Abstract
In the rivers of northern Italy, the presence of water is typically continuous all year long, although in the last five decades there has been a conspicuous increase in drought periods, mainly caused by human impacts and climate change. The aim of this study was to assess the impact of the length of the drought periods on macroinvertebrate assemblages. We collected invertebrates in four reaches of the Po river, characterised by different periods of absence of surface water. We found significant differences among the stations in invertebrate abundance and taxa richness, with a decrease in the more drought‐affected stream reaches. Collector‐gatherers significantly increased as the drought period lengthened, while the opposite occurred for scrapers and shredders. The areas with a discontinuous presence of water were mainly colonised by small, fast‐growing, plurivoltine organisms. A main result of our study is that only a few taxa appear to be able to survive in reaches with intermittent flow, underlining the great ecological difference between perennial and naturally intermittent streams. Our results suggest that the recent increase of droughts will likely cause an impoverishment of benthic communities in prealpine rivers.
Introduction
In the last few decades, the biological monitoring of running water systems has become increasingly important in many countries. Methods based on the analysis of benthic macroinvertebrate communities have become an indispensable complement to traditional chemical and physical techniques in the evaluation of human impact. The Water Framework Directive of the European Community WFD 2000/60 (E.U. Citation2000) underlines the importance of this approach, which has been part of the Italian legislation (D. Lgs. 152/99). The number of indices (approximately 50) based on benthic macroinvertebrate communities is about five times that of any other group and is still growing (Mandaville Citation1999).
The descriptive power of macroinvertebrates in freshwater monitoring is well known (Allan Citation1995), but one of the main disadvantages is that in some cases benthic invertebrates can show huge temporal variation in community structure. These variations can obscure pollution related to hydrological stress (Hancock Citation2002; Hancock & Boulton Citation2005). This is particularly evident in temporary streams, in which flow occurs either seasonally (intermittent streams) or in response to irregular rain (episodic streams) (Hose et al. Citation2005). Intermittent streams, with a regularly occurring dry period (Williams Citation1996), are distributed in coastal and southern regions of Italy with Mediterranean‐type climate, and throughout the world in arid and semi‐arid regions (Gasith & Resh Citation1999).
We can distinguish between droughts in intermittent streams, also called ‘seasonal droughts’, and droughts in perennial streams (‘supra‐seasonal droughts’) which are unpredictable and longer. While the ‘seasonal drought’ is really just an extreme part of the water regime of certain types of environments and is probably not even perceived as drought by many organisms (Lake Citation2000), unpredictable droughts can constitute a severe stress for the whole aquatic ecosystem.
Benthic communities in intermittent lotic environments have evolved adaptations and strategies to survive the dry period: for example, it has been hypothesised that the hyporheic zone (i.e. the interstitial habitat beneath and lateral to a stream‐river channel) is a refuge for organisms that remain in the dry channel (Williams & Hynes Citation1974). In intermittent streams, faunal recovery after seasonal droughts is relatively rapid (Boulton & Lake Citation1992a). Aquatic organisms of intermittent streams appear to be better adapted to drought conditions (Fletcher Citation1986). Boulton (Citation1989, Citation2003) investigated faunal succession after predictable drought in Australian intermittent streams and demonstrated the importance of over‐summering refuges and life‐cycle solutions: most macroinvertebrates survived the summer drought in refuges, such as the interstitial zone, permanent pools along the river and crayfish burrows filled with water, while some specialised taxa had desiccation‐resistant stages that emerged from re‐flooded sediments.
In a study of the ecological impact of droughts in flowing waters, Lake (Citation2000) indicated various phases and effects. In a first phase, the diminution of flushing flows increases the number of lentic habitats and changes in water chemistry are observed, for example, lowering of dissolved oxygen concentration and increasing water temperature. As water level falls further, hydrological connectivity is disrupted and the stream becomes a series of fragmented pools. The critical phase of a drought occurs when surface water disappears and whole sections of the stream desiccate, although hyporheic flow may persist. Direct effects of drought are the decreased water level, which disrupts hydrological connectivity, and the reduced availability of habitats for the aquatic biota (Stanley et al. Citation1994). Indirect impacts include increased predation and interspecific competition and the change in the nature of food resources (Miller & Golladay Citation1996; Lake Citation2000; Hancock Citation2002).
In perennial lotic systems, such as Italian prealpine rivers, the presence of water is typically continuous along the year, with some degree of seasonal variation in discharge. However, in the last five decades there has been a conspicuous increase in drought periods, mainly caused by global change in climate and human impacts (e.g. agricultural water extractions in the middle stream reaches, hydroelectric uses in the upper stream reaches).
The Po river is the largest Italian river and tenth largest in Europe. It drains a basin of over 70,000 km2. The Po originates in the high mountains of the Monviso group, at 2020 m a.s.l. In its upper reach, it is a typical mountain stream with fast‐flowing oxygenated waters. After 13 km, the river enters the alluvial plain and, in the area of Martiniana Po and Revello (approximately 350 m a.s.l.), a highly permeable riverbed produces a substantial hyporheic flow. In the last few decades, drought has become a regular event in this reach due to the presence of dams and irrigation canals (Parco del Po Cuneese, personal observation). For about 5–10 km, the Po riverbed can completely lack water for many weeks or even months of the year. These ‘supra‐seasonal’ droughts are mainly human‐induced and affect prealpine lotic systems usually immune to this phenomenon.
There are two well‐known responses of the aquatic biota to drought: resistance, the capacity of the biota to withstand the drought, and resilience, the ability to recover from the drought (Lake, Citation2000). Communities in intermittent streams show high resistance and strong resilience to seasonal droughts (Pires et al. Citation2000), but less is known about the biotic responses to droughts in perennial streams.
The aim of this study was to assess the impact of the length of drought periods on benthic communities in the Po River, a lotic system in which droughts are a recent and human‐induced phenomenon. This research is focused to provide new data on the potential use of the most common biological monitoring methods under altered hydrological conditions.
Materials and methods
In the study area, the Po River is a third‐order stream. We selected an 11‐km long reach, with a catchment area at the most downstream point of 311.3 km2. The catchment consists mainly of mountains belonging to the Monviso group (3841 m a.s.l.). The riparian vegetation is dominated by Robinia pseudoacacia, Alnus glutinosa and Corylus avellana. Details on grain‐size composition of sediments, major elements, nutrients and trace metals are provided in Vignati et al. (Citation2003). The stream has altered flow regimes in the lowest part of the study reach, but is otherwise unaffected by pollution.
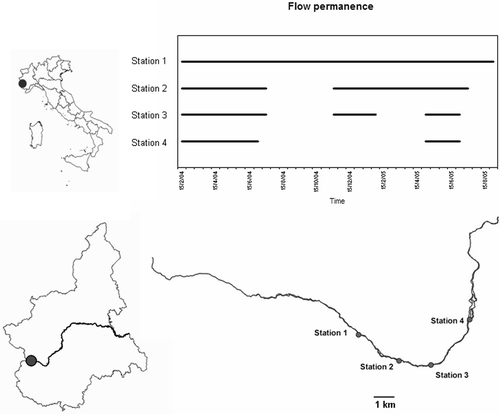
From January 2004 to September 2005, samples were collected in four sites (Figure , Table ):
Site 1: Sanfront (UTM: 367154, 4946144). Water flow is present throughout the year. | |||||
Site 2: Martiniana (UTM: 370861, 4943760). Surface water flow usually ceases for 2–3 months every year. | |||||
Site 3: Revello (UTM: 373797, 4943389). Surface water flow usually ceases for 4–5 months every year. | |||||
Site 4: Saluzzo (UTM: 377344, 4947535). Surface water flow usually ceases for 6–8 months every year. | |||||
Table I. Main physical factors in the four stations of the Po river.
The four stations are close to each other (11 km from Station 1 to Station 4). As an indicator of drought length, we considered the number of days in which the station was without flowing surface water: 0 in Station 1, 150 in Station 2 (26.3% of the study period), 240 in Station 3 (42.1% of the study period) and 330 in Station 4 (57.8% of the study period). Chemical samplings were also performed in five temporal replicates in sites 1–4.
Quantitative methods were used to investigate the composition and structure of the macroinvertebrate assemblages in the four stations. We collected 325 Surber samples in the stream reach using a 0.06 m2 sampler with a 500 μm mesh: 99 samplings from Station 1, 99 samplings from Station 2, 67 samplings from Station 3 and 60 samplings from Station 4. The difference in the number of samples was due to the difference in drought period length among stations. On each sampling date, a mean of four surface samples were collected randomly. Samples were collected proportionately to the importance of the different microhabitats in this stream reach (generally three from erosive microhabitats and one from lateral, depositional microhabitats).
All organisms were counted and identified to genus level, except for Chironomidae, Simuliidae and early instars of some Trichoptera and Diptera, that were identified to the family level. Each taxon was also assigned to a Functional Feeding Group (FFG: scrapers, shredders, collector‐gatherers, filterers and predators) according to Merritt and Cummins (Citation1996). Moreover, all taxa were entered into seven biological and seven ecological groups, according to the species traits approach of Usseglio‐Polatera et al. (Citation2000).
In order to assess differences in taxon richness and abundances among sites, ANOVA was performed on log‐transformed data.
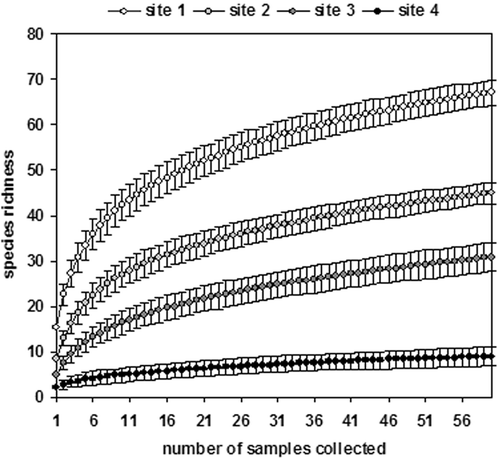
Taxon richness accumulation curves, generated with EstimateS 6.0 software (Colwell Citation1997), were used to compare sampling efficiency among sites.
Results
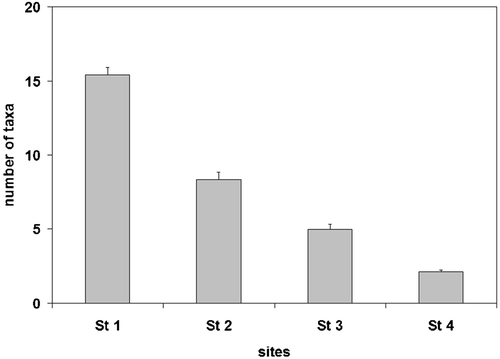
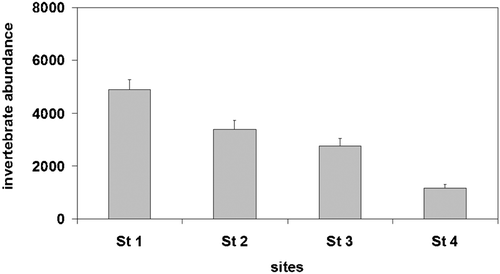
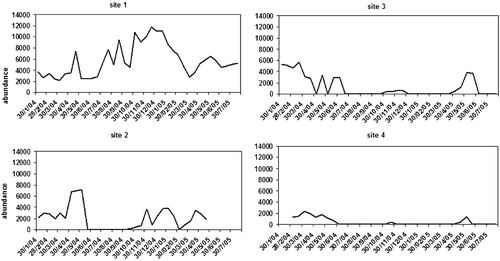
We detected no significant differences in the chemical quality of the water among sites (Table ). In total, we collected 53,580 macroinvertebrates in the natural riverbed of the four sites (Table ). The abundance of macroinvertebrates decreased along the stream reach (Figure ), with significant differences among the four sites (ANOVA F 3,321 = 32.51, P<0.001). The density of the natural macroinvertebrate coenosis was highest in Site 1 (mean = 4886.8±371.2 SE ind./m2) and lowest in Site 4 (mean = 1152.3±148.8 ind./m2).
Table II. Main chemical parameters in the four stations of the Po river.
Table III. List of macroinvertebrates collected in the natural riverbed of the four stations. Biological and ecological traits are given according to Usseglio‐Polatera et al. (Citation2000); Functional Feeding Groups according to Merritt and Cummins (Citation1996).
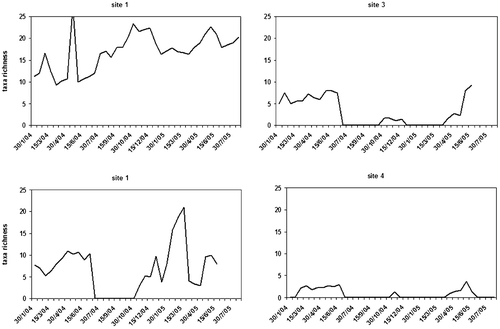
Taxon richness also decreased from the most upstream to the most downstream station (ANOVA F 3,321 = 159.6, P<0.001; Figure ). The number of taxa was highest in samples taken from Station 1 (15.4±0.5) and lowest in samples taken from Station 4 (2.10±0.13).
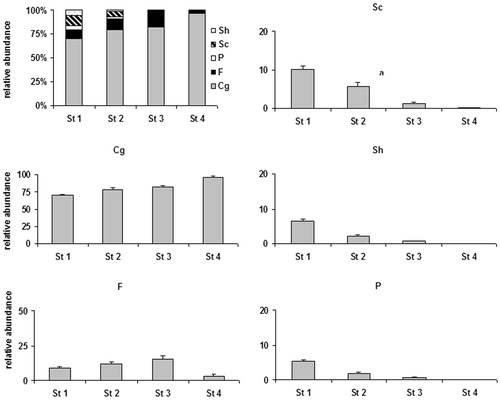
The functional composition of the macroinvertebrate community was very different among sites (Figure ). Although Collector‐gatherers (Cg) was the most abundant FFG throughout the river section, its percentage abundance significantly increased downstream (ANOVA F 3,321 = 55.3, P<0.001). Cg represented 70.05±1.34% of all individuals in Site 1, 79.23±2.02% in Site 2, 82.5±2.25% in Site 3 and 96.38±1.22% in Site 4. The opposite trend was observed for Scrapers (Sc), Predators (P) and Shredders (Sh). Scrapers decreased drastically throughout the river section (ANOVA F 3,321 = 75.9, P<0.001); they were well represented in the reaches with permanent or semi‐permanent flow (10.2±0.77 in Site 1 and 5.76±0.93 in Site 2), while they decreased in Site 3 (1.19±0.43) and were almost absent in Site 4 (0.11±0.07). The relative importance of Predators also significantly diminished downstream (ANOVA F 3,321 = 115.4, P<0.001): the mean percentage of this FFG was 5.29±0.35 in Site 1, 1.96±0.39 in Site 2 , 0.58±0.11 in Station 3 and almost 0 in Site 4. Shredders showed a similar trend (ANOVA F 3,321 = 88.3, P<0.001), with a conspicuous presence in Site 1 (5.53±0.64) and a significant progressive reduction in Sites 2 (1.35±0.23) and 3 (0.35±0.09). No shredders were found in the driest Site 4 throughout the study period. Filterers, mainly represented by Trichoptera Hydropsychidae and Diptera Simuliidae, were most frequent in the intermediate Sites 2 and 3, where they constituted 11.69±1.86% and 15.34±1.22%, respectively. The mean percentage of filterers was 8.94±0.93% in Site 1 and 3.45±1.21% in Site 4.
Figure 4. Percent functional composition of the four station(upper left) and relative importance of the five Functional Feeding Groups in the four stations (Cg = Collectors–gatherers; F = Filterers; P = Predators; Sc = Scrapers; Sh = Shredders).
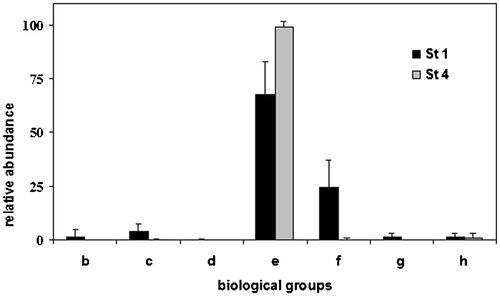
The biological and ecological traits of the benthic macroinvertebrates (Usseglio‐Polatera et al. Citation2000) also differed significantly among the four stations. Considering the biological traits, the most abundant group in all stations was the e group (small or medium‐sized, short‐lived, mono‐ or plurivoltine crawlers with aquatic respiration and cemented eggs) followed by the f group (medium‐sized, monovoltine organisms with aquatic respiration and eggs or larvae that avoid adverse conditions by having a quiescent phase). Interestingly, seven biological groups were well represented in Station 1, while Station 4 was nearly dominated by taxa belonging to only one group (Figure ). For example, the presence of organisms belonging to the b group (large or medium‐sized, crawlers or burrowers, ovoviviparous) significantly decreased downstream (ANOVA F 3,321 = 31.3, P<0.001). Organisms in the c group (medium‐sized monovoltine crawlers) and g group (small or medium‐sized swimmers, mostly with aerial respiration, shredders or piercers) showed the same trend, with a significant reduction downstream (respectively, c group: ANOVA F 3,321 = 88.8, P<0.001, and g group: ANOVA F 3,321 = 28.1, P<0.001). Organisms in the d group (large and medium‐sized with predaceous habits, with semivoltine and long life cycles) were present in Stations 1 (0.17% of the community) and 2 (0.02% of the community) but were completely absent in Stations 3 and 4.
Figure 5. Relative importance of taxa belonging to different‘biological groups’ in the stations 1 and 4, according to Usseglio‐Polatera et al. (Citation2000).
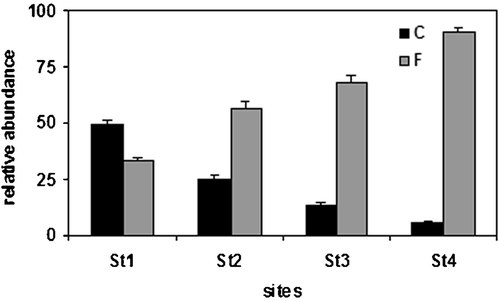
There were also evident differences among stations in the ecological traits of the sampled taxa: in particular, the two most abundant groups showed an inverse trend (Figure ). Organisms belonging to the F ecological group (taxa avoiding high current velocities, living in lentic or stagnant habitats) increased significantly in importance from upstream to downstream (ANOVA F 3,321 = 72.9, P<0.001), while organisms belonging to the C ecological group (taxa with rhithronic, epipotamic and oligotrophic habits, inhabiting mineral substrata with slow to medium current velocities) decreased significantly (ANOVA F 3,321 = 110.4, P<0.001). Furthermore, rheophilous taxa, inhabiting coarse mineral substrata in oligotrophic environments (A ecological group), decreased significantly from Station 1 to Station 4 (ANOVA F 3,321 = 66.8, P<0.001), while eurythermic taxa, inhabiting low flowing waters (E ecological group), and taxa inhabiting aquatic macrophytes, mud or organic substrata (G ecological group) were present in Stations 1 and 2 but were almost absent in the two downstream stations.
Figure 6. Differences among the four station in the presence of the representative‘ecological groups’ C and F.
Species accumulation curves showed that more taxa were likely to be found with additional samplings (Figure ), and the curves agreed with the other data in that the upstream stations showed the greatest taxon richness. The recovery was quite different among stations, excluding Station 1 where surface flow was always present (see Figures and ).
Discussion
Analysing climatological data from 1901 to 1999, Lloyd‐Hughes and Saunders (Citation2002) stated that large parts of Europe were affected by droughts during the twentieth century, but droughts were less common in the Alps than in other European regions. Many scientists have suggested that, with the increase in global warming, extreme hydrological events, such as floods and droughts, will become more common in Europe (Watson et al. Citation1998); for example, the Deutscher Verband für Wasserwirtschaft and Kulturbau (Citation1998) provided evidence that droughts have increased in frequency, duration and severity in the last few decades.
This trend can be related to two distinct phenomena: changes in seasonal meteorological conditions and the increase of artificial influences in catchments (Hisdal et al. Citation2001). Both phenomena could be contributing to the progressive increase in the duration and frequency of droughts in the upper Po river: this area has a highly permeable substratum but human influences, such as water subtraction for agricultural use in the area near Sanfront, probably also play a major role (Parco del Po Cuneese, personal communication).
Recent studies have underlined a progressive increase in the number of permanent streams becoming intermittent because of withdrawal and diversion of water (Robson & Matthews Citation2004). North‐western Italian alpine lotic systems do not usually experience regular drought; for this reason, the reach analysed in this study is an interesting model to investigate the response of benthic communities to the disappearance of surface water in a naturally perennial lotic environment.
Significant differences were observed in taxon richness, ecological diversity and invertebrate abundance; since there was no significant difference in water chemistry among sites, they are likely to be related to changes in stream hydrology along the sites. In the area of Sanfront (Site 1), where flow is permanent throughout the year, the Po showed a rich and diversified benthic community, with a high number of taxa covering many ecological niches. Moving downstream, with an increasing drought length period, we recorded a progressive impoverishment of the benthic coenoses, with the disappearance of many taxa. One of the main reasons for this progressive reduction is probably that periodic droughts disadvantage some taxa with peculiar ecological preferences. The absence of water disrupts some ecological processes, such as the microbial breakdown of allochthonous inputs (Boulton & Lake Citation1992b) or the establishment of permanent periphytic biofilms (Wood & Petts Citation1999). Thus, specialised functional feeding groups such as Shredders and Scrapers were well represented in Site 1 but drastically decreased downstream. Furthermore, the lack of water leads to lower microhabitat availability in the downstream stations: for example, organic‐rich muddy substrata and macrophytes disappeared downstream, as did their invertebrate inhabitants. Areas with gravel substrate also diminished or disappeared in the lower stations because of the reduction in flow velocity and the massive sedimentation of large areas of the riverbed.
Analysis of the biological and ecological traits (Usseglio‐Polatera et al. Citation2000) also yielded interesting results. Firstly, colonisation patterns and mechanisms likely played a key role in producing the recorded difference among stations. It is well known that stream invertebrate populations are in continuous redistribution (Bo et al. Citation2006; Fenoglio et al. Citation2002, Citation2004), but different dispersal strategies may drastically shape the benthic communities in areas with different flow duration. Taxa with aquatic dispersal diminished downstream while the abundance of taxa with aerial dispersal increased, since they are more able to colonise intermittent environments: Crustacea, Irudinea and Mollusca (b biological group) diminished and finally disappeared in the driest stations, which were dominated by Chironomidae, Simuliidae and Ephemeroptera. Large semivoltine taxa with long larval life, which are unable to survive in periodically dry environments, showed the same decrease downstream: for example, Corduliidae and Coenagrionidae damselflies (d biological group) were present in Site 1 but absent in the rest of the study area. The sites with a discontinuous presence of water were mainly colonised by small, fast growing, plurivoltine organisms (d biological group).
Rhithronic, rheophilous taxa that generally inhabit coarse substrata (C ecological group) decreased from Stations 1 to 4, while there was an increase of semi‐lentic taxa, able to survive in eurythermic environments with fluctuating water levels. Only a few taxa could survive drought periods by colonising the sub‐substratum in this pedemontane stream (Fenoglio et al. Citation2006). The assemblages found during periods without running water consisted almost entirely of small or medium‐sized, short‐lived organisms with aquatic respiration, mainly collector‐gatherers. Chironomidae constituted more than 2/3 of all invertebrates, confirming previous findings (Weigelhofer & Waringer Citation2003; Bo et al. Citation2006).
Since Italian and European legislation mandates that benthic communities must be used in the biological monitoring of running water quality, the data emerging from our study have significant practical relevance. While the chemical assessment of this part of the Po river revealed no evident human impact on the lotic system, the biological data underlined an evident alteration. The Italian rules (D. Lgs. 152/99) states that the biological quality of a stream reach must be measured as the mean of four seasonal samplings. When water is absent in the Po, no samplings are conducted: we suggest that the biological quality on these occasions should be scored as “0”, and these data should be included in the calculation of the annual means. In this way, biological monitoring could truly improve our knowledge of the ecological conditions of a lotic environment.
A main result of our study is that, in prealpine environments that are usually permanent, few taxa appear to be able to colonise reaches with artificially induced intermittent flow. This underlines the great ecological difference between naturally perennial and intermittent streams, since invertebrate communities in the latter have evolved strategies to survive recurrent losses of water (Boulton Citation1989; Stanley et al. Citation1994; Hose et al. Citation2005). Although some studies have claimed that periodic droughts may be important in maintaining the diversity of freshwater systems (Everard Citation1996), our study provides evidence that artificial drought severely disturbs the benthic coenoses in Italian prealpine rivers.
More studies are needed to fully understand the evolution of macrobenthic communities in this changing environment, investigating for example the role of the hyporheic zone as a refuge and the recolonisation strategies of different aquatic invertebrates.
Acknowledgements
We thank S. Carletto, N. Decarolis, M. Decostanzi, M. Pessino, F. Sgariboldi for assistance in field work and G. Ferro for Hydraenidae identification. This work was supported by Parco del Po Cuneese and Regione Piemonte CIPE ‘Ricerca scientifica applicata’ grants.
References
- Allan , J. D. 1995 . Stream ecology: Structure and function of running waters , London : Chapman & Hall .
- Bo , T. , Cucco , M. , Fenoglio , S. and Malacarne , G. 2006 . Colonisation patterns and vertical movements of stream invertebrates in the interstitial zone: A case study in the Apennine, NW Italy. . Hydrobiologia , 568 : 67 – 78 .
- Boulton , A. J. 1989 . Over‐summering refuges of aquatic macroinvertebrates in two intermittent streams in central Victoria. . The Transactions of the Royal Society of South Australia , 113 : 23 – 34 .
- Boulton , A. J. 2003 . Parallels and contrasts in the effects of drought on stream macroinvertebrate assemblages. . Freshwater Biology , 48 : 1173 – 1185 .
- Boulton , A. J. and Lake , P. S. 1992a . The ecology of two intermittent streams in Victoria, Australia. III. Temporal changes in faunal composition. . Freshwater Biology , 27 : 123 – 138 .
- Boulton , A. J. and Lake , P. S. 1992b . Benthic organic matter and detritivorous macroinvertebrates in two intermittent streams in south‐east Australia. . Hydrobiologia , 241 : 107 – 118 .
- Colwell RK. 1997. EstimateS: Statistical estimation of species richness and shared species from samples. Version 6.0b1. http://viceroy.eeb.uconn.edu/estimates
- Deutscher Verband für Wasserwirtschaft and Kulturbau . 1998 . “ How to work on a drought mitigation strategy. ” . In ICID Guide
- E.U . 2000 . Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. . Official Journal of the European Communities , 327 : 1 – 72 .
- Everard , M. 1996 . The benefits of drought to the aquatic environment. . Freshwater Forum , 7 : 33 – 50 .
- Fenoglio , S. , Agosta , P. , Bo , T. and Cucco , M. 2002 . Field experiments on colonization and movements of stream invertebrates in an Apennine river (Visone, NW Italy). . Hydrobiologia , 474 : 125 – 130 .
- Fenoglio , S. , Bo , T. and Bosi , G. 2006 . Deep interstitial habitat as refuge for Agabus paludosus (Fabricius, 1801) (Coleoptera: Dytiscidae) during summer droughts. . The Coleopterist Bulletin , 6 : 37 – 41 .
- Fenoglio , S. , Bo , T. , Gallina , G. and Cucco , M. 2004 . Vertical distribution in the water column of drifting stream macroinvertebrates. . Journal of Freshwater Ecology , 19 : 485 – 492 .
- Fletcher , A. R. 1986 . “ Effects of introduced fish in Australia. ” . In Limnology in Australia , Edited by: De Deckker , P and Williams , W. D . 231 – 238 . Melbourne : CSIRO Publ .
- Gasith , A. and Resh , V. H. 1999 . Streams in Mediterranean climate regions: Abiotic influences and biotic responses to predictable seasonal events. . Annual Review of Ecology and Systematics , 30 : 51 – 81 .
- Hancock , P. J. 2002 . Human impacts on stream–groundwater exchange zone. . Environmental Management , 29 : 763 – 781 .
- Hancock , P. J. and Boulton , A. J. 2005 . The effects of an environmental flow release on water quality in the hyporheic zone of the Hunter River, Australia. . Hydrobiologia , 552 : 75 – 85 .
- Hisdal , H. , Stalh , K. , Tallaksen , L. M. and Demuth , S. 2001 . Have streamflow droughts in Europe become more severe or frequent? . International Journal of Climatology , 21 : 317 – 333 .
- Hose , G. C. , Jones , P. and Lim , R. P. 2005 . Hyporheic macroinvertebrates in riffle and pool areas of temporary streams in south eastern Australia. . Hydrobiologia , 532 : 81 – 90 .
- Lake , P. S. 2000 . Disturbance, patchiness, and diversity in streams. . Journal of North American Benthological Society , 19 : 573 – 592 .
- Lloyd‐Hughes , B. and Saunders , M. A. 2002 . A drought climatology for Europe. . International Journal of Climatology , 22 : 1517 – 1592 .
- Mandaville , S. M. 1999 . Bioassessment of freshwaters using benthic macroinvertebrates—A primer. , Halifax, , Canada : Chebucto Community Net Ed . First Ed. Project E‐1, Soil & Water Conservation Society of Metro Halifax
- Merritt , R. W. and Cummins , K. W. 1996 . An introduction to the aquatic insects of North America , Dubuque, , USA : Kendall/Hunt .
- Miller , A. M. and Golladay , S. W. 1996 . Effects of spates and drying on macroinvertebrate assemblages of an intermittent and a perennial prairie stream. . Journal of North American Benthological Society , 15 : 670 – 689 .
- Pires , A. M. , Cowx , I. G. and Coelho , M. M. 2000 . Benthic macroinvertebrate communities of intermittent streams in the middle reaches of the Guadiana Basin (Portugal). . Hydrobiologia , 435 : 167 – 175 .
- Robson , B. and Matthews , T. 2004 . Drought refuges affect algal recolonization in intermittent streams. . River Research and Application , 20 : 753 – 763 .
- Stanley , E. H. , Buschman , D. L. , Boulton , A. J. , Grimm , N. B. and Fisher , S. G. 1994 . Invertebrate resistance and resilience to intermittency in a desert stream. . American Midland Naturalist , 131 : 288 – 300 .
- Usseglio‐Polatera , P. , Bournaud , M. , Richoux , P. and Tachet , H. 2000 . Biological and ecological traits of benthic fresh‐water macroinvertebrates: relationships and definition of groups with similar traits. . Freshwater Biology , 43 : 175 – 205 .
- Vignati , D. , Pardos , M. , Diserens , J. , Ugazio , G. , Thomasì , R. and Dominik , J. 2003 . Characterisation of bed sediments and suspension of the river Po (Italy) during normal and high flow conditions. . Water Research , 37 : 2847 – 2864 .
- Watson , R. T. , Zinyowera , M. C. and Moss , R. H. 1998 . The regional impact of climate change: an assessment of vulnerability IPCC. , Cambridge : Cambridge University Press .
- Weigelhofer , G. and Waringer , J. 2003 . Vertical distribution of benthic macroinvertebrates in riffles versus deep runs with differing contents of fine sediments (Weidlingbach, Austria). . International Review of Hydrobiology , 88 : 304 – 313 .
- Williams , D. D. 1996 . Environmental constraints in temporary fresh waters and their consequences for the insect fauna. . Journal of the North American Benthological Society , 15 : 634 – 650 .
- Williams , D. D. and Hynes , H. B. N. 1974 . The occurrence of benthos deep in the substratum of a stream. . Freshwater Biology , 4 : 233 – 256 .
- Wood , P. J. and Petts , G. E. 1999 . The influence of drought on chalk stream macroinvertebrates. . Hydrological Processes , 13 : 387 – 399 .