation of a CSF, leading to meiotic arrest, followed by MAPK stimulation with the action of the p90 Rsk. The only important difference between the two systems remaining that of the formation of 1 MeA only in starfishes.
Acknowledgements
This work was financed in part by funds of Italian Ministry for University and Research to professor Giovanni Giudice (60%).
References
- Barkoff , A. , Ballantyne , S. and Wickens , M. 1998 . Meiotic maturation in Xenopus requires polyadenylation of multiple mRNAs. . EMBO Journal , 17 : 3168 – 3175 .
- Barkoff , A. F. , Dickson , K. S. , Gray , N. and Wickens , M. 2000 . Translational control of cyclin B1 mRNA during meiotic maturation: Coordinated repression and cytoplasmic polyadenylation. . Developmental Biology , 220 : 97 – 109 .
- Bayaa , M. , Booth , R. A. , Sheng , Y. and Liu , X. J. 2000 . The classical progesterone receptor mediates Xenopus oocyte maturation through a nongenomic mechanism. . Proceedings of the National Academy of Sciences of the United States of America , 97 : 12607 – 12612 .
- Becker , B. E. , Romney , S. J. and Gard , D. L. 2003 . XMAP215, XKCM1, NuMA, and cytoplasmic dynein are required for the assembly and organization of the transient microtubule array during the maturation of Xenopus oocytes. . Developmental Biology , 261 : 488 – 505 .
- Bodart , J. F. , Rodeau , J. L. , Vilain , J. P. and Flament , S. 2001 . c‐Mos proteolysis is independent of the CA(2+) rise induced by 6‐DMAP in Xenopus oocytes. . Experimental Cell Research , 266 : 187 – 192 .
- Boulware , M. J. and Marchant , J. S. 2005 . IP3 receptor activity is differentially regulated in endoplasmic reticulum subdomains during oocyte maturation. . Current Biology , 15 : 765 – 770 .
- Castro , A. , Mandart , E. , Lorca , T. and Galas , S. 2003 . Involvement of Aurora A kinase during meiosis I–II transition in Xenopus oocytes. . The Journal of Biological Chemistry , 278 : 2236 – 2241 .
- Chiba , K. , Nakano , T. and Hoshi , M. 1999 . Induction of germinal vesicle breakdown in a cell‐free preparation from starfish oocytes. . Developmental Biology , 205 : 217 – 223 .
- Culp , P. A. and Musci , T. J. 1999 . c‐mos and cdc2 cooperate in the translational activation of fibroblast growth factor receptor‐1 during Xenopus oocyte maturation. . Molecular Biology of the Cell , 10 : 3567 – 3881 .
- De Smedt , V. , Poulhe , R. , Cayla , X. , Dessauge , F. , Karaiskou , A. , Jessus , C. and Ozon , R. 2002 . Thr‐161 phosphorylation of monomeric Cdc2. Regulation by protein phosphatase 2C in Xenopus oocytes. . The Journal of Biological Chemistry , 277 : 28592 – 28600 .
- Dickson , K. S. , Bilger , A. , Ballantyne , S. and Wickens , M. P. 1999 . The cleavage and polyadenylation specificity factor in Xenopus laevis oocytes is a cytoplasmic factor involved in regulated polyadenylation. . Molecular and Cellular Biology , 19 : 5707 – 5717 .
- Duesbery , N. S. , Choi , T. , Brown , K. D. , Wood , K. W. , Resau , J. , Fukasawa , K. , Cleveland , D. W. and Vande Woude , G. F. 1997 . CENP‐E is an essential kinetochore motor in maturing oocytes and is masked during mos‐dependent, cell cycle arrest at metaphase II. . Proceedings of the National Academy of Sciences of the United States of America , 94 : 9165 – 9170 .
- Duesbery , N. S. and Vande Woude , G. F. 2002 . Developmental biology: an arresting activity. . Nature , 416 : 804 – 805 .
- El‐Jouni , W. , Jang , B. , Haun , S. and Machaca , K. 2005 . Calcium signaling differentiation during Xenopus oocyte maturation. . Developmental Biology , 288 : 514 – 525 .
- Faure , S. , Vigneron , S. , Doree , M. and Morin , N. 1997 . A member of the Ste20/PAK family of protein kinases is involved in both arrest of Xenopus oocytes at G2/prophase of the first meiotic cell cycle and in prevention of apoptosis. . EMBO Journal , 16 : 5550 – 5561 .
- Ferby , I. , Blazquez , M. , Palmer , A. , Eritja , R. and Nebreda , A. R. 1999 . A novel p34(cdc2)‐binding and activating protein that is necessary and sufficient to trigger G(2)/M progression in Xenopus oocytes. . Genes & Development , 13 : 2177 – 2189 .
- Ferrell , J. E. Jr. 1999 . Xenopus oocyte maturation: New lessons from a good egg. . Bioessays , 21 : 833 – 842 .
- Fisher , D. L. , Mandart , E. and Doree , M. 2000 . Hsp90 is required for c‐Mos activation and biphasic MAP kinase activation in Xenopus oocytes. . EMBO Journal , 19 : 1516 – 1524 .
- Fisher , D. L. , Morin , N. and Doree , M. 1999 . A novel role for glycogen synthase kinase‐3 in Xenopus development: Maintenance of oocyte cell cycle arrest by a beta‐catenin‐independent mechanism. . Development , 126 : 567 – 576 .
- Frank‐Vaillant , M. , Haccard , O. , Ozon , R. and Jessus , C. 2001 . Interplay between Cdc2 kinase and the c‐Mos/MAPK pathway between metaphase I and metaphase II in Xenopus oocytes. . Developmental Biology , 231 : 279 – 288 .
- Frank‐Vaillant , M. , Haccard , O. , Thibier , C. , Ozon , R. , Arlot‐Bonnemains , Y. , Prigent , C. and Jessus , C. 2000 . Progesterone regulates the accumulation and the activation of Eg2 kinase in Xenopus oocytes. . Journal of Cell Science , 113 : 1127 – 1138 .
- Frank‐Vaillant , M. , Jessus , C. , Ozon , R. , Maller , J. L. and Haccard , O. 1999 . Two distinct mechanisms control the accumulation of cyclin B1 and Mos in Xenopus oocytes in response to progesterone. . Molecular Biology of the Cell , 10 : 3279 – 3288 .
- Gadea , B. B. and Ruderman , J. V. 2005 . Aurora kinase inhibitor ZM447439 blocks chromosome‐induced spindle assembly, the completion of chromosome condensation, and the establishment of the spindle integrity checkpoint in Xenopus egg extracts. . Molecular Biology of the Cell , 16 : 1305 – 1318 .
- Garrington , T. P. and Johnson , G. L. 1999 . Organization and regulation of mitogen‐activated protein kinase signalling pathways. . Current Opinion in Cell Biology , 11 : 211 – 218 .
- Gavin , A. C. , Ni Ainle , A. , Chierici , E. , Jones , M. and Nebreda , A. R. 1999 . A p90(rsk) mutant constitutively interacting with MAP kinase uncouples MAP kinase from p34(cdc2)/cyclin B activation in Xenopus oocytes. . Molecular Biology of the Cell , 10 : 2971 – 2986 .
- Giudice , G. 1991 . Biologia dello Sviluppo , Bologna : Grasso .
- Hake , L. E. and Richter , J. D. 1994 . CPEB is a specificity factor that mediates cytoplasmic polyadenylation during Xenopus oocyte maturation. . Cell , 79 : 617 – 627 .
- Harada , K. , Oita , E. and Chiba , K. 2003 . Metaphase I arrest of starfish oocytes induced via the MAP kinase pathway is released by an increase of intracellular pH. . Development , 130 : 4581 – 4586 .
- Hashimoto , Y. and Takisawa , H. 2003 . Xenopus Cut5 is essential for a CDK‐dependent process in the initiation of DNA replication. . EMBO Journal , 22 : 2526 – 2535 .
- Hiraoka , D. , Hori‐Oshima , S. , Fukuhara , T. , Tachibana , K. , Okumura , E. and Kishimoto , T. 2004 . PDK1 is required for the hormonal signaling pathway leading to meiotic resumption in starfish oocytes. . Developmental Biology , 276 : 330 – 336 .
- Hochegger , H. , Klotzbucher , A. , Kirk , J. , Howell , M. , le Guellec , K. , Fletcher , K. , Duncan , T. , Sohail , M. and Hunt , Tì. 2001 . New B‐type cyclin synthesis is required between meiosis I and II during Xenopus oocyte maturation. . Development , 128 : 3795 – 3807 .
- Howard , E. L. , Charlesworth , A. , Welk , J. and MacNicol , A. M. 1999 . The mitogen‐activated protein kinase signaling pathway stimulates mos mRNA cytoplasmic polyadenylation during Xenopus oocyte maturation. . Molecular and Cellular Biology , 9 : 1990 – 1999 .
- Johnson , J. and Capco , D. G. 1997 . Progesterone acts through protein kinase C to remodel the cytoplasm as the amphibian oocyte becomes the fertilization‐competent egg. . Mechanisms of Development , 67 : 215 – 226 .
- Kalinowski , R. R. , Berlot , C. H. , Jones , T. L. , Ross , L. F. , Jaffe , L. A. and Mehlmann , L. M. 2004 . Maintenance of meiotic prophase arrest in vertebrate oocytes by a Gs protein‐mediated pathway. . Developmental Biology , 267 : 1 – 13 .
- Kanatani , H. 1973 . Maturation‐inducing substance in starfishes. . International Review of Cytology , 35 : 253 – 298 .
- Kanatani , H. , Shirai , H. , Nakanishi , K. and Kurokawa , T. 1969 . Isolation and identification on meiosis inducing substance in starfish Asterias amurensis. . Nature , 221 : 273 – 274 .
- Kishimoto , T. 1998 . Cell cycle arrest and release in starfish oocytes and eggs. . Seminars in Cell & Developmental Biology , 9 : 549 – 557 .
- Kishimoto , T. 2003 . Cell‐cycle control during meiotic maturation. . Current Opinion in Cell Biology , 15 : 654 – 663 .
- Kishimoto , T. 2004 . More than G1 or G2 arrest: useful starfish oocyte system for investigating skillful MAP kinase. . Biology of the Cell , 96 : 241 – 244 .
- Kubiak , J. Z. and Ciemerych , M. A. 2001 . Cell cycle regulation in early mouse embryos. . Novartis Foundation Symposium , 237 : 79 – 89 .
- Kubota , Y. and Takisawa , H. 2003 . Block to DNA replication in meiotic maturation: A unified view for a robust arrest of cell cycle in oocytes and somatic cells. . Bioessays , 25 : 313 – 316 .
- Kuge , H. , Brownlee , G. G. , Gershon , P. D. and Richter , J. D. 1998 . Cap ribose methylation of c‐mos mRNA stimulates translation and oocyte maturation in Xenopus laevis. . Nucleic Acids Research , 26 : 3208 – 3214 .
- Lemaitre , J. M. , Bocquet , S. and Mechali , M. 2002 . Competence to replicate in the unfertilized egg is conferred by Cdc6 during meiotic maturation. . Nature , 419 : 718 – 722 .
- Liu , J. and Maller , J. L. 2005 . Calcium elevation at fertilization coordinates phosphorylation of XErp1/Emi2 by Plx1 and CaMK II to release metaphase arrest by cytostatic factor. . Current Biology , 15 : 1458 – 1468 .
- Lorca , T. , Castro , A. , Martinez , A. M. , Vigneron , S. , Morin , N. , Sigrist , S. , Lehner , C. , Doree , M. and Labbe , J. C. 1998 . Fizzy is required for activation of the APC/cyclosome in Xenopus egg extracts. . EMBO Journal , 17 : 3565 – 3575 .
- Lutz , L. B. , Kim , B. , Jahani , D. and Hammes , S. R. 2000 . G protein beta gamma subunits inhibit nongenomic progesterone‐induced signalling and maturation in Xenopus laevis oocytes. Evidence for a release of inhibition mechanism for cell cycle progression. . Journal of Biological Chemistry , 275 : 41512 – 41520 .
- Ma , C. , Cummings , C. and Liu , X. J. 2003 . Biphasic activation of Aurora‐A kinase during the meiosis I–meiosis II transition in Xenopus oocytes. . Molecular and Cellular Biology , 23 : 1703 – 1716 .
- Martinez , S. E. , Yuan , L. , Lacza , C. , Ransom , H. , Mahon , G. M. , Whitehead , I. P. and Hake , L. E. 2005 . XGef mediates early CPEB phosphorylation during Xenopus oocyte meiotic maturation. . Molecular Biology of the Cell , 16 : 1152 – 1164 .
- Masui , Y. 2000 . The elusive cytostatic factor in the animal egg. Nature reviews. . Molecular Cell Biology , 1 : 228 – 232 .
- Matten , W. T. , Copeland , T. D. , Ahn , N. G. and Vande Woude , G. F. 1996 . Positive feedback between MAP kinase and Mos during Xenopus oocyte maturation. . Developmental Biology , 179 : 485 – 492 .
- Mita , M. , Yoshikuni , M. and Nagahama , Y. 1999 . 1‐Methyladenine production from ATP by starfish ovarian follicle cells. . Biochimica et Biophysica Acta , 1428 : 13 – 20 .
- Mori , M. , Hara , M. , Tachibana , K. and Kishimoto , T. 2006 . p90Rsk is required for G1 phase arrest in unfertilized starfish eggs. . Development , 133 : 1823 – 1830 .
- Muslin , A. J. , MacNicol , A. M. and Williams , L. T. 1993 . Raf‐1 protein kinase is important for progesterone‐induced Xenopus oocyte maturation and acts downstream of mos. . Molecular and Cellular Biology , 13 : 4197 – 4202 .
- Nakajo , N. , Oe , T. , Uto , K. and Sagata , N. 1999 . Involvement of Chk1 kinase in prophase I arrest of Xenopus oocytes. . Developmental Biology , 207 : 432 – 444 .
- Nakajo , N. , Yoshitome , S. , Iwashita , J. , Iida , M. , Uto , K. , Ueno , S. , Okamoto , K. and Sagata , N. 2000 . Absence of Wee1 ensures the meiotic cell cycle in Xenopus oocytes. . Genes & Development , 14 : 328 – 338 .
- Nebreda , A. R. and Ferby , I. 2000 . Regulation of the meiotic cell cycle in oocytes. . Current Opinion in Cell Biology , 12 : 666 – 675 .
- Noguchi , T. and Mabuchi , I. 2001 . Reorganization of actin cytoskeleton at the growing end of the cleavage furrow of Xenopus egg during cytokinesis. . Journal of Cell Science , 14 : 401 – 412 .
- Palmer , A. , Gavin , A. C. and Nebreda , A. R. 1998 . A link between MAP kinase and p34(cdc2)/cyclin B during oocyte maturation: p90(rsk) phosphorylates and inactivates the p34(cdc2) inhibitory kinase Myt1. . EMBO Journal , 17 : 5037 – 5047 .
- Papin , C. , Rouget , C. , Lorca , T. , Castro , A. and Mandart , E. 2004 . XCdh1 is involved in progesterone‐induced oocyte maturation. . Developmental Biology , 272 : 66 – 75 .
- Peter , M. , Labbe , J. C. , Doree , M. and Mandart , E. 2002 . A new role for Mos in Xenopus oocyte maturation: targeting Myt1 independently of MAPK. . Development , 29 : 2129 – 2139 .
- Radziwill , G. , Steinhusen , U. , Aitken , A. and Moelling , K. 1996 . Inhibition of Raf/MAPK signaling in Xenopus oocyte extracts by Raf‐1‐specific peptides. . Biochemical and Biophysical Research Communications , 227 : 20 – 26 .
- Ranginwale , M. , Smith , S. , Flom , J. , Chie , L. , Kanovsky , M. , Chung , D. , Friedman , F. K. , Robinson , R. C. , Brandt‐Rauf , P. W. Yamaizumi , Z. 2001 . Differences in patterns of activation of MAP kinases induced by oncogenic ras‐p21 and insulin in oocytes. . Experimental Cell Research , 269 : 162 – 169 .
- Reimann , J. D. , Gardner , B. E. , Margottin‐Goguet , F. and Jackson , P. K. 2001a . Emi1 regulates the anaphase‐promoting complex by a different mechanism than Mad2 proteins. . Genes & Development , 15 : 3278 – 3285 .
- Reimann , J. D. , Freed , E. , Hsu , J. Y. , Kramer , E. R. , Peters , J. M. and Jackson , P. K. 2001b . Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. . Cell , 105 : 645 – 655 .
- Reimann , J. D. and Jackson , P. K. 2002 . Emi1 is required for cytostatic factor arrest in vertebrate eggs. . Nature , 416 : 850 – 854 .
- Reverte , C. G. , Yuan , L. , Keady , B. T. , Lacza , C. , Attfield , K. R. , Mahon , G. M. , Freeman , B. , Whitehead , I. P. and Hake , L. E. 2003 . XGef is a CPEB‐interacting protein involved in Xenopus oocyte maturation. . Developmental Biology , 255 : 383 – 398 .
- Sagata , N. , Oskarsson , M. , Copeland , T. , Brumbaugh , J. and Vande Woude , G. F. 1988 . Function of c‐mos proto‐oncogene product in meiotic maturation in Xenopus oocytes. . Nature , 335 : 519 – 525 .
- Santella , L. , Kyozuka , K. , Hoving , S. , Munchbach , M. , Quadroni , M. , Danese , P. , Zamparelli , C. , James , P. and Carafoli , E. 2000 . Breakdown of cytoskeletal proteins during meiosis of starfish oocytes and proteolysis induced by calpain. . Experimental Cell Research , 259 : 117 – 126 .
- Schwab , M. S. , Roberts , B. T. , Gross , S. D. , Tunquist , B. J. , Taieb , F. E. , Lewellyn , A. L. and Maller , J. L. 2001 . Bub1 is activated by the protein kinase p90(Rsk) during Xenopus oocyte maturation. . Current Biology , 11 : 141 – 150 .
- Sheets , M. D. , Fox , C. A. , Hunt , T. , Vande Woude , G. and Wickens , M. 1994 . The 3'‐untranslated regions of c‐mos and cyclin mRNAs stimulate translation by regulating cytoplasmic polyadenylation. . Genes & Development , 8 : 926 – 938 .
- Sheng , Y. , Tiberi , M. , Booth , R. A. , Ma , C. and Liu , X. J. 2001 . Regulation of Xenopus oocyte meiosis arrest by G protein betagamma subunits. . Current Biology , 11 : 405 – 416 .
- Tian , J. , Kim , S. , Heilig , E. and Ruderman , J. V. 2000 . Identification of XPR‐1, a progesterone receptor required for Xenopus oocyte activation. . Proceedings of the National Academy of Sciences of the United States of America , 97 : 14358 – 14363 .
- Tunquist , B. J. and Maller , J. L. 2003 . Under arrest: Cytostatic factor (CSF)‐mediated metaphase arrest in vertebrate eggs. . Genes & Development , 17 : 683 – 710 .
- Walter , S. A. , Guadagno , S. N. and Ferrell , J. E. Jr. 2000 . Activation of Wee1 by p42 MAPK in vitro and in cycling Xenopus egg extracts. . Molecular Biology of the Cell , 11 : 887 – 896 .
- Wang , J. and Liu , X. J. 2003 . A G protein‐coupled receptor kinase induces Xenopus oocyte maturation. . Journal of Biological Chemistry , 278 : 15809 – 15814 .
- Wang , J. and Liu , X. J. 2004 . Progesterone inhibits protein kinase A (PKA) in Xenopus oocytes: demonstration of endogenous PKA activities using an expressed substrate. . Journal of Cell Science , 117 : 5107 – 5116 .
- Waga , S. , Masuda , T. , Takisawa , H. and Sugino , A. 2001 . DNA polymerase epsilon is required for coordinated and efficient chromosomal DNA replication in Xenopus egg extracts. . Proceedings of the National Academy of Sciences of the United States of America , 98 : 4978 – 4983 .
- Whitmire , E. , Khan , B. and Coue , M. 2002 . Cdc6 synthesis regulates replication competence in Xenopus oocytes. . Nature , 419 : 722 – 725 .