Abstract
Most ascidian larvae settle and begin adhesion by means of three mucus secreting and sensory organs, the adhesive papillae or palps. However, the adhesive papillae of Botrylloides genus larvae, despite their name, have only a sensory function. By immunohistochemical localization of serotonin and β‐tubulin, we demonstrated that the adhesive papillae of Botrylloides leachi contain two distinct types of neurons with different localization and possibly a different function. The central neurons emerge from the tunic at the apex of papillae and probably have a mechanosensory function. The lateral neurons contain serotonin and may play a role in the mechanism of metamorphosis triggering. Moreover, by histological analysis we found numerous secreting cells, clustered in the centre of the area among the three papillae, forming a glandular organ. This organ could perform the attachment of the larva to the substrate, which is traditionally considered to be operated by the anterior epidermis of the larva, with a sucker‐like mechanism.
Introduction
Ascidians (Chordata: Tunicata) are sessile marine animals that develop through a swimming larva, which constitutes the dispersal phase of their life cycle. After hatching, the larva searches the substrate by means of its adhesive papillae or palps, three mucus secreting organs projecting from the anterior epidermis. During a species‐specific period of time, the ascidian tadpole explores the substrate by a quick series of touches and then temporarily attaches by means of mucus secreted by the palps. The morphology of these organs is very variable among species and they can be classified in 10 types according to their histological characters (see Burighel & Cloney (Citation1997) for a review). A main distinction can be traced between simple conic papillae, typical of solitary ascidians, and eversible ones, frequently found in compound species and characterized by the capability of rapid evertion and exposure of the sticky mucus (Cloney Citation1978).
With few exceptions, all adhesive papillae are formed by elongated secreting cells and by sensory primary neurons. It has been proposed that sensory cells in the palps may be mechanosensory neurons and play an important role in the selection of a suitable substrate where to adhere (Groppelli et al. Citation2003). It has also been suggested that they are involved in the transmission of the signal that triggers metamorphosis, possibly by releasing signaling molecules (Degnan et al. Citation1997). One putative signaling molecule is the monoamine serotonin, which can trigger metamorphosis in the larvae of the solitary ascidian Phallusia mammillata (Zega et al. Citation2005). The neurotransmitter expression of the papillary neurons is known from only two species. Phallusia mammillata larvae have adhesive papillae with both cholinergic and serotonergic neurons (Groppelli et al. Citation2001; Pennati et al. Citation2001). However, Ciona intestinalis larvae have adhesive papillae with cholinergic neurons (Coniglio et al. Citation1998; Groppelli et al. Citation2001).
The adhesive papillae of larvae belonging to the Botrylloides genus represent an exception to the general rule that these organs have both sensory and secreting function. Grave (Citation1934) described the adhesive papillae as containing sensory neurons, lacking secretory cells and proposed that the adhesion in Botrylloides was performed with a suction‐like mechanism, operated by the region among the palps, which acted as a sucker. However, Cloney and Torrence (Citation1984) reported that this region had a sticky activity when probed with a needle.
Thus, the mechanism of adhesion in larvae of the Botrylloydes genus has not been clarified yet. The aim of the present work is to describe the morphology and the function of the adhesive papillae of Botrylloides leachi (Savigny, 1816) to address the question of adhesion and to contribute to define the neural phenotype of the adhesive papillae primary neurons in this species.
Materials and methods
Animals
Adults of Botrylloides leachi were collected in the lagoon of Venice, Italy, in July 2005. They were maintained at 18°C in small aquaria with Millipore filtered natural sea water (0.45 µm mesh size) and continuously monitored to gather spontaneously spawned larvae. The larvae were fixed in 4% paraformaldheyde dissolved in 0.1 M phosphate buffer saline (PBS) pH 7.4, for 1.5 h with gentle shaking at room temperature.
Histology
Fixed larvae were dehydrated in an alcohol series and embedded in Technovit 7100 plastic (Heraeus Kulzer GmbH, Werheim, Germany) for sectioning. Four micron thick sections were stained with 0.5% methylene blue in water for a few minutes and mounted in Entellan (Merck, Italy).
Immunofluorescence
The fixed larvae were washed several times in PBS and then processed for double immunolocalization of serotonin and β‐tubulin. First, cellulose of the tunic was partially digested with 5 mg of cellulase (Sigma, Italy) in 10 ml of PBS pH 5.5 for 10 min at 37°C in order to facilitate the antibody penetration. The larvae were cooled on ice and washed three times with PBS. The larvae were treated for 20 min with 0.25% Triton‐X and 0.1% Tween‐20 dissolved in PBS pH 7.4, to increase the permeability. The larvae were preincubated for 2 h at room temperature in heat‐inactivated Normal Goat Serum (NGS). Larvae were incubated in a humid chamber for 36 h at 4°C with anti‐serotonin antibody (Medac, Italy), made in rabbit, diluted 1:200 in 50% PBS/50% NGS. Larvae were washed three times in PBS at room temperature and then incubated overnight at 4°C with monoclonal anti‐β‐tubulin (clone 2‐28‐33, Sigma, Italy) diluted 1:200 in 50% PBS/50% NGS. Then they were exposed to a mix of the two secondary antibodies, goat anti‐rabbit FITC and goat anti‐mouse TRITC (Calbiochem, Italy), both diluted 1:250 in PBS, overnight at 4°C in darkness in a humid chamber. Specimens were mounted in 1,4‐diazabicyclo[2,2,2]octane (DABCO, Sigma, Italy) plus MOWIOL (Calbiochem, Italy) on microscope slides and were examined using a confocal laser scanning microscope Leica TCS‐NT (Leica Microsystems, Heidelberg, Germany), equipped with laser argon/krypton, 75 mW multiline. Control experiments were performed pre‐adsorbing anti serotonin antibody with 10 µM serotonin (Sigma, Italy); in these cases no positive signal was detected.
Results
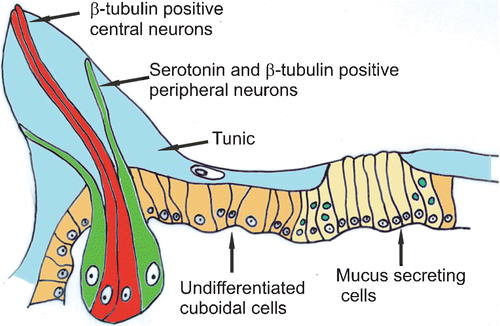
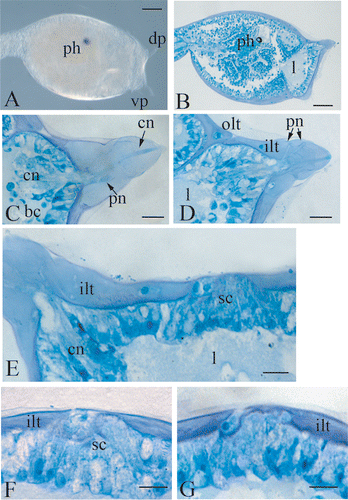
The larva of Botrylloides leachi bears three long papillae, two dorsal and one ventral, arranged according the apices of an equilateral triangle. Each papilla is 90 µm long and 90 µm wide at the base (Figure ), and is completely covered by two layers of tunic, an inner one and an outer one. The anterior part of the trunk region consists of a monolayer epithelium, which lays on a basal lamina. Under the basal lamina, a wide lacuna is present, filled with liquid in which blood cells can occasionally be localized (Figure ). Each papilla is formed by club‐shaped cells, with the nuclei localized in the lower third. Among these cells, two different cellular types are recognizable. The cells of the first type are disposed in the centre of the papilla and present a long distal process, which passes through the two layers of the tunic and emerges with small protrusions at the apex of the papilla. These processes form a rod like structure along the axis of the papilla (Figure ).
Figure 1 A, trunk region of a Botrylloides leachi larva, showing the disposition of the left dorsal and the ventral papilla. The right dorsal one is not focused. B, longitudinal histological section of the trunk region showing the wide hemocoelic region under the anterior ectoderm. C,D, two sections at different level of a dorsal right papilla. E, histological section of the anterior ectoderm, showing the cluster of secreting cells protruding from the tunic. F,G, histological sections at different level of the cluster of secreting cells. bc, blood cells, cn, central neurons; dp, dorsal papilla; ilt, inner layer of the tunic; l, lacuna; ph, photolith; olt ,outer layer of the tunic; pn, peripheral neurons; sc, secreting cells, vp, ventral papilla. Scale bar: A,B 100 µm; C–G 20 µm.
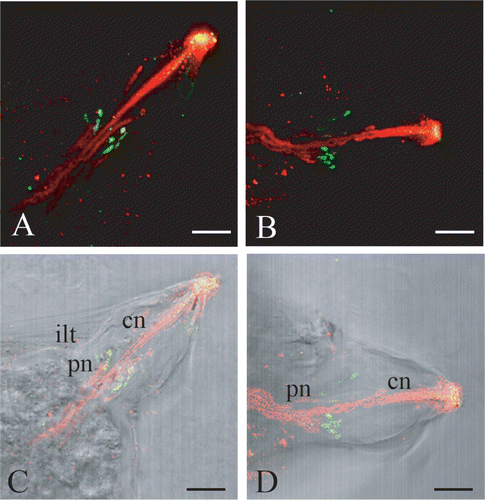
In larvae of Botrylloides leachi, palp neurons were evidenced with monoclonal anti‐β‐tubulin antibody, which efficiently labels neural structures in ascidian larvae (Pennati et al. Citation2001). The immunostaining with this antibody revealed a strong signal in the centre of each papilla, in correspondence with the cytoplasmatic projections of both cell types that form the papillae. In fact, some labelled fibres extend to the apex of the papilla, while others stop at one‐third of its length (Figure ). No other cells in the anterior epidermis showed positive signal for β‐tubulin. Immunolocalization of serotonin showed the presence of this monoamine in small spots inside the inner layer of the tunic, as shown by the superimposition of confocal laser images with those obtained by transmission microscopy (Figure ). These positive spots are present along some of the β‐tubulin positive fibres and are clustered at one‐third of the length of the papilla (Figure ).
Figure 2 Confocal laser images of dorsal right(A) and left (B) papillae showing the localization of serotonin (FITC signal, in green) and of β‐tubulin (TRITC signal, in red). The yellow colour at the apex is an artifact due to unspecific adhesion of both secondary antibodies in the fenestration of the tunic, where distal processes of central neurons reach the surface. C,D, superimposition of A and B to light microscopy imagines. cn, central neurons; ilt, inner layer of the tunic; pn, peripheral neurons. Scale bar: 20 µm.
The reconstruction of the morphology of the papillae and of the central cluster of secreting cells as it emerges from our analysis is schematized in the drawing of Figure . In the papillae two different neurons are shown: the central neurons labelled only with β‐tubulin and possessing elongated sensory‐like projections which reach the apex of the papilla; the peripheral neurons labelled with β‐tubulin and containing serotonin in their distal endings. These terminal endings do not reach the apex of the papilla but stop at one‐third of its length where they form a ring.
Discussion
The morphological analysis revealed that the papillae of Botrylloides leachi contain at least two types of neuronal cells labelled with β‐tubulin: the central neurons which emerge from the apices of the papillae and do not contain serotonin, and the peripheral neurons, containing serotonin. From our results it is not clear if the endings of peripheral neurons emerge completely from the papillae or if they pass through only the inner layer of the tunic. No secreting cells are present in the papillae. Thus it is confirmed that the papillae of this species have only a sensory function and, in agreement with Grave (Citation1934), they could correctly be named sensory papillae to distinguish them from the glandular or adhesive papillae of other species.
The secreting function is retained by another specialized region of the anterior ectoderm, comprised among the three papillae, where secreting cells are clustered. The in vivo observations made by Cloney and Torrence (Citation1984) on the sticky properties of the anterior ectoderm are now confirmed by histological evidence. As a consequence, the sucker‐like mechanism proposed by Grave (Citation1934) to explain the attachment of this larva should be reconsidered. The anatomical conformation of the anterior region, which forms a cup‐like cavity, could possibly contribute to larval attachment to the substrate by creating a vacuum, but the definitive adhesion is operated by the mucus secreted by the central region.
The presence of two types of nervous cells in the papillae could be explained taking into account that, as well as the secretory function, the papillae of ascidian larvae also perform a mechanosensory function participating in the mechanism that triggers metamorphosis (Burighel & Cloney Citation1997). We propose that the two types of neurons identified in Botrylloides leachi could be specialized for different tasks. In particular, the central neurons, whose terminal endings protrude from the apex of the papilla, are the first ones to come into contact with the substrate during the exploratory period of the larva. Thus, it is reasonable to hypothesize that they may have a mechanosensory or chemosensory function. Instead, the terminal endings of the peripheral neurons stop at one‐third the length of the papilla, and thus they do not make contact with the substrate during the series of quick touches by which the larva tests the substrate. It is only after the larva has firmly attached to a suitable substrate by means of glandular organ that the papillae are pushed against the substrate itself and the peripheral neurons could be stimulated. Consequently, it is possible to hypothesize that, when the adhesion becomes permanent, these neurons are stimulated and may participate to the signalling cascade that triggers metamorphosis, possibly by releasing a signalling molecule.
The peripheral neurons contain serotonin, which is known to be involved in inducing metamorphosis in the larvae of many animal phyla, from hydrozoans to molluscs and crustaceans (Couper & Leise Citation1996; Walther et al. Citation1996; Yamamoto et al. Citation1999). Serotonin is present in small spots at the distal end of peripheral neurons, maybe in storage vesicles. The presence of serotonin has been reported in the papillary neurons of the ascidian Phallusia mammillata (Pennati et al. Citation2001), but data regarding the presence of serotonin in other ascidian species are scarce. Stach (Citation2005) described serotonin immunolocalization in the CNS of the larvae of Herdmania momus and Ascidia interrupta, but he did not mention the presence of this neurotransmitter in PNS. In the ascidian Phallusia mammillata a serotonin receptor agonist, DOI hydrochloride, was able to induce the beginning of metamorphosis (Zega et al. Citation2005). In the planula larva of the hydroid Phialidium gregarium, serotonin was localized with a punctuated distribution in ectodermal cells, supposed to be sensory cells. In this species it was proposed that 5‐HT is released after contact with an appropriate external cue and it determines cell depolarization thus inducing metamorphosis (McCauley Citation1997).
Thus in larvae of Botrylloides leachi serotonin released by peripheral neurons may act as an internal signal that stimulates the metamorphosis process, after the permanent adhesion has been attained. This hypothesis need to be experimentally validated, may be exposing larvae to serotonin, even if the short exploratory period of Botrylloides leachi will make difficult the experimental procedure.
Acknowledgments
The confocal microscopy images were obtained using the facilities of C.I.M.A. (Advanced Microscopy Center of the University of Milano). We thank Dr. Umberto Fascio for the confocal microscopy images. We thank Prof. Brunetti for the identification of the species. The work was supported by grants from the University of Milano (FIRST 2005).
References
- Burighel , P. and Cloney , R. A. 1997 . “ Urochordata: Ascidiacea. ” . In Microscopic anatomy of invertebrates. Vol. 15. Hemichordata, Chaetognatha, and the invertebrate chordates. , Edited by: Harrison , F. W and Ruppert , E. E . 221 – 347 . New York : Wiley‐Liss Inc .
- Cloney , R. A. 1978 . “ Ascidian metamorphosis: Review and analysis. ” . In Ascidian metamorphosis: Review and analysis , Edited by: Chia , F. S and Rice , M . 255 – 282 . Amsterdam : Elsevier‐North Holland Biomedical Press .
- Cloney , R. A. and Torrence , S. A. 1984 . “ Ascidian larvae: Structure and settlement. ” . In Marine biodeterioration: Interdisciplinary study , Edited by: Costolow , J. D and Tipper , R. C . 141 – 148 . Annapolis, MD : Naval Institute press .
- Coniglio , L. , Morale , A. , Angelini , C. and Falugi , C. 1998 . Cholinergic activation of settlement in Ciona intestinalis metamorphosing larvae. . Journal of Experimental Zoology , 280 : 314 – 320 .
- Couper , J. M. and Leise , E. M. 1996 . Serotonin injections induce metamorphosis in larvae of the gastropod Ilyassana obsoleta. . Biological Bulletin , 191 : 178 – 186 .
- Degnan , B. M. , Souter , D. , Degnan , S. M. and Long , S. C. 1997 . Induction of metamorphosis with potassium ions requires development of competence and an anterior signalling centre in the ascidian Herdmania momus. . Development, Genes & Evolution , 206 : 370 – 376 .
- Grave , C. 1934 . The Botryllus type of ascidian larva. . Carnegie Institute Washington Publication , 435 : 143 – 156 .
- Groppelli , S. , Pennati , R. , Sotgia , C. and De Bernardi , F. 2001 . AChE localization in the adhesive papillae of the ascidian larvae: Effects of citral, a retinoic acid synthesis inhibitor. . Invertebrate Reproduction & Development , 40 : 95 – 102 .
- Groppelli , S. , Pennati , R. , Scarì , G. , Sotgia , C. and De Bernardi , F. 2003 . Observations on the settlement of Phallusia mammillata larvae: Effects of different lithological substrata. . Italian Journal of Zoology , 70 : 321 – 326 .
- McCauley , D. W. 1997 . Serotonin plays an early role in the metamorphosis of the hydrozoan Phialidium gregarium. . Developmental Biology , 190 : 229 – 240 .
- Pennati , R. , Groppelli , S. , Sotgia , C. , Candiani , S. , Pestarino , M. and De Bernardi , F. 2001 . Serotonin localization in Phallusia mammillata larvae and effect of 5‐HT antagonists during larval development. . Development, Growth & Differentiation , 43 : 647 – 656 .
- Stach , T. 2005 . Comparison of the serotonergic nervous system among Tunicata: Implication of its evolution within Chordata. . Organism, Diversity & Evolution , 5 : 15 – 24 .
- Walther , M. , Ulrich , R. , Kroiher , M. and Berking , S. 1996 . Metamorphosis and pattern formation in Hydractinia echinata, a colonial hidroid. . International Journal of Developmental Biology , 40 : 313 – 322 .
- Yamamoto , H. , Shimizu , K. , Tachibana , A. and Fusetani , N. 1999 . Roles of dopamine and serotonin in larval attachment of the barnacle, Balanus amphitrite. . Journal of Experimental Zoology , 284 : 746 – 758 .
- Zega , G. , Pennati , R. , Groppelli , S. , Sotgia , C. and De Bernardi , F. 2005 . Dopamine and serotonin modulate the onset of metamorphosis in the ascidian Phallusia mammillata. . Developmental Biology , 282 : 246 – 256 .