Abstract
Biometric evaluation, ovary histomorphometry, plasma 17β‐estradiol (E2) and androgens (A) concentrations were investigated through the gonadal maturation and spawning of the wild chub, Leuciscus cephalus. A lower concentration of plasma E2 was recorded from January (oogonia) to March (previtellogenic oocytes), while estradiol concentrations increased in April, when the ovary mostly contained vitellogenic oocytes. In July, when it was prevalently composed of post‐ovulatory follicles, there was a decline in E2 plasma concentrations. Low concentrations of this steroid remained in the plasma until January, while high concentrations of androgens reached a peak in February and April preceding and following E2. The observed seasonal changes in plasma steroids, reflecting the pattern of follicles size development, were to a large extent consistent with observations made in other cyprinids. Since they define the reproductive cycle of the European female chub, they will be useful in the construction of a maturity schedule for wild populations.
Introduction
To develop a baseline reproductive biology for wild populations, maturity schedule studies on the process of gonad maturity, using steroid patterns, gonadosomatic index, macroscopic characteristics and histology are usually applied (Hunter et al. Citation1992). The sex steroids plasma concentrations are useful indicators of steroidogenic secretion during each stage of the sexual cycle and in association with change in gonadal morphology; in addition, they foster an understanding of the endocrine control of reproduction in teleosts (Sisneros et al. Citation2004).
Seasonal changes in circulating concentrations of gonadal steroid hormones during the reproductive cycle have been described for a variety of freshwater and marine teleost species (Fostier et al. Citation1983; Sisneros et al. Citation2004). In marine teleosts, reproductive periodicity of gonadal steroid hormone concentrations has been documented for wild‐caught populations of flatfish (Harmin et al. Citation1995), mullet (Dindo & MacGregor Citation1981), salmon (Ueda et al. Citation1984), cod (Pankhurst & Conroy Citation1987), orange roughy (Pankhurst & Conroy Citation1988), rockfish (Nagahama et al. Citation1991; Takano et al. Citation1991), sardines (Matsuyama et al. Citation1994; Murayama et al Citation1994), grouper (Johnson et al. Citation1998), kingfish (Poortenaar et al. Citation2001), tuna (Stequert et al. Citation2001), eelpout (Larsson et al. Citation2002), damselfish (Pankhurst et al. Citation1999), goby (Pierantoni et al. Citation1990), and toadfish (Modesto & Canario Citation2003), showing the relevance of these studies that provide critical information for formulating management plans to sustain the populations of endangered species.
Moreover, alterations of steroid hormones have implications in studying the effects of the environmental parameters (pollution, temperature, photoperiod, etc.) on the reproductive biology of fishes (Shimizu et al. Citation1994) by acting on gametogenesis and spawning (Wen & Lin Citation2001; Tollefsen et al. Citation2002).
17β‐Estradiol induces vitellogenin synthesis in adult female fishes and a correlation has been reported between its plasma concentrations and relative gonadal weights (Pavlidis et al. Citation2000; Aruke & Goksoyr Citation2003; Moncaut et al. Citation2003; Berg et al. Citation2004). Testosterone (T) has been identified in plasma of female teleosts and may be utilized as the major aromatizable androgen (Sen et al. Citation2002) and as an indicator of final oocyte maturation (Scott et al. Citation1998).Seasonal gonadal morphology of various wild cyprinids has been performed and is reviewed in Rinchard and Kestemont (Citation1996).
Chub is a cyprinid species, whose biology has been well studied and whose various interesting aspects have been examined (Pottinger et al. Citation2000; Jensen et al. Citation2001; Mancini et al. Citation2005). However, nothing was found about the sex steroid patterns and seasonal gonadal morphology of the female of this species, whereas the role of sex steroids in controlling the maturation cycle in the male was already reported in Guerriero et al. (Citation2005).
In the present study, chub females were collected throughout one year and gonadosomatic index (GSI) and histological analyses were used to describe the sexual cycle. Seasonal changes in plasma 17β‐estradiol and androgens were monitored and correlated to GSI and stage of gonadal maturity.
Materials and methods
Animals and sampling procedures
Mature female chub (length: 140–200 mm) were collected monthly from a large outdoor pond of the River Tiber (central Italy) from July 2004 to June 2005 by using a backpack electric shocker. In order to avoid the effects of fish size on the morphological parameters measured within a species, at each monthly sampling, specimens of similar size were selected. For analysis, only samples (age: 3+) reported as adult by Bianco and Santoro (Citation2002) were utilized. Experimental procedures were conducted in accordance with the Guidelines for the use of Animals in Research (Animal Behaviour Citation1998). Fish were anesthetized in 2‐phenoxyethanol (Sigma), and blood (0.3–0.5 ml) was collected by cardiac puncture in heparinized glass capillaries. Plasma was obtained after centrifugation at 800× g for 15 min at 4°C and immediately stored at −20°C until radioimmunoassay was performed. Total length (mm), body mass, gonadal mass (g) and gonadosomatic index (GSI: defined here as 100×gonadal mass/body mass) were recorded (Table ) and ovaries were fixed in Bouin's fixative solution for histological examination.
Table I. Mean total lengths (mm), body and gonadal mass (g) of the female chub Leuciscus cephalus by collection.
Histological analysis
Gonads were dissected out, weighed separately and plunged into Bouin's fixative solution for histological analysis. Ovaries were enclosed in paraffin–celloidin and the sections (7 µm) were stained with hematoxylin–eosin. In preliminary studies, no differences were detected in gonadal development between anterior, middle, and posterior sections of the chub right and left ovary. Therefore, tissue samples from the middle of right gonad were prepared, each gonad being classified according to the most advanced stage of oocytes present (Table ). Ovarian development was examined by histomorphometric analysis. Two ovarian parameters were examined: (1) mean oocyte diameter by relative proportion in each month, by randomly counting 50 cells per ovary; and (2) distribution percentage of oocyte size, assessed by measuring 50 profiles of each oocyte type, and then dividing the percentage of a given stage by the corresponding mean diameter. The microscopic characteristics for determining the maturity stages and describing oocytes were reported according to the classification of Yamamoto (Citation1956).
Table II. Maturity stages of ovaries of wild‐caught chub Leuciscus cephalus. Oocyte stage, appearance and oocyte diameter (mean±SD) detected monthly.
Radioimmunoassay (RIA)
Steroids (17β‐estradiol and androgens) were extracted from plasma by double ether extraction. Ether (1.5 ml) was added to 200 µl of plasma, vortexed for 30 s and supercooled in a −20°C methanol bath. The extract was reconstituted with 200 µl standard diluent. Radioimmunoassay of plasma steroids followed the method of D'Istria et al. (Citation1974), the reliability of which for fish plasma was assessed and reported by Guerriero et al. (Citation1998, Citation2005).
The following sensitivities were recorded: 17β‐estradiol, 5 pg (intrassay, 9%; interassay, 13%); androgens, 7 pg (intrassay, 7%; interassay, 13%). Tritiated steroids were purchased from Amersham Biosciences. The antisera were provided by G. Bolelli (Physiopathology of Reproduction Service, University of Bologna, Bologna, Italy). Testosterone antiserum reacted also with 5α‐dihydrotestosterone (about 80%); therefore the term androgens will be used throughout the paper.
Statistical analysis
The statistical errors are expressed as mean±SD. The correlation coefficient was considered significant at the 5% significance level. One‐way analysis of variance (ANOVA) was used to test for significant monthly changes.
Results
Histomorphological analysis
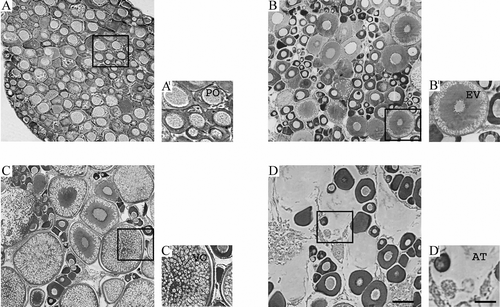
In chub, the cavity of the ovary was lined with germinal epithelium. The ovarian stage, the oocyte type and the mean diameter detected are reported in Table . Oocytes at different maturational stages were embedded into a connective tissue, rich in blood capillaries, nerves and smooth muscle fibers, with a variable monthly mean diameter (Figure ). In December–January the ovary showed small, spherical oocytes (oogonia), with a mean diameter of 0.17 mm. The nucleus was spherical, basophilic and centrally placed containing several nucleoli. Follicle cells forming a discontinuous layer surrounded oocytes with some previtellogenic oocytes (Figure ). In February, oocytes started increasing in size due to yolk storage, as suggested by the presence of vacuoles at the periphery of the ooplasm. The nucleus was placed centrally, containing several visible nucleoli and the oocytes were surrounded by a layer of follicle cells. At this stage, 42% of oocytes were at early‐vitellogenesis stage (previtellogenic oocytes; mean diameter 0.31 mm) and 58% of oogonia were still present. In March, yolk kept accumulating in the oocytes (vitellogenic oocytes; mean diameter 0.57 mm); oogonia were still 53% present previtellogenesis oocytes were reduced by 18% and vitellogenic oocytes became 29%. The centrally placed nucleus contained several visible nucleoli. Follicle cells formed a multilayer around the oocytes, the granulosa and an internal and external theca layer were visible and separated by a basal lamina (Figure ), while oocytes presented an early endogenous vitellogenesis. In April, 51% of oogonia, 10% of previtellogenesis oocytes and 39% of vitellogenic oocytes (mean diameter 1.04 mm) were present. The ooplasma was filled with yolk and oocytes were ready to be ovulated. The granulosa layer was thicker than in the previous period and the theca layers were well evident. In May, 55% of oogonia, 2% of previtellogenic oocytes and 43% vitellogenic oocytes (mean diameter 1.14 mm) were found (Figure ), and oocytes were completing the endogenous vitellogenesis at different levels, corresponding to the various spawning phases. In June, 20% of oogonia, 5% of previtellogenesis oocytes (mean diameter 0.49 mm) and 75% of postovulatory follicles and atretic follicles were detected. From July to November, only oogonia (mean diameter 0.50 mm) were present (100%), embedded in an abundant connective tissue (Figure ).
Figure 1 Histological sections(A–D) of ovaries of the chub Leuciscus cephalus during A, undeveloped stage: immature (primitive oogonia, PO); B, early recovery of gonadal activity stage: maturing (early vitellogenic, EV); C, ripening stage: mature (vitellogenic, VO); D, resting stage: post‐mature (atretic, AT). Stained with hematoxylin and eosin. Scale bars: A–D = 50 µm and A'–D' = 100 µm.
Changes in gonadosomatic index and steroid plasma concentrations
Variations in the gonadosomatic index (GSI) during the experimental period are shown in Figure . The GSI rapidly increased in May (13.4±1.2, P<0.05) showing a sharp decrease in June (5.1±0.4, P<0.05), after ovulation. Similar behavior for oocyte mean diameter appeared with a peak in May (Figure ).
Figure 2 Monthly changes ofφ = mean oocyte diameter and GSI = gonadosomatic index (A), plasma levels of E2 = 17β‐estradiol (B); and A = androgen (C) in the chub Leuciscus cephalus. Each value represents the mean of three determinations in triplicate of each sample (n = 20)±SD. * Level of significance versus the mean values observed in the preceding month (P<0.05).
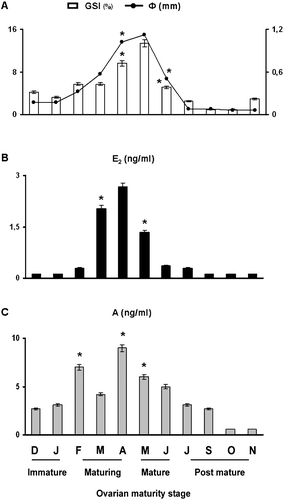
Specific changes of sex hormone plasma concentrations occurred during the different phases of the gonadal development. Plasma 17β‐estradiol (Figure ) increased from January (0.12±0.01 ng/ml, P<0.05) to April (2.67±0.10 ng/ml, P<0.05) and rapidly decreased in June after ovulation. Androgen concentrations (Figure ) reached bimodal peaks: in the stasis period, February (7.0±0.49 ng/ml, P<0.05), and at full vitellogenesis, April (9.0±0.54 ng/ml, P<0.05), just after ovulation.
For all maturity stages, fish size, GSI, 17β‐estradiol and androgens were positively correlated with each other (P<0.05).
Discussion
In this investigation the histology of the ovary, the sex steroid plasma concentrations and the gonadosomatic index were employed to develop a baseline reproductive biology for the female chub living in the River Tiber (central Italy) increasing information on maturity schedules of wild populations.
Reproductive biology of fishes has always been widely investigated (Nash Citation1999; McBride & Thurman Citation2003; Sisneros et al. Citation2004), but recently, the exploitation of some valuable cyprinid species for commercial purposes has made their investigation particularly relevant (Ronnback et al. Citation2002; Berry Citation2003). In addition, cyprinids are a family of evolutionary interest, often used as tools for genetic and physiological investigations (Roldan et al. Citation2000; Tsigenopoulos et al. Citation2002; Mancini et al. Citation2005). Knowledge about the role of sex steroids in maturation cycle control, especially when spawning time is altered by environmental or hormonal manipulation, could be of theoretical and practical interest, as for other species (Casini et al. Citation2002).
The histological examination of the chub ovary throughout the year led us to divide the sexual cycle into four physiological periods of the maturity stages in the ovary, mainly based on monthly distribution of gametes at different stages of development: (1) the immature or stasis period (December–January), characterized by the presence of oogonia; (2) the maturing or recrudescent period (February–April), when oocytes are previtellogenic and vitellogenic; (3) the mature or spawning period (May–June), during which spawning was observed several times; (4) the post‐ovulatory or post‐spawning period (July–November), when the gonads appeared less organized and characterized by oogonia. It is interesting to note that only a few publications deal with the whole histological and biochemical evaluation on the reproductive cycle of cyprinids (Degani et al. Citation1998; Guerriero et al. Citation1998, Citation2005; Jensen et al. Citation2001).
In the single spawners, the spawning period length is generally determined by analyzing GSI curves. However, in multiple‐spawning fish, GSI seems to be a less reliable indicator of fish maturity; only histological analysis provides a precise assessment of ovarian maturity and distinguishes between fish in an intermediate spawning stage and those in a post‐spawning stage. Moreover, GSI greatly underestimates the reproductive investment in serial spawners (Rinchard & Kestemont Citation1996). In chub, an example of a multi‐spawner, both oocyte diameter and GSI increases occur during vitellogenesis, to reach a peak in May. This species shows group‐synchronous oocytes that, under specific environmental conditions, spawn several times throughout the spawning period. In April and May, mature female chub presented eggs at different developmental stages. About 30% was represented by mature eggs, ready for spawning, with a diameter of 1.0–1.1 mm and the remaining part with different range of diameter (0.1–0.5 mm). The multi‐spawning strategy of different cyprinind species is necessary to guarantee reproductive success, above all for the specimens which live in rivers where some spawnings could be destroyed by meteorological events.
The GSI profile found in River Tiber chub indicates that the spawning period occurs in May–June, whereas it was between April and June in chub of the Retina River in Greece and in the Savur stream in Turkey (Unlu & Balci Citation1993). However, a slightly different spawning period has been reported for other populations of chub from Great Britain, Belgium, Spain and Turkey. In these geographical areas, the spawning period begins a little later than in Greece and in Italy (present data) and it extends longer in time (Unlu & Balci Citation1993; Encina & Granado‐Lorencio Citation1997). Such slight variations could be explained by the relationship existing between the reproduction cycle and environmental variations of biotic and abiotic factors, such as water temperature, photoperiod, and food resources (Tveinten & Johnsen Citation2001; Wen & Lin Citation2001; Tollefsen et al. Citation2002).
However, our results related to the spawning period of chub present in the River Tiber are in agreement with others reported for the plasma sex steroid pattern in different cyprinids (Aida Citation1988; Rinchard & Kestemont Citation1996). They confirm seasonal data of several species of teleosts indicating seasonal concentration changes of circulating sex hormones and their importance for reproduction (Fostier et al. Citation1983; Kagawa et al. Citation1983; Okuzawa et al. Citation1989; Guerriero et al. Citation1998; Nash Citation1999; Pankhurst et al. Citation1999; Consten et al. Citation2002). In fact, acute changes in reproductive activity of teleosts are commonly accompanied by a shift in reproductive hormones concentrations (Tsigenopoulos et al. Citation2002).
The present work indicates plasma 17β‐estradiol in female chub of the same order as those reported for cyprinid species of similar size (Rinchard et al. Citation1997).
The analysis of plasma 17β‐estradiol and GSI pattern showed a good correlation from January to April, indirect evidence implicating 17β‐estradiol as being in control of the synthesis of vitellogenic proteins (Casini et al. Citation2002). Similarly, a peak in 17β‐estradiol plasma concentrations associated with the synthesis of vitellogenic proteins has been described in many other teleosts (Pavlidis et al. Citation2000; Berg et al. Citation2004). In the chub, as in salmonids, the 17β‐estradiol concentrations decrease after ovulation; in fact, the post‐ovulatory ovary contains only very small yolkless oocytes. The observed decrease in estradiol represents, in other species, a characteristic of final oocyte maturation when progesterone analogues and other maturation‐inducing hormone increase (Nagahama et al. Citation1994; Mylonas et al. Citation1997). Furthermore, Scott et al. (Citation1998) showed a decrease of plasma estradiol levels in Pleuronectes platessa with increasing proportions of oocytes in final maturation in the ovaries, as was observed in our specimens (Figures , ).
One other interesting finding is that seasonal changes of androgen plasma concentrations in the female chub prove similar to those of the present study reported in goldfish, plaice and amago salmon (Borg Citation1994). The profile of plasma androgen level in fish caught in the River Tiber (Figure ) was similar to the pattern expected during maturation of group‐synchronous species (Wallace & Selman Citation1981). Both plasma 17β‐estradiol and androgen were present at elevated levels in reproductively active female chub and concentrations of the two steroids were significantly and positively correlated (P<0.05).
The first increase of androgen levels during oocyte development can be related to its role as precursor of 17β‐estradiol synthesis (Barannikova et al. Citation2000; Sen et at. Citation2002); the second peak was also measured in most fish during final oocyte maturation, an effect of the release of T into the plasma when this was no longer needed for aromatization. According to Kobayashi et al. (Citation1989), this acute rise in testosterone indicates that oocytes were fully mature and ready to ovulate. Although fish were captured by means of an electric system to limit the stress of capture, the sampling procedure and handling may have induced a significant drop in some sexual steroids, as reviewed by Kestemont et al. (Citation1999) in fish such as Pagrus auratus and Morone saxatilis.
Gonadosomatic index (GSI) evaluation, combined with histomorphological analysis and endocrine pattern, suggested that May was the main spawning season, with few chances of early spawning in March or later spawning in July.
From these data, the maturation mechanism of the Italian chub could be clarified. Histological examination of the ovary combined with sex steroids plasma determinations enabled us to update the maturing schedule for the wild populations. Sex steroids fluctuations in the plasma proved correlated with the maturity stage of gonads and were in general agreement with what is known in other teleosts.
Acknowledgments
Many thanks are due to Dr. Massimo Rampacci (AGEI–Roma) for assistance during sampling, identifying and stocking of fish. This work was supported by a grant from Federico II University, Naples, Italy.
References
- Aida , M. 1988 . A review of plasma hormone changes during ovulation in cyprinid fishes. . Aquaculture , 74 : 11 – 21 .
- Aruke , A. and Goksoyr , A. 2003 . Eggshell and egg yolk proteins in fish: hepatic proteins for the next generation: Oogenetic, population, and evolutionary implications of endodisruption. . Comparative Hepatology , 2 : 4 – 7 .
- Barannikova , I. A. , Diubin , V. P. , Baiunova , L. V. and Semenkova , T. B. 2000 . Steroids in the reproductive function regulation in fish. Rossiskii Fiziologicheski¿ Zhurnal Imeni I.M. . Sechenova , 86 : 968 – 978 .
- Berg , A. H. , Westerlund , L. and Olsson , P. E. 2004 . Regulation of arctic char (Salvelinus alpinus) egg shell proteins and vitellogenin during reproduction and in response to 17β‐estradiol and cortisol. . General and Comparative Endocrinology , 135 : 276 – 285 .
- Berry , C. 2003 . Fishy business. . QJM , 96 : 83 – 84 .
- Bianco , P. G. and Santoro , E. 2002 . The reproductive biology of the Italian roach, European chub and Appenninian barb in the river basin of Cilento and Vallo di Diano National Park in southern Italy (Pisces; Cyprinidae). . Biologia Ambientale , 18 : 85 – 91 .
- Borg , B. 1994 . Androgens in teleost fishes. . Comparative Biochemistry and Physiology , 109 : 219 – 245 .
- Casini , S. , Fossi , M. C. , Mori , G. and Bjornstad , A. 2002 . Vitellogenin induction in Cyprinus carpio treated with 17beta‐estradiol and 4‐nonylphenol. . Environmental Monitoring and Assessment , 75 : 235 – 239 .
- Consten , D. , Keuning , E. D. , Bogerd , J. , Zandbergen , M. A. , Lambert , J. G. , Komen , J. and Goos , H. J. 2002 . Sex steroids and their involvement in the cortisol‐induced inhibition of pubertal development in male carp, Cyprinus carpio L. . Biology of Reproduction , 67 : 465 – 472 .
- D'Istria , M. , Delrio , G. , Botte , V. and Chieffi , G. 1974 . Radioimmunoassay of testosterone, 17β‐oestradiol and oestrone in the male and female plasma of Rana esculenta during sexual cycle. . Steroids and Lipids Research , 5 : 42 – 48 .
- Degani , G. , Boker , R. and Jackson , K. 1998 . Growth hormone, sexual maturity and steroids in male carp (Cyprinus carpio). . Comparative Biochemistry and Physiology , 120C : 433 – 440 .
- Dindo , J. J. and MacGregor , R. 1981 . Annual cycle of serum gonadal steroids and serum lipids in striped mullet. . Transactions of the American Fisheries Society , 110 : 403 – 409 .
- Encina , L. and Granado‐Lorencio , C. 1997 . Seasonal variations in the physiological status and energy content of somatic and reproductive tissues of chub. . Journal of Fish Biology , 50 : 511 – 522 .
- Fostier , A. , Jalbert , B. , Billard , R. , Breton , B. and Zohar , Y. 1983 . “ The gonadal steroids. ” . In Fish physiology. , Edited by: Hoar , W. S , Randall , D. J and Donaldson , E. M . 117 – 170 . New York, London : Academic Press .
- Guerriero , G. , Ferro , R. and Ciarcia , G. 2005 . Correlations between plasma levels of sex steroids and spermatogenesis during the sexual cycle of the chub, Leuciscus cephalus L. . Zoological Studies , 44 : 228 – 233 .
- Guerriero , G. , Paolucci , M. , Botte , V. and Ciarcia , G. 1998 . The reproductive cycle of the cyprinid Alburnus albidus: Morphological changes of the male gonads and plasma sex steroid fluctuations. . Italian Journal of Zoology , 65 : 223 – 226 .
- Animal Behaviour . " Guidelines for the treatment of animals in behavioural research and teaching. ." Animal Behaviour , 55 . pp. 251 – 257 . .
- Harmin , S. A. , Crim , L. W. and Wiegand , W. D. 1995 . Plasma sex steroid profiles and the seasonal reproductive cycle in male and female winter flounder, Pleuronectes americanus. . Marine Biology , 121 : 601 – 610 .
- Hunter , J. R. , Macewicz , D. J. , Chyan‐huel Lo , N. and Kimbrel , C. A. 1992 . Fecundity, spawning, and maturity of female Dover sole Microstomus acificus, with an evaluation of assumption and precision. . Fishery Bulletin , 90 : 101 – 128 .
- Jensen , K. M. , Korte , J. J. , Kahl , M. D. , Pasha , M. S. and Ankley , G. T. 2001 . Aspects of basic reproductive biology and endocrinology in the fathead minnow (Pimephales promelas). . Comparative Biochemistry and Physiology , 128C : 127 – 141 .
- Johnson , A. K. , Thomas , P. and Wilson , R. R Jr. 1998 . Seasonal cycles of gonadal development and plasma sex steroid levels in Epinephelus morio, a protogynous grouper in the eastern Gulf of Mexico. . Journal of Fish Biology , 52 : 502 – 518 .
- Kagawa , H. , Young , G. and Nagahama , Y. 1983 . Changes in plasma steroid hormone levels during gonadal maturation in goldfish Carassiuss auratus. . Bulletin of the Japanese Society of Scientific Fisheries , 49 : 1783 – 1787 .
- Kestemont , P. , Rinchard , J. , Feys , B. and Fostier , A. 1999 . Spawning migrations, sexual maturity and sex steroid levels in female roach Rutilus rutilus from the River Meuse. . Aquatic Sciences , 61 : 111 – 121 .
- Kobayashi , M. , Aida , K. and Hanyu , I. 1989 . Hormone changes during ovulation and effects of steroid hormones on plasma gonadotropin levels and ovulation in goldfish. . General and Comparative Endocrinology , 67 : 301 – 307 .
- Larsson , D. G. J. , Mayer , I. , Hyllner , S. J. and Forlin , L. 2002 . Seasonal variations of vitelline envelope proteins, vitellogenin, and sex steroids in male and female eelpout (Zoarces viviparus). . General and Comparative Endocrinology , 125 : 184 – 196 .
- Mancini , L. , Caimi , S. , Ciardullo , S. , Zeiner , M. , Bottoni , P. , Pancioni , L. , Cautadella , S. and Caroli , S. 2005 . A pilot study on the contents of selected pollutants in fish from the Tiber River (Rome). . Microchemical Journal , 79 : 171 – 145 .
- Matsuyama , M. , Fukuda , T. , Ikeura , S. , Nagahama , Y. and Matsuura , S. 1994 . Spawning characteristics and steroid hormone profiles in the wild female Japanese Sardine Sardinops melanostictus. . Fisheries Science , 60 : 703 – 706 .
- McBride , R. S. and Thurman , P. E. 2003 . Reproductive biology of Hemiramphus brasiliensis and H. balao (Hemiramphidae): Maturation, spawning frequency, and fecundity. . The Biological Bulletin , 204 : 57 – 67 .
- Modesto , T. and Canario , A. V. M. 2003 . Morphometric changes and sex steroid levels during the annual reproductive cycle of the Lusitanian toadfish, Halobatrachus didactylus. . General and Comparative Endocrinology , 131 : 220 – 231 .
- Moncaut , N. , Nostro , F. L. and Maggese , M. C. 2003 . Vitellogenin detection in surface mucus of the South American cichlid fish Cichlasoma dimerus (Heckel, 1840) induced by estradiol‐17beta. Effects on liver and gonads. . Aquatic Toxicology , 63 : 127 – 137 .
- Murayama , T. , Shiraishi , M. and Aoki , I. 1994 . Changes in ovarian development and plasma levels of sex steroid hormones in the wild female Japanese sardine (Sardinops melanostictus) during the spawning period. . Journal of Fish Biology , 45 : 235 – 245 .
- Mylonas , C. C. , Scott , A. P. and Zohar , Y. 1997 . Plasma gonadotropin II, sex steroids, and thyroid hormones in wild striped bass (Morone saxatilis) during spermiation and final oocyte maturation. General and Comparative Endocrinology</pub-tl>108223236 .
- Nagahama , Y. , Takemura , A. , Takano , K. , Adachi , S. and Kusakari , M. 1991 . Serum steroid hormone levels in relation to the reproductive cycle of Sebastes taczanowskii and S. schlegeli. . Environmental Biology of Fishes , 30 : 31 – 38 .
- Nagahama , Y. , Yoshicuni , M. , Yamashita , M. and Tanaka , M. 1994 . “ Regulation of oocyte maturation in fish. ” . In Fish physiology. , Edited by: Sherwood , N , Hew , C. L , Farrell , A. P and Randall , D. J . 393 – 439 . New York : Academic Press .
- Nash , J. P. 1999 . “ Seasonal reproduction, fish. ” . In Encyclopedia of reproduction. , Edited by: Knobil , E and Neill , J. P . 329 – 340 . San Diego : Academic Press .
- Okuzawa , K. , Furukawa , K. , Aida , K. and Hanyu , I. 1989 . Effects of photoperiod and temperature on gonadal maturation, and plasma steroid and gonadotropin levels in a cyprinid fish, the honmoroko Gnathopogon caerulescens. . General and Comparative Endocrinology , 75 : 139 – 147 .
- Pankhurst , N. W. and Conroy , A. M. 1987 . “ Seasonal changes in reproductive condition and plasma levels of sex steroids in the blue cod, Parapercis colias (Bloch and Schneider) (Mugiloididae). ” . In Fish Physiology and Biochemistry Vol. 4 , 15 – 26 .
- Pankhurst , N. W. and Conroy , A. M. 1988 . Endocrine changes during gonadal maturation and spawning in the orange roughy (Hoplostethus atlanticus Collett), a teleost from the midslope waters off New Zealand. . General and Comparative Endocrinology , 70 : 262 – 273 .
- Pankhurst , N. W. , Hilder , P. I. and Pankhurst , P. M. 1999 . Reproductive condition and behaviour in relation to plasma levels of gonadal steroids in the spiny damselfish Acanthochromis polyacanthus. . General and Comparative Endocrinology , 115 : 53 – 69 .
- Pavlidis , M. , Greewood , L. , Mourot , B. , Kokkari , C. , Le Menn , F. , Divanach , P. and Scott , A. P. 2000 . Seasonal variations and maturity stages in relation to different serum levels of gonadal steroids, vitellogenin, and thyroid hormones in the common dentex (Dentex dentex). . General and Comparative Endocrinology , 118 : 14 – 25 .
- Pierantoni , R. , Fasano , S. , Minacci , S. , Di Matteo , L. , D'Antonio , M. , Bottazzi , F. and Chieffi , G. 1990 . Regulation of the testicular activity in the marine teleost fish, Gobius paganellus. . General and Comparative Endocrinology , 80 : 1 – 8 .
- Poortenaar , C. W. , Hooker , S. H. and Sharp , N. 2001 . Assessment of yellowtail kingfish (Seriola lalandi lalandi) reproductive physiology, as a basis for aquaculture development. . Aquaculture , 201 : 271 – 286 .
- Pottinger , T. G. , Carrick , T. R. , Appleby , A. and Yeomans , W. E. 2000 . High blood cortisol levels and low cortisol receptor affinity: is the chub, Leuciscus cephalus, a cortisol‐resistant teleost. . General and Comparative Endocrinology , 120 : 108 – 117 .
- Rinchard , J. and Kestemont , P. 1996 . Comparative study of reproductive biology in single‐ and multiple‐spawner cyprinid fish. I. Morphological and histological features. . Journal of Fish Biology , 49 : 883 – 894 .
- Rinchard , J. , Kestemont , P. and Heine , R. 1997 . Comparative study of reproductive biology in single and multiple‐spawner cyprinid fish. II. Sex steroid and plasma protein phosphorus concentrations. . Journal of Fish Biology , 50 : 169 – 180 .
- Roldan , M. I. , Perrotta , R. G. , Cortey , M. and Pla , C. 2000 . Molecular and morphologic approaches to discrimination of variability patterns in chub mackerel, Scomber japonicus. . Journal of Experimental Marine Biology and Ecology , 253 : 63 – 74 .
- Ronnback , P. , Bryceson , I. and Kautsky , N. 2002 . Coastal aquaculture development in eastern Africa and the Western Indian Ocean: Prospects and problems for food security and local economies. . Ambio , 31 : 537 – 542 .
- Scott , A. P. , Witthames , P. R. , Turner , R. J. and Canario , A. V. M. 1998 . Plasma concentrations of ovarian steroids in relation to oocyte final maturation and ovulation in female plaice sampled at sea. . Journal of Fish Biology , 52 : 128 – 145 .
- Sen , U. , Mukherjee , D. , Bhattacharyya , S. P. and Mukherjee , D. 2002 . Seasonal changes in plasma steroid levels in Indian major carp Labeo rohita: influence of homologous pituitary extract on steroid production and development of oocyte maturational competence. . General and Comparative Endocrinology , 128 : 123 – 134 .
- Shimizu , A. , Aida , K. and Hanyu , I. 1994 . Effects of photoperiod and temperature on gonadal activity and plasma steroid levels in an autumn spawning bitterling, Acheilognathus rhombea, during different phases of its annual reproductive cycle. . General and Comparative Endocrinology , 93 : 137 – 150 .
- Sisneros , J. A. , Forlano , P. M. , Knapp , R. and Bass , A. H. 2004 . Seasonal variation of steroid hormone levels in an intertidal‐nesting fish, the vocal plainfin midshipman. . General and Comparative Endocrinology , 136 : 101 – 116 .
- Stequert , B. , Rodriguez , J. N. , Cuisset , B. and Le Menn , F. 2001 . Gonadosomatic index and seasonal variations of plasma sex steroids in skipjack tuna (Katsuwonus pelamis) and yellow fin tuna (Thunnus albacares) from the western Indian ocean. . Aquatic Living Resources , 14 : 313 – 318 .
- Takano , K. , Takemura , A. , Furihata , M. , Nakanishi , T. and Hara , A. 1991 . Annual reproductive and spawning cycles of female Sebastiscus marmoratus. . Environmental Biology of Fishes , 30 : 39 – 48 .
- Tollefsen , K. E. , Meys , J. F. , Frydenlund , J. and Stenersen , J. 2002 . Environmental estrogens interact with and modulate the properties of plasma sex steroid‐binding proteins in juvenile Atlantic salmon (Salmo salar). . Marine Environmental Research , 54 : 697 – 701 .
- Tsigenopoulos , C. S. , Rab , P. , Naran , D. and Berrebi , P. 2002 . Multiple origins of polyploidy in the phylogeny of southern African barbs (Cyprinidae) as inferred from mtDNA markers. . Heredity , 88 : 466 – 473 .
- Tveinten , H. and Johnsen , H. K. 2001 . Thermal influences on temporal changes in plasma testosterone and oestradiol‐17β concentrations during gonadal recrudescence in female common wolffish. . Journal of Fish Biology , 59 : 175 – 178 .
- Ueda , H. , Hiroi , O. , Hara , A. , Yamauchi , K. and Nagahama , Y. 1984 . Changes in serum concentrations of steroid hormones, thyroxine, and vitellogenin during spawning migration of the chum salmon, Oncorhynchus keta. . General and Comparative Endocrinology , 53 : 203 – 211 .
- Unlu , E. and Balci , K. 1993 . Observation on the reproduction of Leuciscus cephalus orientalis (Cyprinidae) in Savur stream (Turkey). . Cybium , 17 : 241 – 250 .
- Wallace , R. A. and Selman , K. 1981 . Cellular and dynamic aspects of oocyte growth in teleosts. . American Zoologist , 21 : 325 – 343 .
- Wen , H. and Lin , H. 2001 . Effect of environmental factors on gonadal maturation as well as its ovulation and spawning in teleosts. . Ying Yong Sheng Tai Xue Bao , 12 : 151 – 155 .
- Yamamoto , K. 1956 . Studies on the formation of fish eggs I. Annual cycle in the development of ovarian eggs in the flounder, Liopsetta obscura. . Journal of the Faculty of Science Hokkaido University Series VI , 12 : 362 – 373 .