Abstract
Spermatogenesis of the marine sponge Halichondria semitubulosa (formerly Pellina semitubulosa) has been investigated at the ultrastructural level. This process can be observed in March when, among the choanocyte chambers of the aquiferous system, spermatic cysts are visible. They are delimited by pinacocyte‐like cells and include elements in progressive development: spermatocytes of the first and second order, spermatids and spermatozoa. The early phase of spermatogenesis was not detected. Spermatocytes of the first order show an elongated shape, several small mitochondria and patched chromatin; spermatocytes of the second order, frequently connected by bridges, show denser chromatin, a single large mitochondrion with numerous tightly adherent cristae, glycogen, and round‐shaped inclusions with a central electron‐dense core. In the spermatids the chromatin tends to be packed in the central region. Spermatozoa have a uniformly dense nucleus in close association with the large mitochondrion. Sperm maturation takes place synchronously within the same cyst but asynchronously within the same specimen.
Introduction
In the recent publication Systema Porifera, the genus Pellina Schmidt, 1870 has been moved to the family Halichondriidae, as synonymous of the genus Halichondria Fleming, 1828 (Erpenbeck & Van Soest Citation2002). The former Pellina semitubulosa (Lieberkühn, 1859) belongs now to the subgenus Halichondria, which encompasses the species with “surface smooth or digitate, no continuous cover with conical papillae”. Consequently, the correct spelling of the specific name is Halichondria (Halichondria) semitubulosa Lieberkühn, 1859.
Halichondria semitubulosa is a fairly common species in the shallow water of the southern Mediterranean Sea. The specimens are small in size and may grow as epibionts on algal thalli. This species has been the subject of previous investigations focused on the architecture of the aquiferous system (Sciscioli et al. Citation1997), and on the silica content and spicule size variations (Mercurio et al. Citation2000). In the former paper, based on the fine organization of the choanocyte chambers, the water flow dynamics and related filter‐feeding activity were described. In the latter, the evaluation of the silica content proved a trend correlated to water temperature values. In particular, the study of the sexual reproduction trend of this species, using routine histological procedures, revealed active spiculogenesis, probably due to sponge body remodelling after gamete and larval release (Mercurio et al. Citation2000).
Sexual reproduction in Porifera has long been an active research area (see reviews in Fell Citation1974; Sarà Citation1974, Citation1990; Reiswig Citation1983, Simpson Citation1984; Ereskovsky Citation2000; Leys & Ereskovsky Citation2006), even though the detection of gametes, mainly of male gametes, is so sporadic as to limit our knowledge to a few cases. Ultrastructural investigations provided more data on the fine organization of the spermatozoa and showed their diversified morphology according to species (Boury‐Esnault & Jamieson Citation1999).
The aim of the present study was to gain an understanding of the spermatogenesis in H. semitubulosa to contribute to the knowledge of the biology of this species.
Material and methods
Specimens of H. semitubulosa were collected using scuba equipment in a sheltered basin located on the south‐western coast of Italy (Porto Cesareo Basin, Ionian Sea, Apulia). In agreement with previous research on this species (cited as Pellina semitubulosa), sampling was performed monthly, from March to June 2005, a period in which sperm cysts were already detected (Mercurio et al. Citation2000). Immediately after collection sponge fragments were fixed for 2 h in 2.5% glutaraldehyde in cacodylate buffer (0.7 M) and artificial seawater, to a final pH of 7.4. The same buffer was used for post‐fixation in 1% osmium tetroxide (1 h) and desilification (30 min) in 5% hydrofluoric acid (HF). Afterwards selected material was dehydrated in a graded ethanol series and embedded in araldite for transmission electron microscopy (TEM) investigation. Ultra‐thin sections, obtained with a Leica EM UC6 ultramicrotome, were collected on formvar‐coated copper grids and stained with uranyl acetate and lead citrate. The thin sections were examined with a Philips EM 208 electron microscope.
Results
In the sampling site, H. semitubulosa shows a massive and branching form with short oscular chimneys (Figure ). In this species the aquiferous system is particularly developed and the choanocyte chambers are numerous, very close to one another and about 35–40 µm in diameter (Figure ). In March, spermatic cysts are evident in the choanosomal area where they are located amidst the choanocyte chambers (Figure ). The spermatic cysts measure about 90–100 µm and contain male elements in various phases of maturity. Gametes do not occupy the whole cyst but they tend to accumulate in certain areas, in such a way that the cyst includes empty vacuoles (Figure ). On occasion, the space interposed between choanocyte chambers and cysts shows electron‐dense debris apparently included in a cytoplasmic matrix (Figure ).
Figure 1. Halichondria semitubulosa. A, sponge specimens in their natural environment (scale bar, 2 cm); B, semithin section showing choanocytes chambers (ChC) very close to one another (scale bar, 12 µm); C, Semithin section showing a spermatocyst (SC) near a choanocytes chamber (ChC) (scale bar, 12 µm); D, ultrathin section showing a spermatocyst (SC) and a choanocytes chamber (ChC). Note the debris (De) included in the cytoplasmic matrix. Sp I, spermatocytes of the first order (scale bar, 2 µm).
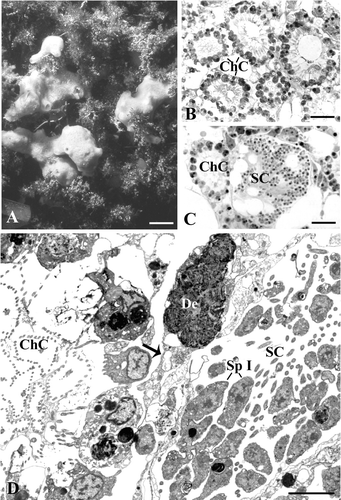
Each cyst is delimited by elongated pinacocyte‐like cells that form a thin coat separating the cysts from one another (Figure ). Inside the cyst, numerous flagella oriented towards the inner cyst region are visible (Figure ).
Figure 2. TEM micrographs of spermatogenetic phases.A, two spermatocysts (SC) at different stage of maturation. Note the layer of pinacocyte‐like cells (P) delimiting the cysts. F, flagella (scale bar, 5 μm); B, Spermatocytes of the first order (Sp I). M, mitochondria; N, nucleus (scale bar, 0.7 µm); C, spermatocytes of the second order (Sp II) connected by a cytoplasmic bridge (arrow). M, mitochondrion; N, nucleus (scale bar, 0.5 µm); D, a large mitochondrion (M) in the spermatocyte of the second order. Gl, glycogen; I, round‐shaped electron‐dense inclusions; N, nucleus (scale bar, 0.2 µm); E, spermatid. Note the presence of glycogen in the basal region of the tail (arrows). Gl, glycogen; I, electron‐dense inclusions; M, mitochondrion; N, nucleus (scale bar, 0.4 µm).
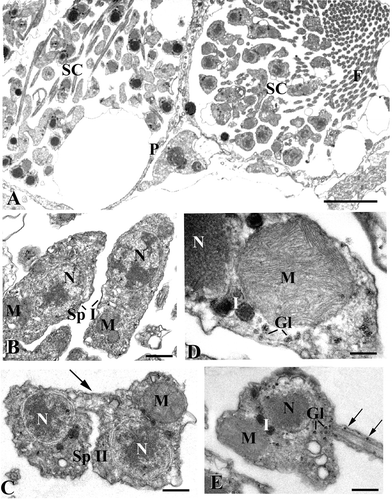
Transition from spermatocytes to spermatozoa
The cells in the spermatocysts develop synchronously. Spermatocytes of the first order tend to be elongated in shape (5 µm along the major axis) and are characterized by a large cytoplasmic component, nucleus with patched chromatin and several small mitochondria (Figure ).
Spermatocytes of the second order (Figure ) have a minor cytoplasmic component and show a single large mitochondrion (about 1 μm) located just below the cell membrane. During the formation of such elements, binucleate cells, connected by cytoplasmic bridges (Figure ) showing a thickening of the membrane by opaque material, can be observed.
A zoomed view of a spermatocyte of the second order gives evidence for the fine organization of the mitochondrion, which results in numerous and tightly adherent cristae (Figure ). Glycogen rosettes are scattered in the cytoplasm along with round‐shaped electron‐dense inclusions showing a central opaque core delimited by a more translucent region (Figure ).
Spermatids conserve the general characteristics of the spermatocytes of the second order but the chromatin tends to be compact in the central area (Figure ) and the glycogen rosettes enter even in the basal flagellar region (Figure ).
Spermatozoa are characterized by a uniformly electron‐dense nucleus and the inclusions tend to gather in proximity to it (Figure ).
Figure 3. TEM micrographs.A, spermatozoon with electron‐dense nucleus (N) and inclusions (I) (scale bar, 0.5 µm); B, section of a spermatozoon showing the co‐presence of nucleus (N) and mitochondrion (M). I, inclusions (scale bar, 0.4 µm); C, an almost empty cyst including only some spermatozoa (S) (scale bar, 6 µm).
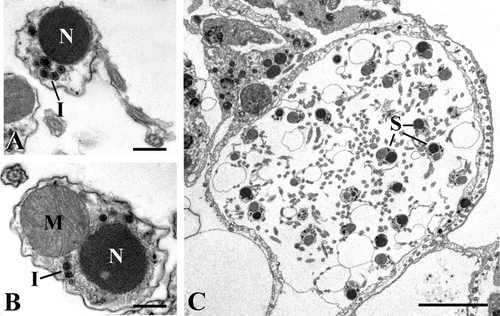
The orientation of the sperm in the cyst did not allow us to obtain sections encompassing sperm with their complete organization, including head and tail. Most sections show the co‐presence of nucleus and mitochondrion (Figure ), thus suggesting that they are in tight association.
At the end of the spermatogenic process, almost empty cysts are observed. They are still delimited by their coat and include only a few mature spermatozoa (Figure ).
Discussion
Spermatogenesis in sponges has been poorly studied, owing to the lack of determined germ cells and sex organs, which makes it difficult to trace the gamete differentiation from somatic cells. This research field is even more limited at the ultrastructural level, which is necessary to better describe the succession events of spermatogenesis. However in some demosponges the ultrastructural data give evidence of the origin of spermatozoa from choanocytes (Tuzet et al. Citation1970; Diaz et al. Citation1973; Diaz & Connes, Citation1980; Gaino et al. Citation1984, Citation1986; Paulus & Weissenfels Citation1986; Paulus Citation1989; Barthel & Detmar ;Citation1990; Kaye & Reiswig Citation1991; Boury‐Esnault & Jamieson Citation1999; Usher et al. Citation2004), even though this feature is still controversial. Indeed, some observations suggest that archaeocytes may be at the origin of the germinal cell line (Hoppe & Reichert Citation1987; Ilan Citation1995). The point in case is represented by those carnivorous cladorhizids and in other Poecilosclerida sponges, which lack an aquiferous system and choanocyte chambers (Vacelet & Boury‐Esnault Citation1996; Vacelet Citation2006). Likewise, in Hexactinellida, which possess anucleate choanocytes, spermatogenesis takes place in archaeocytes located in the mesohyl below the chambers (Boury‐Esnault & Vacelet Citation1994). It seems acceptable that both origins exist, depending on the species.
In H. semitubulosa the early stage of spermatogenesis, ranging from spermatogonia, was not observed. In fact, in all the reproductive specimens the spermatic cysts included germinal cells from spermatocytes of the first order to spermatozoa. Consequently, at present, we have no data about the actual origin of the sperm in this species. However, in consideration of the architecture of its aquiferous system, consisting of an astonishing number of choanocytes chambers, and of the lack of archeocyte‐like cells grouped together in the mesophyl matrix, we can speculate a possible origin of the male gametes from the transformation of the choanocytes of an entire choanocyte chamber.
The synchrony of spermatogenesis takes place within the same cyst and frequently between different cysts. It is well known that synchrony and asynchrony vary according to species and can occur at population, individual and cyst levels (Boury‐Esnault & Jamieson Citation1999).
The pathway of spermatogenesis is similar to that observed in other demosponges in which ultrastructural investigations have shown that the fine organization of gametes during maturation varies according to species and the spermatozoon differs mainly for the presence or absence of apical vesicles, the shape of the nucleus, the condensed chromatin and the number, size and shape of mitochondria (see review in Boury‐Esnault & Jamieson Citation1999).
As observed by Barthel and Detmer (Citation1990) during gamete maturation of the congeneric H. panicea (Pallas, 1766), neither Golgi apparatus was observed in H. semitubulosa nor an acrosome. In both species the reduction of the chondriome to a single mitochondrion has occurred. In contrast, the spermatozoon of H. semitubulosa differs remarkably from that of H. panicea because in the latter sponge two types of sperm can be recognized: a long, narrow type with an extremely elongated nucleus flanked by a long mitochondrion; and a more‐or‐less ovoid type due to the rolling up of the nucleus into a helical formation (Barthel & Detmer Citation1990).
To date, the presence of an acrosome has been observed in two Homoscleromorpha, namely in Oscarella lobularis (Baccetti et al. Citation1986; Gaino et al. Citation1986) and in Pseudocorticium jarrei (Boury‐Esnault & Jamieson Citation1999), whereas in the Poecilosclerida Crambe crambe the apical zone is said to contain an opaque vesicle (Tripepi et al. Citation1984), which could be interpreted as an acrosome. In the remaining species investigated so far, dense vesicles persist near the nucleus and were tentatively considered a rudimentary acrosome (Diaz & Connes Citation1980, Gaino et al. Citation1984). In H. semitubulosa, vesicles with a more electron‐dense core already can be observed in spermatocytes of the first order, and their number increases in the following stages up to the final mature sperm. We cannot exclude that these components could represent an infant form of acrosome.
In conclusion, the different structural organization of the sperm shown by the two congeneric species H. semitubulosa and H. panicea suggests that male gametes express species‐specific morphological traits. Ultrastructural investigations on the sponge spermatogenesis, even though fragmentary due to the lack of gonads in Porifera, could provide a set of characteristics useful for tracing phylogenetic trends in this basal Metazoa.
Acknowledgments
The authors wish to thank Professor Giuseppe Corriero for useful suggestions and discussions. The research was supported by University of Bari funds (Fondi di Ateneo per la Ricerca Scientifica 2005).
References
- Baccetti , B. , Gaino , E. and Sarà , M. 1986 . A sponge with acrosome: Oscarella lobularis. . Journal of Ultrastructure and Molecular Structure Research , 94 : 195 – 198 .
- Barthel , D. and Detmer , A. 1990 . The spermatogenesis of Halichondria panicea (Porifera, Demospongiae). . Zoomorphology , 110 : 9 – 15 .
- Boury‐Esnault , N. and Jamieson , B. G. M. 1999 . “ Porifera. ” . In Reproductive biology of invertebrates, vol. 9 part A: Progress in male gamete ultrastructure and phylogeny , Edited by: Adiyodi , K. G and Adiyodi , R. G . 1 – 20 . New York : John Wiley & Sons .
- Boury‐Esnault , N. and Vacelet , J. 1994 . “ Preliminary studies on the organization and development of a hexactinellid sponge from a Mediterranean cave, Oopsacas minuta. ” . In Sponges in time and space. Biology, chemistry, paleontology , Edited by: van Soest , R. W. M , van Kempen , Th. M. N and Braekman , J. C . 407 – 416 . Rotterdam : Balkema .
- Diaz , J. P. , Connes , R. and Paris , J. 1973 . Origine de la lignée germinale chez une Démosponge de l'étang de Thau: Suberites massa Nardo. . Comptes‐Rendus de l'Académie des Sciences de Paris , 277 : 661 – 664 .
- Diaz , J. P. and Connes , R. 1980 . Etude ultrastructurale de la spermatogénèse d'une Démosponge. . Biologie Cellulaire , 38 : 225 – 230 .
- Ereskovsky , A. V. 2000 . Reproduction cycles and strategies of the cold‐water sponges Halisarca dujardini (Demospongiae, Halisarcida), Myxilla incrustans and Iophon piceus (Demospongiae, Poecilosclerida) from the White Sea. . Biological Bulletin , 198 : 77 – 87 .
- Erpenbeck , D. and Van Soest , R. W. M. 2002 . “ Family Halichondriidae Gray, 1867. ” . In Systema Porifera. A guide to the classification of sponges , Edited by: Hooper , J. N. A and van Soest , R. W. M . 787 – 815 . New York : Kluwer Academic‐Plenum Publishers .
- Fell , P. E. 1974 . “ Porifera. ” . In Reproduction of marine invertebrates, vol. 1 , Edited by: Giese , A. C and Pearse , J. S . 51 – 132 . New York : Academic Press .
- Gaino , E. , Burlando , B. , Zunino , L. , Pansini , M. and Buffa , P. 1984 . Origin of male gametes from choanocytes in Spongia officinalis (Porifera, Demospongiae). . International Journal of Invertebrate Reproduction and Development , 7 : 83 – 93 .
- Gaino , E. , Burlando , B. , Buffa , P. and Sarà , M. 1986 . Ultrastructural study of spermatogenesis in Oscarella lobularis (Porifera, Demospongiae). . International Journal of Invertebrate Reproduction and Development , 10 : 297 – 305 .
- Hoppe , W. F. and Reichert , J. J. M. 1987 . Predictable annual mass release of gametes by the coral reef sponge Neofibularia nolitangere (Porifera: Demospongiae). . Marine Biology , 94 : 277 – 285 .
- Ilan , M. 1995 . Reproductive biology, taxonomy and aspects of chemical ecology of Latrunculiidae (Porifera). . Biological Bulletin , 188 : 306 – 312 .
- Kaye , H. R. and Reiswig , H. M. 1991 . Sexual reproduction in four Caribbean commercial sponges. I. Reproductive cycles and spermatogenesis. . Invertebrate Reproduction and Development , 19 : 1 – 11 .
- Leys , S. P. and Ereskovsky , A. V. 2006 . Embryogenesis and larval differentiation in sponges. . Canadian Journal of Zoology , 84 : 262 – 287 .
- Mercurio , M. , Corriero , G. , Scalera Liaci , L. and Gaino , E. 2000 . Silica content and spicule size variations in Pellina semitubulosa (Porifera: Demospongiae). . Marine Biology , 137 : 87 – 92 .
- Paulus , W. 1989 . Ultrastructural investigation of spermatogenesis in Spongilla lacustris and Ephydatia fluviatilis (Porifera, Spongillidae). . Zoomorphology , 109 : 123 – 130 .
- Paulus , W. and Weissenfels , N. 1986 . The spermatogenesis of Ephydatia fluviatilis (Porifera). . Zoomorphology , 106 : 155 – 162 .
- Reiswig , H. M. 1983 . “ Porifera. ” . In Reproductive biology of invertebrates, vol. 2: Spermatogenesis and sperm function , Edited by: Adiyodi , K. G and Adiyodi , R. G . 1 – 21 . Chichester : John Wiley & Sons .
- Sarà , M. 1974 . Sexuality in the Porifera. . Bollettino di Zoologia , 4 : 327 – 348 .
- Sarà , M. 1990 . “ Porifera: Sexual differentiation and behaviour. ” . In Reproductive biology of invertebrates, vol. 5 , Edited by: Adiyodi , K. G and Adiyodi , R. G . 1 – 29 . New Delhi, , India : Oxford and IBH Publishing .
- Sciscioli , M. , Lepore , E. , Corriero , G. , Scalera Liaci , L. and Gaino , E. 1997 . Ultrastructural organization of choanocyte chambers in the haplosclerid Pellina semitubulosa (Porifera, Demospongiae): a cue for water flow into the sponge body. . Italian Journal of Zoology , 64 : 291 – 296 .
- Simpson , T. L. 1984 . The cell biology of sponges , New York, , USA : Springer‐Verlag .
- Tuzet , O. , Garrone , R. and Pavans de Ceccatty , M. 1970 . Observations ultrastructurales sur la spermatogenése chez la Démosponge Aplysilla rosea Schulze (Dendroceratides): une métaplasie exemplaire. . Annales des Sciences Naturelles, Zoologie, Paris , 12 : 27 – 50 .
- Tripepi , S. , Longo , O. M. and La Camera , R. 1984 . “ A new pattern of spermiogenesis in the sponge Crambe crambe: Preliminary observations in electron microscopy. ” . In Eighth European Congress on Electron Microscopy, Budapest, Programme Committee Edited by: Csanady , A , Röhlich , P and Szabόo , D . 2073 – 2074 .
- Usher , K. M. , Sutton , D. C. , Toze , S. , Kuo , J. and Fromont , J. 2004 . Sexual reproduction in Chondrilla australiensis (Porifera: Demospongiae). . Marine and Freshwater Research , 55 : 123 – 134 .
- Vacelet , J. 2006 . New carnivorous sponges (Porifera, Poecilosclerida) collected from manned submersibles in the deep Pacific. . Zoological Journal of the Linnean Society , 148 : 553 – 584 .
- Vacelet , J. and Boury‐Esnault , N. 1996 . A new species of carnivorous sponge (Demospongiae: Cladorhizidae) from a Mediterranean cave. . Bulletin de l'Institut Royal des Sciences Naturelles de Belgique, Biologie , 66 : 109 – 115 .