Abstract
Ommatidia of eyes of stomatopods change with adaptation to light or dark. With dark adaptation the crystalline cones shorten and the retina becomes longer. This is due to contractions of myofibrils in veils surrounding the cones, while microtubules within the accessory pigment cells maintain the cytoarchitecture of the ommatidia. Here we describe the changes in Lysiosquillina maculata, a stomatopod living in an extremely bright habitat, which is compared to stomatopods from other ambient light conditions. The postlarval eye of stomatopods grows over the larval eye, gradually substituting it. Growth starts from a morphogenetic furrow at the inner border with the stalk. Similarly, in the adult eye as well at the inner margin between the stalk and the cornea, a morphogenetic furrow is inserted, which at its interior contains a proliferation tissue with clusters of developing ommatidia. At its distal side the proliferation tissue appears to generate the dioptric apparatus, i.e. veils, corneagenous cells and cones, and at its more proximal part the basement membrane, accessory pigment cells and retinular cells. The ommatidial components grow at different speeds.
Introduction
Stomatopods live at all ocean depths from bright light tropical surface waters to the mesopelagic and bathypelagic zones (Schiff & Hendrickx Citation1997) and in all latitudes. Each eye explores the environment independently from the other, but when a prey enters the visual field of one of the eyes, then the eyes are fixed in a characteristic position. Eyes are divided by a midband in two hemispheres. Pseudopupils are dark regions observed in all apposition compound eyes, when viewing a group of ommatidia along their optical axes (Figure on the left). When the eye moves with respect to the observer, pseudopupils move around the eye, as different groups of ommatidia become successively aligned with the viewing direction. Thus pseudopupils indicate the directions of the optical axes of groups of ommatidia.
Figure 1. Eyes ofLysiosquillina maculata (at left and centre) and L. sulcata (right). At left a triple pseudopupil; centre and right: total internal (dark regions of the eye) and external (bright regions) reflection. All eyes are 10±1 mm.
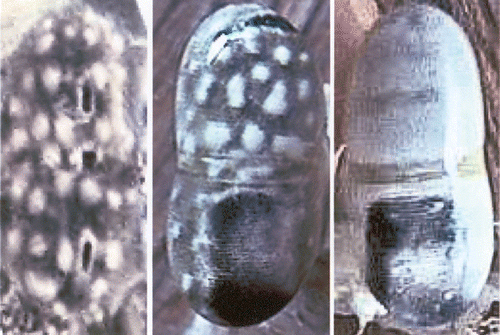
In stomatopod eyes pseudopupils are triple: one in each hemisphere and one in the midband. This means that the three groups of ommatidia in one pseudopupil all look in the same direction, i.e. that of the observer. Ommatidia belonging to one of the three parts of the pseudopupil all are stimulated simultaneously and their visual fields overlap. While the midband subserves colour and polarization vision (Cronin et al. Citation2002), the two hemispheres of each single eye analyse sizes, shapes, distance and motion of the prey (Schiff et al. Citation1989; Iacino et al. Citation1990; Schiff, Citation1997). Near to the midband ommatidia are skewed towards the midband such that visual fields overlap with those of the other half of the eye and with those of the midband ommatidia (Schiff et al. Citation2002). These patterns of superposition of visual fields in the dorsal and ventral hemisphere depend on the region of the eye, and also on the sizes, shapes, location and motion of the object. For an object passing in front of a column of ommatidia (across the midband), maxima of stimulation, due to parallel ommatidia, occur at the centre of each hemisphere (Figure ). For large distances only one maximum occurs and distance evaluation becomes binocular. A configuration of ommatidia is stimulated at each instant and excitation transferred to the lamina ganglionaris, the first visual ganglion (Figure ); from the lamina parallel nervous channels transmit information according to the different tasks to be accomplished (Schiff & Sertorio Citation1993).
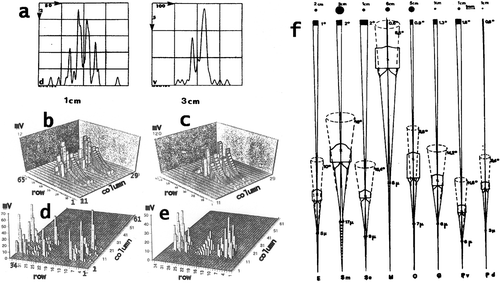
Figure 2. a, Patterns of summed Gaussian response curves constituting the outputs from the ommatidia from the retina when a point light source passes in front of a column of ommatidia (from tip to tip across the midband) in the eye of Squilla mantis at a speed of 10 cm/s. At the left: in a small eye the point passes at a distance of 1 cm, right: the point passes at a distance of 3 cm in front of a large eye. Ordinates: number of ommatidia with overlapping visual fields (i.e. Gaussian sensitivity curves), i.e. ommatidia which would be stimulated simultaneously. Abscissae: time in milliseconds. For small and large eyes similar patterns of overlap result for shorter, respectively, longer distances, which are correlated to the shorter or longer raptorial appendages. b–e, Patterns of stimulated activity in the lamina ganglionaris of: b, c, Squilla mantis (living in dim light); d, e, Gonodactylus oerstedi (from bright light) when a rectangle passes in front of the eye. Ordinate: mV = potentials generated in the lamina, abscissae: columns and rows of ommatidia stimulated by the rectangle. b, target 2×0.5 cm moves at a speed of 10 cm/s at a distance of 1 cm from the eye, ordinate from O to 12 mV; c, a 2×0.5 cm rectangle moves at a distance of 2 cm at a speed of 5 cm/s, Ordinate: 0–120 mV. d, Target size 2×0.5 cm, speed 10 cm/s, distance from eye 1 cm, ordinate: 0–70 mV; e, Target 2×0.5 cm, speed 5 cm/s, distance 2 cm, ordinate 0–50 mV. f, Calculated acceptance angles, apertures and visual fields at indicated distances for different stomatopods: E = Echinosquilla guerini, Sm = Squilla mantis, Se = Squilla empusa, H = Hemisquilla ensigera, O = Odontodactylus scyllarus, G = Gonodactylus spp., Pv, Pd = Pseudosquilla ciliata, Pv posterior, Pd anterior ommatidia. Numbers from top to bottom: sizes of visual fields at indicated distances at approximate striking distances of species, degrees of acceptance angles, degrees of aperture angles, diameters of distal rhabdoms.
The frontal parts of the eyes of species like Lysiosquillina maculata (Fabricius, 1793) and Lysiosquillina sulcata (Manning, 1978) living in extremely bright habitats, undergo a total internal (hemispheres seen dark, Figure centre) or external reflection (bright hemispheres, Figure , right side) within a limited angle of viewing. Stomatopod species from bright habitats usually have long cones and thin, but relatively short rhabdoms (Figure ) and small visual fields (Figure ; Schiff et al. Citation1986). Total numbers of ommatidia are smaller in species from low light conditions, e.g. about 3000 in Squilla mantis (Linnaeus, 1758) but over 6000 in L. maculata. But stomatopod species living in dim habitats have short, thick cones and thick fused rhabdoms (Figure ). But species of the same genus maintain the organization also in different habitats, e.g. Squilla mantis and Squilla empusa (Say, 1818) eyes and ommatidia are very similar, in spite of the fact that S. m. lives in dim and S. e. in brighter waters. Exceptions occur, e.g. Meiosquilla oculinova (Manning, 1968) from only about 30 m depth, has an eye strongly suggesting a dark habitat or a night life, but it was caught in the same luminous habitat as Gonodactylus with a typical bright light eye. Presumably once a genus has developed the eye for one species, successive evolutionary species adapt the eyes to their living conditions. For example: all stomatopods have apposition eyes, which are usually assumed to be bright light compound eyes. Species of stomatopods living in low light or dark conditions still have apposition eyes. They adjust their vision in different ways: sizes and shapes (Manning et al. Citation1984; Schiff & Abbott Citation1989) of the eyes, of the ommatidia, sizes of visual fields (Figure ) and amount of their overlap (Schiff & Di Stefano Citation1992; Schiff & Sertorio Citation1993; Schiff Citation1997; Figure ), which depends on the skewing of ommatidia near the midband. In particular the skewing of ommatidia and the resulting superposition of visual fields allow a spatial and temporal summation of inputs, thus increasing sensitivity without sacrificing resolution. Resolution though is not determined by a point‐by‐point (i.e. mosaic vision) analysis but by the configuration of stimulated ommatidia, which changes with the motion of a prey or other object relative to the eye.
Figure 3. a, The eye of Lysiosquillina maculata showing the varying sizes of ommatidia along a column across the midband. Bar: 1 mm. b, The rhabdom of S. mantis and c, that of L. maculata at same amplification. Bars: 50 µm.
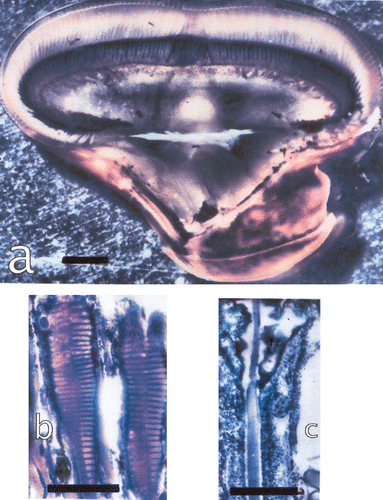
Here we will describe the morphological changes in light/dark adaptation and the growth of the eyes in L. maculata. Light/dark adaptation involves mechanical changes of the dioptric apparatus and the retina. Crustacean eyes continue to grow through lifetime (Keskinen et al. Citation2002) increasing sizes of eyes and ommatidia. Sizes of ommatidia and skewing both change with the sizes of the animal. When the animal grows, the pattern of skewing changes and adapts the eye to the lengths of the extended raptorial appendices (Schiff et al. Citation1989; Di Stefano et al. Citation1990). These last subserve the high‐speed strike for hitting a prey (Caldwell & Dingle Citation1976).
Materials and methods
One L. maculata was collected at Rangiroa (French Polynesia). Two eyes from a dark‐adapted and two eyes from a light‐adapted animal were obtained by the kindness of Roy Caldwell, as were also the juvenile animals. Eyes were fixed in formaldehyde 4%. In the present paper we use a glycol methacrylate resin embedding method; this method produces 2 μm sections where most of the extracellular, cellular and some of the subcellular structures are visible in light microscopy, as described previously (Bonucci Citation1981; Dore et al. Citation2005).
For the conventional GlucoseAminoGlycans (GAGs) histochemistry (Bonucci, Citation1981), the following staining procedures were used: Periodic Acid Schiff (PAS) and PASM reaction for studying neutral GAGs, PAS reaction after amylase (Sigma, type XII‐A) digestion to exclude any possible glycogen, alcian blue 1% (AB) staining at pH 2.5 for the detection of acidic GAGs, alcian blue at pH 1.0 for the demonstration of sulphated GAGs, a stain with AB pH 2.5 following strong methylation plus saponification (MS) to confirm the presence of carboxylic GAGs.
Results
The longest axis in the light‐adapted eyes from tip to tip was 9.1 mm, of the dark‐adapted eyes 8.6 mm, for the eye from Rangiroa it was 11 mm.
The ommatidia
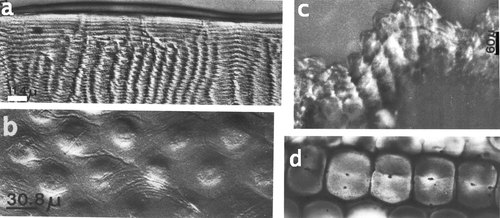
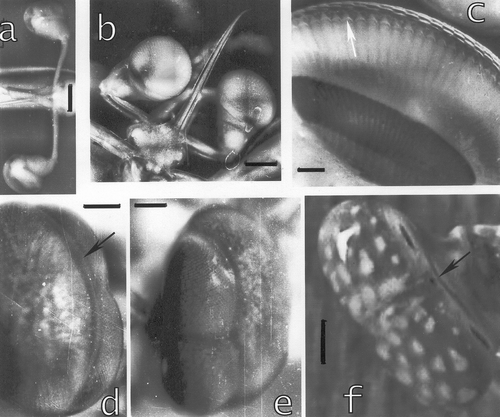
Sizes of ommatidia and their components vary considerably with the size (age) of the animal, the position within the eye and with light/dark adaptation. Ommatidia in the two halves of the eye vary in size and skewing. From the midband towards the tips skewing diminishes and sizes first increase by 8%, then decrease by over 40% (Figure ). Mean values of the components of the ommatidia are given in Table . The ommatidium starts with a hexagonal, multilayered corneal facet. Facets of different stomatopod species also change according to the availability of light in their habitat. All stomatopods have aspherical lenses which correct spherical aberration, i.e. blue and red light would be focused at the same point in the rhabdoms. An aspherical lens has flattened margins and a convex central part. In stomatopods from bright habitats as Odontodactylus scyllarus (Linnaeus, 1758), Pseudosquilla ciliata (Fabricius, 1787) (Figure ) or L. maculata, the outer surface of a facet is rather flat; stomatopods from lower light conditions have more convex surfaces with a stronger flattening of the facet margins (Figure ). An extreme case can be observed in Meiosquilla oculinova where the central parts of the facets bulge outward (Figure ).
Table I. Mean values of sizes of ommatidial components from the eyes of two L. maculata, one adapted to light (LA), the other adapted to the dark (DA).
Figure 4. a, Section through a corneal facet of Odontodactylus scyllarus, bar: 11.4 µm; b, Pseudosquilla ciliata, bar: 30.8 µm; c, Meiosquilla oculinova, corneal surface seen from laterally, bar 60 µm ; d, S. mantis (diameter of facet is about 100 µm). Note the external surface facet with different refractive indices in a.
The crystalline cones are attached to each other in one direction parallel to the midband (Figure ). Towards the interior of the eye the two wedge‐shaped corneagenous cells surround the distal cone. The crystalline cone, composed by four cells, is attached with a thin tip to the centre of the corneal facet. Towards proximal the cone widens and contains an enforced four‐part ring. The four parts are situated around the widest part of the cone (Figure , stars). They contain the cone nuclei and are inserted also towards the interior of the cone. The enforcements form a compact mass which then, towards the interior of the cone, extends in little branches. Each cone is enclosed by a membranous network which stains heavily in toluidine blue (Figure ). Towards the retina the cones have multiple grooves of the enveloping membranes towards the interior (Figure ). The cones have four extensions which run along the rhabdom corners and are finally attached to the basement membrane. In the other two directions (at 60°) cones are aligned but they are separated by enlargements of the veils which contain the veil nuclei.
Figure 5. a, A section through a cone in the eye of a large L. maculata with the four enforcements, bar: 25 µm. b, A piece of the cone enveloping network membrane in a tangential section of a cone, bar: 25 µm. c, Section through cones from a small L. m. with the four enforcements in each cone, bar: 10 µm. d, Section through cones, from cornea (left) towards the retina. Proximal cones are multiply folded. Cones are surrounded by the myofibril containing ‘veils’, bar: 100 µm. e, Section through cones and the surrounding veils containing six groups of myofibrils (arrow) around each cone, bar: 100 µm. f, Section parallel to the optical axis through a cone and the veils attached to the cornea. Star: one of the four inserted enforcements. Bar: 50 µm. g, Same as f but a section near the optical axis. Stars: two inserted enforcements. Bar: 50 µm. h–i, Cones and veils in longitudinal sections showing also the network of the cone enveloping membrane (triangles). Arrows indicate the myofibrils. Bars: 50 µm.
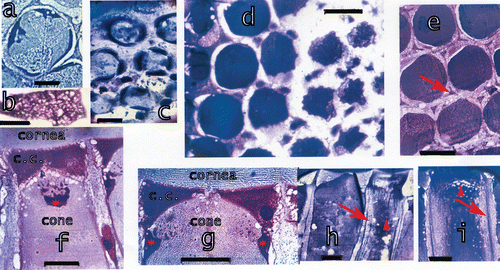
At each of the six corners of the corneal facet a bundle of myofibrils is attached to the cornea (Figure , arrows). These fibrils are immersed in a thin veil which is attached to the cornea and the corneagenous cells and surrounds the corneagenous cells and the cones. These veils are thicker at the first part of the widest part of the cone (Figure ). Still further towards the retina the veils with their myofibrils restrict to form six bundles of myofibrils surrounding each cone (Figure , arrow). Each bundle, however, belongs to three neighbouring ommatidia. At the distal retina these bundles form little brushes connected to the accessory pigment cells.
The six accessory pigment cells in each ommatidium are filled with a black pigment and surround the seven retinular cells, both running to the basement membrane (Figure ). Here the accessory pigment cells are fastened by little boot‐shaped feet. The pattern on the distal side of the basement membrane is shown in Figure . In between the pigment granules microtubules can be observed (Dore et al. Citation2005). Proximal to the basement membrane large amounts of black pigment accumulates, probably constituting a reservoir of pigment for the accessory pigment cells (Figure ). Black pigment is also accumulated around the distal first‐order nerve fibres (i.e. the continuations of the retinular cells). The sheaths of black pigment under the basement membrane and around the first order nerve fibres occur also in other stomatopod species, but apparently only in bright light species and not, for example, in S. mantis.
Figure 6. a, Schematic drawing of dark‐adapted and light‐adapted ommatidia showing also the components of the ommatidia. b, Tangential section at the distal side of the basement membrane, black: the feet of the accessory pigment cells attached to the basement membrane, blue: the proximal retinular cells passing through the basement membrane. Bar: 50 µm. c, Drawing of the proximal rhabdom tips (blue, rh), retinular cells (yellow) and feet of accessory pigment (black) cells. Outlined in red an accessory pigment cell and the rhabdom tips of the three ommatidia connected by this accessory pigment cell. d, The distal retina surface with the rhabdoms, the four lobes of the eighth retinular cell and the four cone extensions on the rhabdom corners (arrowhead). On the left drawing of the section showing the cone extensions on the rhabdom corners and the four lobes of the eighth retinular cells. Seven normal retinular cells and six accessory pigment cells surround each rhabdom. Bar: 20 µm. e, Longitudinal section of the distal retina showing two of the four lobes of the eighth retinular cell, the rhabdom and distal tips of the retinular cells. Bar: 20 µm. The drawing shows the correlation between the lobes of the eighth cell and the rhabdom. f, Section through a dark‐adapted and g, a light‐adapted retina. Perirhabdomal spaces (arrows) are slightly wider in dark‐adapted eyes and black pigment accumulated around the distal rhabdom. Bar in f: 30 µm, in g 20 µm.
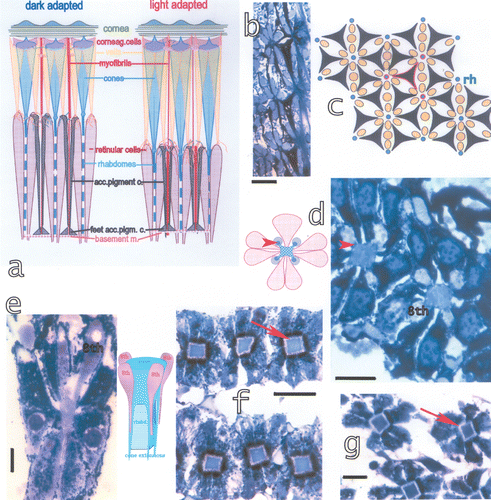
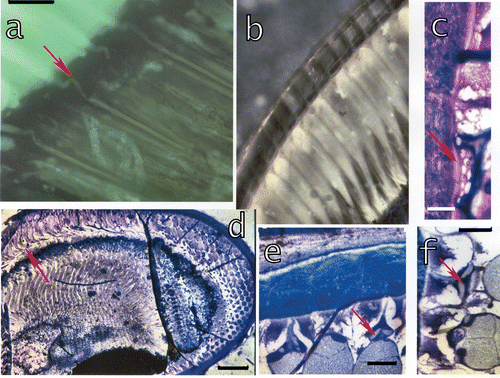
Figure 7. a, Section of a dark‐adapted retina of L. m. seen in epifluorescent light (λe = 305 nm) showing the bent connections (arrow) from the cones to the rhabdoms. Bar: 20 µm. b, In the light‐adapted eyes the crystalline cones suddenly restrict to slim proximal parts which enter the retina and contact the rhabdoms. c, In light‐adapted eyes a thin facet (arrow) underlies the corneal facet and may contribute to the reflection. Bar: 20 µm. d, Section through a juvenile eye of L. m. showing the bending of cones in these quickly growing eyes. Bar: 300 µm. e–f, Connecting structures (arrows) between the cornea and the crystalline cones in a growing eye of S. mantis. These structures possibly form later on the enforcements around the distal tips of the cones. Bar in e 100 µm, in f 50 µm.
The fused rhabdom is surrounded by seven retinular cells which contain pigment granules. The retinular cells penetrate the basement membrane as first‐order nerve fibres, loosing their pigment. Six nerve fibres end in a particular pattern of distribution (Schiff Citation1997) in the neurocartridges of the lamina ganglionaris. The fibres from the seventh and eighth retinular cell end in the medulla externa, the second visual ganglion.
At the junction between the cones and the fused rhabdoms a special rhabdom is inserted, longer in L. maculata than in S. mantis, belonging to the eighth retinular cell. The rhabdom of the eighth retinular cell is thinner than the normal rhabdom. The eighth retinular cell sits on the distal tip of the rhabdome and is four‐lobed, with only one nucleus. In a cross‐section (Figure ) of the distal retina the four cone extensions can be seen tightly attached to the four corners of the distal rhabdom (Figure , arrowheads). The four lobes of the eighth retinular cell are seen on top of the cone extensions and contact the rhabdom via slim extensions. In a section along the ommatidial axis instead the four lobes are bulged at their distal part, but slim to thin extensions which run along the rhabdom (Figure ). In light microscopy the confluence of the four lobes around the rhabdom is not visible.
A thin layer of the retinular cells covers the rhabdom separated by the perirhabdomal space from the bulk of the retinular cells (Figure , arrows). The thin layer is multiply bridged through the ‘empty’ space to the main part of the retinular cells (Schiff & Gervasio Citation1969).
Adaptation
During dark adaptation the cones become shorter and slightly thicker. The accessory pigment cells are pulled up towards the cornea, such that the proximal cones are immersed within the accessory pigment cells. The retina becomes longer (cone/retina = 1.5 in dark and 2.5 in light adaptation), i.e. cones in dark adaptation are twice the length of the retina and in light adaptation three times as long (Figure ). The proximal parts of the cones are bent during dark adaptation (Figure , arrow) within the distal retina. As in other stomatopods the pigments accumulate around the rhabdom during dark adaptation (Figure ). The perirhabdomal space (about 1–2 μm wide) is slightly wider in dark adaptation (Figure , arrows), but the volume of the rhabdom does not seem to change in L. maculata, as in other arthropods or in Squilla (Dore et al. Citation2005). In light‐adapted eyes the cones are thick distally, then towards proximal slim abruptly (Figure ). The retina of the midband distally bulges outside into the layer of cones. These ommatidia as well as the corneagenous cells change very little or not at all with light or dark adaptation.
In light‐adapted eyes a thin fascia underlies the corneal facet (Figure , arrow). This thin fascia has distal and proximal membranes and in between material which stains little with toluidine blue. A similar fascia was observed also at the outside of the corneal facet of L. maculata and in O. scyllarus (Figure ).
Eyes from juvenile L. maculata have strongly bent cones, in light as well as dark adaptation (Figure , arrow), presumably due to a quick growth of the animals, increase in the number and size of the ommatidia and preparation for moulting. No changes for light or dark adaptation could be observed. The large amount of black pigment in Figure is presumably the reservoir of black pigment for the growing accessory pigment cells. With the growth of the animal and its eyes all components of the ommatidia grow considerably and the cones are bent until the next moult. Only few ommatidia are added such that approximate counts of ommatidia are slightly larger than 6000 in small and in about five times larger animal species and eyes.
The visual field
The visual field of an ommatidium can be calculated from the dimensions of its optical equipment (Snyder Citation1979). The calculated visual field is approximately one degree in L. maculata and apparently does not change much in light/dark adaptation.
Morphogenesis of the eyes
The larval eyes of stomatopods are spherical, without the midband dividing the eyes into three parts. Eyes of stomatopod larvae have an extra link on the long stalks (Figure ) such that the eyes have high motility in all directions and stereoscopic vision is presumably binocular and not monocular as in the adult eyes. Facets are relatively larger than those of adult eyes. The connection between cornea and cones is accomplished by a sort of little rooflets hanging from the cornea between the corneagenous cells and fitting over the distal cone (Figure , arrow). Similar structures were seen in an adult eye of S. mantis (Figure ), where they resemble the enforcements of the distal cone (Dore et al. Citation2005).
Figure 8. a, Eyes of an Alima hyalina larva. Bar: 420 µm. b, Eyes of a squilloid larva. Bar: 500 µm. c, The interior of the eye of the squilloid larva. The arrow indicates the little rooflets hanging from the cornea and covering the distal tips of the crystalline cones. Bar: 100 µm. d–e, Postlarval eye of Pseudosquillopsis marmorata. d, Seen from the larval (left) towards the adult (right) eye, e, the adult double eye in the foreground. Bars: 105 µm. f, Adult eye of L. maculata. Bar: 2 mm. Arrows in d and f indicate the morphogenetic furrows in the postlarval and in the adult eye.
In the postlarval eye the adult eye grows over the larval eye starting at the inner border between the stalk and the cornea (Figure arrows). The new eye has smaller facets than the larval eye and is divided by a midband into three parts: midband and dorsal and ventral hemispheres (Figure ). A morphogenetic furrow separates the new adult eye from the larval eye (Figure , arrow). In adult eyes a groove, constituting the morphogenetic furrow, at the inner margin of the eye, between the stalk and the cornea, apparently constitutes the region of growth (Figure , arrow). In the living eye a dark false pseudopupil can be observed here, similar to a second pseudopupil but seen dark from almost any direction, i.e. without well‐oriented optical axes of these (new?) ommatidia and not still functional corneal facets.
As the animals grow, also the ommatidia grow. The dioptric apparatus grows quicker than the retina, such that the proximal parts of the cones and the myofibril containing cells, called also veils, are bent until the next moult. This is more evident in juvenile animals (Figure , arrow) which grow quicker and have more frequent moults. The total number of ommatidia (counted along a column and multiplied by the number in a row) do not change much between a juvenile and a large adult, but in each moult a line of new cornea and new ommatidia are added.
At the inner border between the stalk and the cornea the morphogenetic furrow (Figure , arrow) contains a proliferation tissue (Figure ) at the interior of the eye. This tissue had been previously interpreted as a tissue providing total reflection of light (Schiff et al. Citation2002). With new data it was observed that this tissue is not positioned frontally but at the inner border between the stalk and the cornea. New studies demonstrated its involvement in the growth of ommatidia.
Figure 9. a, The tissue at the interior of the morphogenetic furrow in a section across the midband. From this proliferation tissue little tree‐like extensions (arrowhead) grow towards the interior of the eye. Bar: 100 µm. b, The tissue is organised in hexagonal patterns, typical for the stomatopod eye. Each group produces the veils surrounding the cones. Bar: 10 µm. c, Formation of networks generating the veils around the cones. bar: 20 µm. d, The extensions originating in each of the groups in b contact a network generating myofibril‐containing veils. Myofibrils are already present (arrow). Arrowhead: generation of a corneagenous cell in the network at the contact region with the tissue. Bar: 20 µm. e–f, Extensions from the tissue which have already formed two corneagenous cells, c.c., for each new ommatidium. The corneagenous cells form the crystalline cones (arrows), besides the new cornea. Bars: 50 µm.
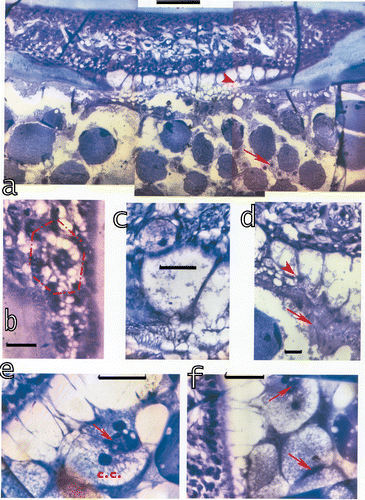
This tissue generates ommatidia towards the cornea and towards the stalk new parts of the eye muscles. This proliferation tissue appears to produce first the myofibril containing cells which grow towards the inside of the eye. The approximately hexagonal clusters or pre‐clusters are distributed in the typical hexagonal pattern of the eye with six clusters surrounding each cluster (Figure ). Each cluster generates first the myofibril containing veils, then the corneagenous cells and within their centre successively the crystalline cones (Figure , arrows). The corneagenous cells (Figure ) produce the new cornea and apparently participate in the formation of the crystalline cones (Figure , arrows; Figure , arrow in Figure ) or at least in the distal enforcements and the web around the crystalline cones (Figure ). In the new cornea the layers of alternating refractive indices are thinner than those in the normal cornea and furthermore, become progressively thinner from outside towards the interior of the eye. Attached to the region between the stalk and the cornea are the myofibril containing cells (Figure ) and the corneagenous cells which all are stretched towards the interior of the eye (Figure ). The myofibril cells form a hexagonal ring around the newly emerging corneagenous cells (Figure ) and the cone, but towards proximal become thin veils covering the cones. These veils contain already the myofibrils (Figure ) which thus surround the cone completely. Still further towards proximal these cells separate into six solid bundles of myofibrils around each cone, each bundle with a nucleus (Figure , arrow). The new cones attach to the rhabdoms (Figure ) and produce the extensions which run to attach to the basement membranes.
Figure 10. Sections parallel to the midband at the morphogenetic furrow show the proliferation tissue generating the dioptric apparatus at its distal part and the formation of the retina at its proximal part.a, Extending from the cornea are the myofibril containing veils and the corneagenous cells containing the beginning of a cone at their interior. The balls of black pigment are presumably the beginning of the formation of the accessory pigment cells. Black pigment surrounds also the beginning of the first‐order nerve fibres, i.e. the continuation of the retinular cells after passage through the basement membrane which last is also generated here. Bar: 100 µm. b–c, The corneagenous cells and the veils are already formed and generate the cones at their interior. Cone formation is advanced in b in the beginning in c; bar in b: 50 µm, in c: 100 µm. d, Contact between the new cone and the rhabdom and the formation of the four cone extensions which then attach to the basement membrane. Bar: 20 µm. e, The new connections between the dioptric and the retinal part. The myofibril containing ‘veils’ attach to the accessory pigment cells, but are still undulated (arrows). These structures will be stretched later on, presumably once the cone extensions and the accessory pigment cells are attached to the basement membrane. Bar: 20 µm.
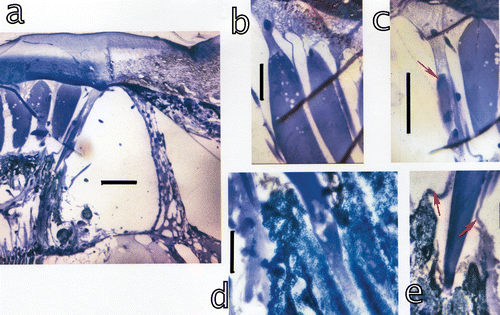
The proliferation tissue divides into two separate layers (Figure ): the distal layer apparently produces the dioptric apparatus, while a proximal layer produces the basement membrane, the accessory pigment cells and successively the retinular cells, which in turn produce the new rhabdoms. The proximal layer of the tissue seems to organize first the basement membrane. The dioptric apparatus seems to grow from the cornea towards the inside of the eye, the sensory part from the proximal layer of the tissue towards the cornea.
The myofibril cells grow towards the accessory pigment cells where they branch rootlike and contact a network (Figure , arrowheads). Six myofibril cells extend as tree‐like extensions from the proliferation tissue (Figure ). When they reach the six new accessory pigment cells they attach to these (Figure ). Before the moult the myofibril cells, when attached to the accessory pigment cells, are bent (Figure , arrows) and apparently stretch after the moult by action of actin and myosin in the dioptric apparatus, the veils, and within the retina. Growing myofibril cells and bent cones may also be stretched and straightened by the cuticular and full animal swelling during and immediately after moult.
Discussion
Light rays reflected from the multilayered cornea are polarized while those refracted and funnelled into the cones are polarized at 90° with respect to the reflected rays. With that the reflection in the frontal part of the eye, besides protecting the ommatidia from extreme light, may also add another functional component when polarization analysis could be important. Reflected light derives from reflection in the multilayered cornea, the thin outer and inner fasciae on the corneal facets and, presumably, from the reflecting pigments on the distal retina surface.
In the lobster eye the proliferation tissue is organized in clusters. Each cluster produces one whole ommatidium (Hafner & Tokarski Citation2001). In the eye of Ligia exotica (Roux, 1828) a proliferation zone produces ommatidia but the different components grow at different speeds (Keskinen et al. Citation2002). This also seems to be the case in stomatopod eyes. Furthermore, it appears that the clusters in the proliferation tissue generate only the myofibril containing veils, the corneagenous cells and the crystalline cones. The basement membrane, the retinular cells and the accessory pigment cells seem to start from a more proximal site. The new cornea with thinner and thinner layers of alternating refractive indices may be due to the deposits of different chitinous layers which then grow when the corneagenous cells produce more chitin. The effect on the transmission of light is that light is not funnelled properly into the new ommatidia. In the normal cornea the thickness of the layers is such that light is reflected and refracted according to the angle of incidence of the light, such that light is funnelled exactly into the thin distal tip of the cone. The deviation of light rays in multilayer structures decreases with thinner layers, as in the new cornea, and less oblique rays. Thus light is not funnelled into the cones. This may be the cause of the false pseudopupil seen from almost any direction.
We think that the shortening and folding of the cones during dark adaptation is provoked by the contraction of the myofibrils in the veils between the cones and the simultaneous relaxation of myofibrils in the retina. Actin and myosin have been observed around and next to the cones in other compound eyes (Baumann Citation1992). Myofibrils containing these molecules are able to contract (Hafner et al. Citation1992). Microtubules as well as the cone enveloping membranes are usually interpreted as more or less rigid structures which could support the cytoarchitecture of the cones or retina and the return to their original shapes after the myofibril contractions (Baumann Citation2001, Citation2004). But the microtubules in the accessory pigment cells may also slide during light adaptation as myosin and actin were found at the rhabdom margins and in the accessory pigment cells in arthropod eyes (Baumann Citation2004).
The cone enveloping membrane and the cone extensions through the retina apparently are associated with GAG molecules. Here the cytoplasm of the corneagenous cells unexpectedly stains heavily for nonsulphated GAGs, i.e. water‐absorbing and ‐releasing molecules (Dore et al. Citation2005). Thus the corneagenous cells may have one or many of the following functions:
-
preparing the new cornea for the next moult, contributing precursor saccharides for the chitin synthesis;
-
building GAG molecules transferred to the corneal structure;
-
building GAGs transferred to the extracellular envelopes of cones and veils;
-
constituting a water‐absorbing and ‐releasing hydraulic cushion involved in the morphological changes in light and dark adaptation; and
-
funnelling light from the borders of the visual field into the cone, as suggested by their transparency and wedge‐shaped structure.
New ommatidia produce a small part of cornea at the stalk junction, as the main part of the cornea is produced by the growing corneagenous cells. The little rooflets observed in some larval and adult eyes of S. mantis apparently connect the new cornea, grown by the corneagenous cells, to the rest of the ommatidium. Corneagenous cells, cone envelopes and the chitinous cornea are all rich in GAGs. But those GAGs forming the cornea may be considered to be of high molecular weight, insoluble molecules, while the GAGs associated with the cone envelopes are less rigid molecules. Possibly in the region of growth the corneagenous cells provide GAG molecules for the growth of the cornea and the organization of the dioptric apparatus.
References
- Baumann , O. 1992 . The submembrane cytoskeleton of pigmented glial cells, primary pigment cells and crystalline cone cells in the honeybee compound eye. . Cell and Tissue Research , 270 : 353 – 363 .
- Baumann , O. 2001 . Distribution of nonmuscle myosin‐II in honeybee photoreceptors and its possible role in maintaining compound eye architecture. . Journal of Comparative Neurology , 435 : 364 – 378 .
- Baumann , O. 2004 . Spatial pattern of nonmuscle myosin‐II distribution during the development of the Drosophila compound eye and implications for retinal morphogenesis. . Developmental Biology , 269 : 519 – 533 .
- Bonucci , E. 1981 . Manuale di Istochimica , Roma : Lombardo Ed .
- Caldwell , R. L. and Dingle , H. 1976 . Stomatopods. . Scientific American , 234 : 80 – 89 .
- Cronin , T. W. , Caldwell , R. L. and Erdmann , M. V. 2002 . Tuning of photoreceptor function in three mantis shrimp species that inhabit a range of depths. I. Visual pigments. . Journal of Comparative Physiology A , 188 : 179 – 186 .
- Di Stefano , G. , Iacino , L. and Schiff , H. 1990 . What the mantis shrimp's eye (possibly) tells its raptorial appendages. . Biological Cybernetics , 63 : 393 – 401 .
- Dore , B. , Schiff , H. and Boido , M. 2005 . Photomechanical adaptation in the eyes of Squilla mantis (Crustacea, Stomatopoda). . Italian Journal Zoology , 72 : 189 – 199 .
- Hafner , G. S. and Tokarski , T. R. 2001 . Retinal development in the lobster Homarus americanus. Comparison with compound eyes of insects and other crustaceans. . Cell and Tissue Research , 305 : 147 – 158 .
- Hafner , G. S. , Tokarski , T. R. and Kipp , J. 1992 . Localization of actin in the retina of the crayfish Procambarus clarkii. . Journal of Neurocytology , 21 : 94 – 104 .
- Iacino , L. , Di Stefano , G. and Schiff , H. 1990 . A neural model for localizing targets in space accomplished by the eye of a mantis shrimp. . Biological Cybernetics , 63 : 383 – 391 .
- Keskinen , E. , Takaku , Y. , Meyer‐Rochow , V. B. and Hariyama , T. 2002 . Postembryonic eye growth in the seashore isopod Ligia exotica (Crustacea, Isopoda). . Biological Bulletin , 202 : 223 – 231 .
- Manning , R. B. , Schiff , H. and Abbott , B. C. 1984 . Cornea shape and surface structure in some stomatopod crustacea. . Journal of Crustacean Biology , 4 : 502 – 513 .
- Schiff , H. 1997 . Mantis shrimp vision: Biology and models for localization and recognition of prey and figure‐ground discrimination. . Trends in Comparative Biochemistry & Physiology , 3 : 26 – 49 .
- Schiff , H. and Abbott , B. C. 1989 . “ Stomatopod vision. ” . In Biology of stomatopods. Selected symposia and monographs. U.Z.I., vol. 3 , Edited by: Ferrero , E. A . 11 – 38 . Modena : Mucchi .
- Schiff , H. and Di Stefano , G. 1992 . Target localization in different luminous environments. . Comparative Biochemistry & Physiology A , 103 : 479 – 486 .
- Schiff , H. and Gervasio , A. 1969 . Functional morphology of the Squilla retina. . Pubblicazioni della Stazione Zoologica di Napoli , 37 : 610 – 629 .
- Schiff , H. and Hendrickx , M. 1997 . An introductory survey of ecology and sensory receptors of tropical Eastern Pacific crustaceans. . Italian Journal of Zoology , 64 : 13 – 30 .
- Schiff , H. and Manning , R. B. 1984 . Description of a unique crustacean eye. . Journal of Crustacean Biology , 4 : 604 – 614 .
- Schiff , H. and Sertorio , C. 1993 . Parallel processing and learning in vertebrate, invertebrate and theoretical sensory networks. . Trends in Comparative Biochemistry & Physiology , 1 : 607 – 633 .
- Schiff , H. , Castelletti , G. , DiStefano , G. and Iacino , I. 1989 . A model for the dynamic properties of integrating fibers: Target localization in a three‐dimensional space—II. Computer simulation. . Comparative Biochemistry and Physiology A , 92 : 342 – 352 .
- Schiff , H. , Dore , B. and Donna , D. 2002 . A mantis shrimp wearing sun‐glasses. . Italian Journal of Zoology , 69 : 205 – 214 .
- Schiff , H. , Manning , R. B. and Abbott , B. C. 1986 . Structure and optics of ommatidia from eyes of stomatopod crustaceans from different luminous habitats. . Biological Bullettin , 170 : 461 – 480 .
- Snyder , A. W. 1979 . “ Physics of vision in compound eyes. ” . In Handbook of sensory physiology, vol. VII/6A. Invertebrate photoreceptors , Edited by: Autrum , H . 225 – 314 . New York : Springer‐Verlag .