Abstract
The regulation of the settlement process in barnacles has attracted research interest due to their role as fouling organisms. The involvement of neurotransmitters in the regulation of settlement of marine invertebrate larvae has been described in several species. In this work, we reported the effects of the neurotransmitters dopamine and serotonin on the settlement of cyprids of the barnacle Balanus improvisus and described differences among cyprids of different ages. Also, we tested the effects of dopaminergic and serotonergic agonists and antagonists on settlement of cyprid larvae. We found that dopamine significantly stimulated settlement of 2‐ and 4‐day‐old cyprids, while serotonin exerted an inhibitory effect, regardless of cyprid age. The agonists and antagonists to the two neurotransmitters were not able to stimulate settlement but in general had either an inhibitory or no effect. We compared our results to those previously reported of the roles of dopamine and serotonin in the settlement of Balanus amphitrite. There appeared to be striking differences in the effects of these neurotransmitters between the two species because it has been reported that serotonin induces settlement in B. amphitrite and that dopamine inhibits it. This suggested that dopamine and serotonin play pivotal roles in settlement of barnacle but may act in different ways in the two species.
Introduction
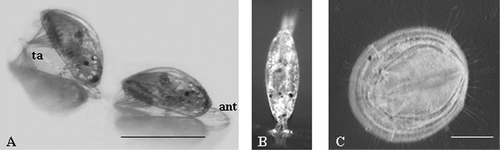
The barnacle Balanus improvisus (Darwin, 1854) is one of the major fouling organisms in Swedish waters and from a fouling control perspective the settlement of this species has attracted recent research interest (Berntsson et al. Citation2000b; Dahlstrom et al. Citation2004b; Sjogren et al. Citation2004a, Citationb). B. improvisus has six naupliar stages before moulting into the non‐feeding cyprid larva (Figure ). The cyprid larva is the final stage in barnacle larval development and it is responsible for site selection and settlement.
Figure 1 Settlement process of cyprid larvae ofBalanus improvisus. A, Cyprid larvae exploring the substrate by flicking their antennulae; scale bar 500 µm (ant: antennulae; ta: thoracic appendages). B, Cyprid larva in permanent attachment, after cement secretion. C, Juvenile after ecdysis; scale bar 300 µm.
Cyprid transportation to available substrates for settlement is facilitated by near‐shore currents and wave actions (Jonsson et al. Citation2004). At the substratum, the cyprid is able to sense and respond to a variety of environmental cues, e.g. bacterial biofilms (Maki et al. Citation1988, Citation1990; O'Connor & Richardson Citation1998; Olivier et al. Citation2000), surface roughness (Berntsson et al. Citation2000a, Citationb) and surface wettablity (Roberts et al. Citation1991; Dahlström et al. Citation2004a). In a number of marine invertebrates, the sensing of specific external chemical inducers associated with the substrate, food sources or conspecifics are particularly important and initiate the settlement process (reviewed by Hadfield Citation2000; Clare & Matsumura Citation2000). For barnacles it has been shown that adult pheromonal cues guide cyprids to sites for settlement (Matsumura et al. Citation1998a,Citationb). The recognition of conspecifics at a settlement site may serve the purpose of ensuring future reproductive success.
Cyprids have a small but well‐organized nervous system composed of some 2000 neurons (Harrison & Sandeman Citation1999) and bear several sensory structures on, for example their antennulae (Clare & Nott Citation1994; Hoeg et al. Citation1998; Figure ). The nature of the receptors with which external cues and pheromones interact is to date not known. However, pharmacological assays have provided evidence that, after pheromone recognition, the signal transduction pathway in cyprids involves cAMP (Rittschof et al. Citation1986; Clare et al. Citation1995). Also, studies suggest that protein kinase C (PKC) participates in the signal transduction pathway leading to metamorphosis since cyprids treated with PKC activators metamorphosed without prior attachment (Yamamoto et al. Citation1995).
It has been shown in larvae of several marine invertebrates that catecholamines, i.e. dopamine, noradrenaline and adrenaline, as well as the indoleamine serotonin can regulate and/or trigger settlement and metamorphosis: hydroids (Edwards et al. Citation1987; Kolberg & Martin Citation1988; McCauley Citation1997), molluscs (Fitt et al. Citation1990; Couper & Leise Citation1996; Pires et al. Citation1997, Citation2000) and ascidians (Kimura et al. Citation2003; Zega et al. Citation2005). The role of cathecolamines and serotonin (5‐HT), in the settlement of the barnacle B. amphitrite has been studied to some extent. Kon‐ya and Endo (Citation1995) demonstrated that L‐DOPA and dopamine had inducing effect on settlement. Kon‐ya et al. (Citation1995) verified that L‐tryptophan and 5‐HT induced settlement in young cyprids. Yamamoto et al. (Citation1996, Citation1999) suggested that serotonin enhanced larval attachment while dopamine inhibited it. Moreover, the presence of both neurotransmitters in cyprids was demonstrated by HPLC analysis.
In this work, cyprid larvae of different ages were treated with dopamine and serotonin in order to study the possible effects of these biogenic amines on settlement as larvae gained competence. Larval competence is the realization of a peculiar physiological state that enables larvae of marine invertebrates to respond to metamorphic stimuli (Hadfield Citation2000). Larval competence is gained with larval age and in B. amphitrite it has been hypothesized that larval competence is attained at day 3 of cyprid life (Rittschof et al. Citation1984; Satuito et al. Citation1996; Miron et al. Citation2000; Smith et al. Citation2000). In the present study, pharmacological agents, i.e. agonists and antagonists directed to dopaminergic and serotonergic receptors as well as transporter proteins involved in serotonergic re‐uptake, were also examined for their effect on B. improvisus settlement.
Materials and methods
Pharmacological agents
Dopamine hydrochloride, (±)‐SKF‐38393 hydrochloride, S‐(−)‐Lisuride, Quinpirole (LY‐141,865), R (+)‐SCH‐23390, Clozapine, 5‐HT (5‐hydroxytryptamine, serotonin), (±)‐8‐Hydroxy‐2‐(di‐n‐propyl‐amino) tetralin hydrobromide (8‐OH‐DPAT HBr), R‐(−)‐DOI Hydrochloride, WAY–100635 maleate, Fluoxetine (LY‐110,140) hydrochloride, Ritanserin were purchased from SIGMA, Italy. Their chemical structures and respective molecular targets in vertebrates are summarized in Table .
Table I. The pharmacological agents tested in the present study, their reported endogenous targets in vertebrates and their respective chemical structures.
Figure 2 Effects of dopamine and serotonin on the settlement rate ofBalanus improvisus cyprids. The experiment used different concentrations of the two agents in combination with different larval age (d0, d2 and d4). Results are shown as mean settlement (%) as observed two days after the beginning of the experiment±SE. *Significantly different from controls.
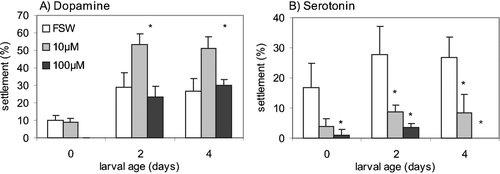
Figure 3 Effects of different concentrations of dopamine receptor agonists and antagonists on the settlement rate ofB. improvisus newly hatched (day 0) cyprids. The bars represent mean settlement (%) observed 6 days after the beginning of the experiment±SD. Concentrations in which settlement is lower than 1% are not presented in the figure but listed in Table . *P⩽0.05; **P⩽0.01; ***P⩽0.001; ****P⩽0.0001.
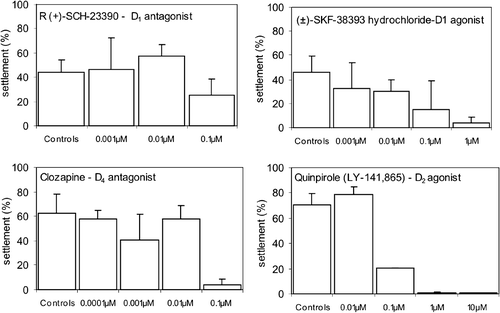
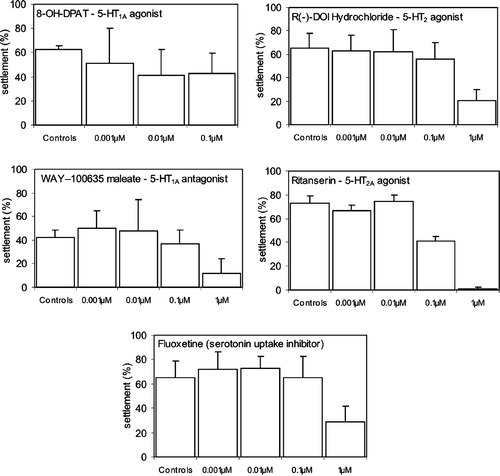
Figure 4 Effects of different concentrations of serotonin receptor agonists, antagonists and of the serotonin uptake inhibitor on the settlement ofB. improvisus newly hatched (day 0) cyprids. The bars represent mean settlement (%) observed 6 days after the beginning of the experiment±SD. Concentrations in which settlement was lower than 1% are not presented in the figure but listed in Table . *P⩽0.01; **P⩽0.001; ***P⩽0.0001.
Larval rearing and settlement assays
The rearing facility at Tjärnö Marine Biological Laboratory keeps adult barnacles of Balanus improvisus in buckets with running seawater (21±2°C). The barnacles are fed daily with nauplii of Artemia sp. and Isochrysis galbana. Under these conditions adults spawn continuously throughout the year and release nauplii larvae. Naupli larvae are then collected and reared as described by Berntsson et al. (Citation2000b). After 6–7 days newly hatched cyprids (in this work named day 0, d0) are collected for use in in vivo bioassays. In the present study, settlement assays were performed in polystyrene Petri dishes (Nunc, Ø 48mm), to which the drugs of interest were added and serially diluted to yield a final volume in the dish of 10 mL of filtered seawater (FSW, salinity 32.1±1‰). To each dish 20±5 cyprids were added. Experiments were maintained in room temperature (21±1°C) for the assay duration, with 12 h light cycle.
In assays with the neurotransmitters dopamine and serotonin day 0, day 2 and day 4 cyprids old (d0, d2, d4) were employed and settlement rate was assessed 2 days after treatment has started; in experiments with the pharmacological agents, i.e. the dopamine and serotonin receptor agonists and antagonists, cyprids were used on the first day after moulting, named day 0 (d0), and settlement rate was assessed 6 days after treatment has started. Each substance was tested at least twice with the same results. All drugs were dissolved in milliQ (mQ) water or dimethyl sulfoxide (DMSO) to obtain 10 mM stock solutions. DMSO alone, diluted to the appropriate concentration, had no effect on settlement propensity. Stock solutions were serially diluted with FSW to give the desired concentration series. In each experiment, larvae from the same batch were used and each treatment was replicated four or five times. When concluding the experiments after an exposure time of 2 and 6 days, cyprid settlement propensity was assessed under a stereo microscope.
Statistical analysis
Significant effects on larval settlement were tested using analysis of variance (ANOVA), following which Tukey's Post Hoc test (significant at P⩽0.05) was used to identify specific effects. In each test settlement rate of treated cyprids was compared to that of the controls containing FSW or FSW with DMSO. Data were also checked for heterogeneity of variances using Cochran's test. In the case where dopamine and serotonin were tested with different ages of larvae, a 2‐factor ANOVA was employed with treatment (controls and concentrations) and age (day 0, 2 and 4) as fixed factors. To check for specific effects of the different factors (treatment and age), a Means Comparison Contrast (MCC) test was performed.
Results
Effects of dopamine and dopamine agonists and antagonists
The mean settlement (%) of cyprids of different ages treated with two different concentrations, i.e. 10 or 100 µM, of dopamine was observed on the second day of the experiment (Figure ). For day 0 cyprids (d0 cyprids) the treatment with 10 µM dopamine resulted in a mean settlement similar to that in control dishes (2‐factor ANOVA, MCC d0 cyprids: F 1,36 = 0.01, P = 0.92; Figure ). In the dishes where d0 larvae were exposed to100 µM of dopamine there was no settlement. However, this result was not significantly different from the mean settlement in the control dishes, which was very low: 9.8%±3.2 SE, (2‐factor ANOVA, MCC d0 cyprids exposed to 100 µM dopamine: F 1,36 = 1.75, p = 0.19) (Figure ). Settlement rate of either day 2 (d2) or day 4 (d4) cyprids treated with 10 µM dopamine was significantly higher than controls (2‐factor ANOVA, MCC d2 cyprids: F 1,36 = 10.6, P = 0.003; d4 cyprids: F 1,36 = 10.5, P = 0.003; Figure ). Dopamine added in 100 µM had no effect on the settlement of neither d2 cyprids (2‐factor ANOVA, MCC F 1,36 = 0.58, P = 0.46) nor d4 cyprids (2‐factor ANOVA, MCC F 1,36 = 0.2, P = 0.65; Figure ).
All dopaminergic agonists and antagonists except one, the D1 receptor antagonist R(+)‐SCH‐23390, exerted a significant inhibitory effect on the settlement of d0 cyprids (Table , Figure ). The D1 receptor agonist, (±)‐SKF‐38393 hydrochloride, had a significant inhibitory effect ranging from 0.1 µM to 10 µM (Table , Figure ). Both S‐(−)‐lisuride, a D2 receptor agonist, and quinpirole, a selective D2‐like receptor agonist, strongly inhibited settlement: the former from 0.01 µM to 10 µM, the latter from 0.1 µM to 10 µM. The D1 receptor antagonist, R (+)‐SCH‐23390, had no effect on settlement rate in any of the concentrations tested, while clozapine, a D4 receptor antagonist, strongly inhibited settlement in concentrations ranging from 0.01 µM to 1µM (Table , Figure ).
Table II. The effects of different dopamine receptor agonists and antagonists on the settlement propensity of cyprid larvae of B. improvisus.
Effects of serotonin and serotonin agonists and antagonists
The mean settlement (%) of cyprids of different ages treated with two concentrations, i.e. 10 or 100 µM, of the neurotransmitter serotonin was observed on the second day of the experiment (Figure ). Treatments of d0, d2, d4 cyprids with 10 µM or 100 µM of serotonin caused a significant inhibitory effect. When treating d0 cyprids with 10 µM of serotonin, there was non significant inhibitory effect on settlement (2‐factor ANOVA, MCC F 1,36 = 3.21, P = 0.0858; Figure ). However, serotonin significantly inhibited the settlement of d2 and d4 larvae when used in 10 µM (2‐factor ANOVA, MCC d2 cyprids: F 1,36 = 6.6, P = 0.015; d4 cyprids: F 1,36 = 6.1, P = 0.019; Figure ). When treating cyprids of age 0, 2 and 4 days with 100 µM of serotonin, the settlement of all ages were significantly inhibited (2‐factor ANOVA, MCC d0 cyprids: F 1,36 = 6.2, P = 0.018; d2 cyprids: F 1,36 = 10.8, P = 0.002; d4 cyprids: F 1,36 = 12.9, P = 0.001; Figure ). Typically, cyprids treated with the highest concentration of serotonin ceased swimming but were still able to move the thoracic appendages. When cyprids were exposed to 10 µM serotonin they displayed normal swimming behaviour.
All serotonergic agonists and antagonists exerted a significant inhibitory effect on the settlement of d0 cyprids (Table , Figure ). In addition, all serotonergic agonists or antagonists tested had lethal effects in concentrations ranging from 1 to 10µM, varying on the substance tested. Treatments with 0.1 µM of the 5‐HT1A agonist, 8‐OH‐DPAT HBr, significantly inhibited settlement while the same concentration of the 5‐HT1A antagonist, WAY–100635 maleate, had no effect on the settlement in concentrations ranging from 0.001 to 1 µM. However, at the concentration of 10µM WAY–100635 significantly inhibited settlement (Table , Figure ). The 5‐HT2 agonist, R‐(−)‐DOI HCl caused a significant settlement inhibition in 1 µM and 10µM (Table , Figure ). Also, the 5‐HT2 antagonist ritanserin, inhibited settlement in the concentrations of 0.1 to 1µM (Table , Figure ). When cyprids were treated the serotonin reuptake inhibitor fluoxetine in 1 µM, settlement was significantly inhibited. Cyprids treated with concentrations below 1 µM settled to the same extent as cyprids in the control dishes (Table , Figure ).
Table III. The effects of different serotonin receptor agonists and antagonists and the effect of one serotonin reuptake inhibitor on the settlement propensity of cyprids of B. improvisus.
Discussion
Dopamine involvement in cyprid attachment and metamorphosis
The biogenic amines dopamine and serotonin are good candidates for participating in the metamorphic signal transduction pathway in barnacle cyprid larvae. In this work we demonstrated that dopamine (10 µM) exerted a significantly stimulatory effect on attachment and metamorphosis of older larvae (2 and 4 days old) of B. improvisus (Figure ). However, newly hatched larvae (day 0) treated with 10 µM of dopamine were not stimulated to settle but instead displayed similar settlement propensity as did control larvae (Figure ). The higher concentration tested, 100 µM, had no effect on settlement of cyprids of any age (0, 2 or 4 days) as compared to the settlement of larvae in control dishes. Thus, in the present study we found a significant stimulatory concentration of dopamine (10 µM) on the settlement propensity of cyprid larvae of B. improvisus but this stimulatory effect was only exerted on older larvae. The different effects of dopamine in relation to larval age found in the present study could be a result of the distinct responsiveness associated with larval age, i.e. the gaining of metamorphic competence. In such a context, treating newly hatched cyprids with dopamine would have no effect since cyprids have not developed the necessary features to be able to respond properly to an agent involved in the metamorphic stimulatory pathway (Hadfield Citation2000).
In the work by Kon‐ya and Endo (Citation1995) it was shown that newly hatched larvae (day 0) of the closely related barnacle species B. amphitrite, which were pulse‐treated with L‐DOPA or dopamine in concentrations comparable to those used in the present work, were induced to metamorphose. However, larvae metamorphosed but failed to settle. The authors concluded that dopamine triggered metamorphosis also in pre‐competent cyprids.
Dopamine is known to stimulate cement release in the barnacle Megabalanus rosa, when directly applied to isolated cement glands (Okano et al. Citation1996). This mechanism may be conserved among barnacles. Recent data on the role of dopamine in B. improvisus, particularly in the searching behaviour, i.e. antennular movements, and on stimulation of cement glands, suggest that dopamine increases the frequency of antennular flicking and also, stimulates cement release (Odling et al., personal communication).
In contrast to the results presented by Kon‐Ya et al. (Citation1995), Yamamoto et al. (Citation1996) showed that dopamine inhibited settlement in B. amphitrite cyprids in concentrations ranging from 0.1 µM to 100 µM. These authors also reported that combined treatments with 100 µM dopamine and 0.1 µM serotonin inhibited settlement, both with day 0 and day 5 cyprids (Yamamoto et al. Citation1999).
Effects of dopamine agonists and antagonists on the settlement of B. improvisus cyprids
The dopamine D1 agonist, (±)‐SKF‐38393, inhibited settlement in B. amphitrite in concentrations from 10 µM to 100µM; the dopamine D2 agonist S‐(−)‐Lisuride inhibited settlement of the same species in concentrations ranging from 0.001 µM to 100 µM (Yamamoto et al. Citation1999). It was also found that S‐(−)‐Lisuride induced cyprid searching behaviour in concentrations from 0.1 µM to 1 µM; however, this stimulated behaviour did not result in attachment and metamorphosis. In the settlement assays presented in this study using B. improvisus cyprids, concentrations of 100 µM of (±)‐SKF‐38393 or S‐(−)‐Lisuride)were lethal to the larvae. When used in lower concentrations both substances inhibited settlement (Tables and ). We observed no stimulation of cyprid searching behaviour when using S‐(−)‐Lisuride in lower concentrations, i.e. from 0.01 to 1 µM (Table ). The remaining dopamine agonists tested herein significantly inhibited cyprid attachment and metamorphosis (Table , Figure ). The two dopamine antagonists tested in the present study, i.e. the selective D1 antagonist R(+)‐SCH‐23390 and the D4 antagonist Clozapine, were both lethal to larvae at 1 µM and 10 µM, respectively. Below the concentration of 1 µM, R(+)‐SCH‐23390 did not affect cyprid settlement, while Clozapine significantly inhibited settlement at 0.1 µM without any significant lethal effects. However, Clozapine is also an antagonist at 5‐HT receptors, e.g. 5‐HT2A and 5‐HT2C, and thus, the settlement inhibition displayed by this compound on cyprids of B. improvisus may be ascribed to it interacting with other systems important to attachment and metamorphosis (Figure ).
Table IV. A comparison between the effects of dopamine and serotonin on B. amphitrite settlement vs B. improvisus settlement. Also, the table includes those pharmacological agents hitherto tested on both species.
In conclusion, the dopamine system appears crucial for the attachment and metamorphosis of cyprids of B. improvisus and may be a prominent part of the metamorphic signal transduction pathway. Based on our results we suggest that dopamine is stimulatory for settlement of competent cyprids, i.e. cyprids of age 2 and 4 days. This stimulatory action may be linked to cement release, and the development of the cement glands as larvae gain competence. However, further research is needed to establish the mechanism behind dopamine's stimulatory action and studies are on their way to trace the action of dopamine both on a behavioural as well as on a neurochemical scale.
Serotonin involvement in attachment and metamorphosis
The results obtained in this study in regard to the effect of serotonin on larval settlement of B. improvisus are intriguing. We found in repeated experiments that serotonin strongly inhibited settlement of B. improvisus cyprids: the results being highly significant for older (day 2 and 4) cyprids and a non‐significant trend towards the same effect on newly hatched cyprids (day 0) (Figure ). This is the opposite to what have been found for cyprids of B. amphitrite, where serotonin stimulated settlement of newly hatched cyprids (Kon‐Ya et al. Citation1995; Table ). When re‐examining the effect of serotonin on B. amphitrite cyprids, this time using competent cyprids (>3 days old), Yamamoto et al. (Citation1996) showed that cyprids were stimulated to settle upon treatment with 100 µM of serotonin. Later, Yamamoto et al. (Citation1999) showed that 5‐HT agonists, i.e. agonists interacting with 5‐HT3, 5‐HT2 and a non‐specific 5‐HT agonist, significantly enhanced settlement, while 5‐HT antagonists, particularly 5‐HT2 antagonists, inhibited it.
Effects of serotonin agonists and antagonists on the settlement of B. improvisus cyprids
In our experiments, the 5‐HT2 agonist R‐(−)‐DOI significantly inhibited settlement at 1 µM (Figure ), while the 5‐HT1A agonist 8‐OH‐DPAT and the serotonin re‐uptake inhibitor fluoxetine had no effect on settlement, neither inhibitory nor stimulatory (Figure ). The concentrations tested of 8‐OH‐DPAT were quite low (0.001–1 µM) due to the significant lethal effect of this compound in 1 µM (Table ). The 5‐HT receptor antagonists tested were the 5‐HT1A antagonist WAY‐100635 and the 5‐HT2A antagonist Ritanserin. These compounds displayed significant settlement inhibition at 1 µM (Figure ). The 5‐HT2A antagonist Ritanserin and the serotonin re‐uptake inhibitor Fluoxetine were lethal to larvae in 10 µM (Table ), while the 5‐HT1A antagonist WAY–100635 and the 5‐HT2 agonist R‐(−)‐DOI were lethal to larvae at 100 µM (Table ).
The importance of serotonin and dopamine in attachment and metamorphosis
In B. amphitrite, titres of both serotonin and dopamine that stimulated cyprids to settle has been quantified using High Performance Liquid Chromatography (HPLC) with electrochemical detection (Yamamoto et al. Citation1999). They showed that cyprid content of serotonin was approximately two orders of magnitude higher than that of dopamine. Preliminary analysis of B. improvisus cyprid content of dopamine and serotonin suggest that, dopamine is much more abundant than serotonin (Mia Dahlström, personal communication). These results may explain the opposite effects that exogenously applied serotonin have on larval attachment and metamorphosis in B. improvisus and B. amphitrite.
Okazaki and Shizuri (Citation2000a, Citationb) isolated six genes in metamorphosing cyprid larvae of B. amphitrite, named bcs genes, which were expressed in a specific order during the settlement process. Serotonin seemed to induce earlier expression of some of these genes. Okazaki and Shizuri (Citation2001) showed that 5‐HT and adults extracts stimulated the expression of the gene encoding AADC (Aromatic L‐amino acid decarboxylase), an enzyme responsible for both serotonin and dopamine synthesis. They suggested that intracellular biosynthesis of serotonin, or dopamine, or both, might be important for the control of settlement and metamorphosis.
Results from the present work show that in B. improvisus serotonin had a general inhibitory effect regardless of cyprid age, while dopamine enhanced settlement but only in d2 and d4 cyprids. All the dopaminergic and the serotonergic drugs tested on d0 cyprids inhibited settlement or had no effect. It is possible that interfering with dopamine and serotonin signalling in young larvae may inhibit searching behaviour and/or settlement.
The results of the effects of serotonin and dopamine found in the present study, are very different from those described in B. amphitrite. For dopamine, the reports on its effect on settlement of B. amphitrite have been contradictory. Kon‐Ya et al. (Citation1995) suggested that dopamine stimulated metamorphosis without prior attachment, i.e. cyprids metamorphosed but failed to adhere to the surface, while Yamamoto et al. (Citation1996) found that dopamine inhibited settlement in concentrations ranging from 0.1 to 100 µM. For serotonin, the reports are unanimous: serotonin induces attachment and metamorphosis in both newly hatched and in older cyprids of B. amphitrite (Kon‐Ya et al. Citation1995; Yamamoto et al. Citation1996).
It is possible that these striking differences are a result of the time required by larvae of the two species to acquire competence. The time to gain metamorphic competence may differ and concomitantly, titres of neurotransmitters change in relation to different responsive states of the cyprid larva. However, taken together, our results suggest that dopamine and serotonin may act in opposite ways in the two species. Further studies on tissue and molecular level as well as detailed studies on how cyprid behaviour is affected will clarify the roles of these two important neurotransmitter systems in the attachment and metamorphosis in B. improvisus cyprids.
Acknowledgements
This work was supported by grants from the Italian MIUR (PRIN 2004), by the Swedish Foundation for Strategic Environmental Research (MISTRA) through the R&D programme Marine Paint (KMB and MD) and by The Swedish Royal Academy of Sciences (MD).
References
- Berntsson , K. M. , Andreasson , H. , Jonsson , P. R. , Larsson , L. , Ring , K. , Petronis , S. and Gatenholm , P. 2000a . Reduction of barnacle recruitment on micro‐textured surfaces: Analysis of effective topographic characteristics and evaluation of skin friction. . Biofouling , 16 : 245 – 261 .
- Berntsson , K. M. , Jonsson , P. R. , Lejhall , M. and Gatenholm , P. 2000b . Analysis of behavioural rejection of micro‐textured surfaces and implications for recruitment by the barnacle Balanus improvisus. . Journal of Experimental Marine Biology and Ecology , 251 : 59 – 83 .
- Clare , A. S. and Matsumura , K. 2000 . Nature and perception of barnacle settlement pheromones. . Biofouling , 15 : 57 – 71 .
- Clare , A. S. and Nott , J. A. 1994 . Scanning electron microscopy of the fourth antennular segment of Balanus amphitrite amphitrite. . Journal of the Marine Biological Association of the UK , 74 : 967 – 970 .
- Clare , A. S. , Thomas , R. F. and Rittschof , D. 1995 . Evidence for the involvement of cyclic AMP in the pheromonal modulation of barnacle settlement. . Journal of Experimental Biology , 198 : 655 – 664 .
- Couper , J. M. and Leise , E. M. 1996 . Serotonin injections induce metamorphosis in larvae of the gastropod mollusc Ilyassana obsoleta. . Biological Bulletin , 191 : 178 – 186 .
- Dahlström , M. , Jonsson , H. , Jonsson , P. R. and Elwing , H. 2004a . Surface wettability as a determinant in the settlement of the barnacle Balanus improvisus (DARWIN). . Journal of Experimental Marine Biology and Ecology , 305 : 223 – 232 .
- Dahlström , M. , Jonsson , P. R. , Lausmaa , J. , Arnebrant , T. , Sjögren , M. , Holmberg , K. , Mårtensson , L. G. E. and Elwing , H. 2004b . Impact of polymer surface affinity of novel antifouling agents. . Biotechnology and Bioengineering , 86 : 1 – 8 .
- Edwards , N. C. , Thomas , M. B. , Long , B. A. and Amyotte , S. J. 1987 . Catecholamines induce metamorphosios in the hydrozoan Halocordyle disticha but not in Hydractinia echinata. . Development Genes and Evolution , 196 : 381 – 384 .
- Fitt , W. K. , Coon , S. L. , Walch , M. , Weiner , R. M. , Colwell , R. R. and Bonar , D. B. 1990 . Settlement behavior and metamorphosis of oyster larave (Crassostrea gigas) in response to bacterial supernatants. . Marine Biology , 109 : 389 – 394 .
- Hadfield , M. G. 2000 . Why and how marine invertebrate larvae metamorphose so fast. . Cell and Developmental Biology , 11 : 437 – 443 .
- Harrison , P. J. H. and Sandeman , D. C. 1999 . Morphology of the nervous system of the barnacle cyprid larva (Balanus amphitrite Darwin) revealed by light and electron microscopy. . Biological Bulletin , 197 : 144 – 158 .
- Hoeg , J. T. , Hosfeld , B. and Gram‐Jensen , P. 1998 . TEM studies on the lattice organs of cirripede cyprid larvae (Crustacea, Thecostraca, Cirripedia). . Zoomorphology , 118 : 195 – 205 .
- Jonsson , P. R. , Berntsson , K. M. and Larsson , A. I. 2004 . Linking larval supply to recruitment: Flow‐mediated control of initial adhesion of barnacle larvae. . Ecology , 85 : 2850 – 2859 .
- Kimura , Y. , Yoshida , M. and Morisawa , M. 2003 . Interaction between noradrenaline and adrenaline and the β1‐adrenergic receptor in the nervous system triggers early metamorphosis in the ascidian, Ciona savignyi. . Developmental Biology , 258 : 129 – 140 .
- Kolberg , K. J. S. and Martin , V. J. 1988 . Morphological, cytochemical and neuropharmacological evidence for the presence of catecholamines in hydrozoan planulae. . Development , 103 : 249 – 258 .
- Kon‐ya , K. and Endo , M. 1995 . Catecholamines as settlement inducers of barnacle larvae. . Journal of Marine Biotechnology , 2 : 79 – 81 .
- Kon‐ya , K. , Miki , W. and Endo , M. 1995 . L‐tryptophan and related compounds induce larval settlement of the barnacle Balanus amphitrite Darwin. . Fisheries Science , 61 : 800 – 803 .
- Maki , J. S. , Rittschof , D. , Costlow , J. D. and Mitchell , R. 1988 . Inhibition of attachment of larval barnacles, Balanus amphitrite, by bacterial surface films. . Marine Biology , 97 : 199 – 206 .
- Maki , J. S. , Rittschof , D. , Samuelsson , M. O. , Szewzyk , U. , Yule , A. B. , Kjelleberg , S. , Costlow , J. D. and Mitchell , R. 1990 . Effect of marine bacteria and their exopolymers on the attachment of barnacle cyprid larvae. . Bulletin of Marine Science , 46 : 499 – 511 .
- Matsumura , K. , Nagano , M. and Fusetani , N. 1998 . Purification of a larval settlement‐inducing protein complex (SIPC) of the barnacle, Balanus amphitrite. . Journal of Experimental Zoology , 281 : 12 – 20 .
- Matsumura , K. , Nagano , M. , Kato‐Yoshinaga , Y. , Yamazaki , M. , Clare , A. S. and Fusetani , N. 1998b . Immunological studies on the settlement‐inducing protein complex (SIPC) of the barnacle Balanus amphitrite and its possible involvement in larva–larva interactions. . Proceedings of the Royal Society B , 265 : 1825 – 1830 .
- McCauley , D. W. 1997 . Serotonin plays an early role in the metamorphosis of the hydrozoan Phialidium gregarium. . Developmental Biology , 190 : 229 – 240 .
- Miron , G. , Walters , L. J. , Tremblay , R. and Bourget , E. 2000 . Physiological conditions and barnacle behavior: A preliminary look at the relationship between TAG/DNA ratio and larval substratum exploration in Balanus amphitrite. . Marine Ecology Progress Series , 198 : 303 – 310 .
- O'Connor , N. J. and Richardson , D. L. 1998 . Attachment of the barnacle (Balanus amphitrite Darwin) larvae: Response to bacterial films and extracellular materials. . Journal of Experimental Marine Biology and Ecology , 226 : 115 – 129 .
- Okazaki , Y. and Shizuri , Y. 2000a . Structure of six cDNas exspressed specifically at cyprid larva barnacle Balanus amphitrite. . Gene , 250 : 127 – 135 .
- Okazaki , Y. and Shizuri , Y. 2000b . Effect of inducers and inhibitors on the expression of bcs genes involved in cyprid larval attachment and metamorphosis of the barnacle Balanus amphitrite. . International Journal of Developmental Biology , 44 : 451 – 456 .
- Okazaki , Y. and Shizuri , Y. 2001 . Identification of the aromatic L‐amino acid decarboxylase (AADC) gene and its expression in the attachment and metamorphosis of the barnacle, Balanus amphitrite. . Development Growth and Differentiation , 43 : 33 – 41 .
- Okano , K. , Shimizu , K. , Satuito , C. G. and Fusetani , N. 1996 . Visualization of cement gland exocytosis in the cyprid cement gland of the barnacle Megabalanus rosa. . Journal of Experimental Biology , 199 : 2131 – 2137 .
- Olivier , F. , Tremblay , R. , Bourget , E. and Rittschof , D. 2000 . Barnacle settlement: field experiments on the influence of larval supply, tidal level, biofilm quality and age on Balanus amphitrite cyprids. . Marine Ecology Progress Series , 199 : 185 – 204 .
- Pires , A. , Coon , S. L. and Hadfield , M. G. 1997 . Catecholamines and dihydroxyphenyalanine in metamorphosing larva of the nudibranch Phestilla sibogae (Gastropoda: Opistobranchia). . Journal of Comparative Physiology A , 181 : 187 – 194 .
- Pires , A. , Croll , R. P. and Hadfield , M. G. 2000 . Catechoalmines modulate metamorphosis in the opistobranch gastropod Phestilla sibogae. . Biological Bulletin , 198 : 319 – 331 .
- Rittschof , D. , Branscomb , E. S. and Costlow , J. D. 1984 . Settlement and behaviour in relation to flow and surface in larval barnacles, Balanus amphitrite Darwin. . Journal of Experimental Marine Biology and Ecology , 82 : 131 – 146 .
- Rittschof , D. , Maki , J. , Mitchell , R. and Costlow , J. D. 1986 . Ion and neuropharmacological studies of barnacle settlement. . Netherlands Journal of Sea Research , 20 : 269 – 275 .
- Roberts , D. , Rittschof , D. , Holm , E. and Schimdt , A. R. 1991 . Factors influencinginitial larval settlement: Temporal, spatial and surface molecular components. . Journal of Experimental Marine Biology and Ecology , 150 : 203 – 211 .
- Satuito , C. G. , Shimizu , K. , Natoyama , K. , Yamazaki , M. and Fusetani , N. 1996 . Age‐related settlement succes by cyprids of the barnacle Balanus amphitrite, with special reference to consumption of cyprid storage protein. . Marine Biology , 127 : 125 – 130 .
- Sjogren , M. , Dahlstrom , M. , Goransson , U. , Jonsson , P. R. and Bohlin , L. 2004a . Recruitment in the field of Balanus improvisus and Mytilus edulis in response to the antifouling cyclopeptides barettin and 8,9‐dihydrobarettin from the marine sponge Geodia barretti. . Biofouling , 20 : 291 – 297 .
- Sjogren , M. , Goransson , U. , Johnson , A. L. , Dahlstrom , M. , Andersson , R. , Bergman , J. , Jonsson , P. R. and Bohlin , L. 2004b . Antifouling activity of brominated cyclopeptides from the marine sponge Geodia barretti. . Journal of Natural Products , 67 : 368 – 372 .
- Smith , P. A. , Clare , A. S. , Rees , H. H. , Prescoot , M. C. , Wainwright , G. and Thorndyke , M. C. 2000 . Identification of methyl farnesoate in the cyprid larva of the barancle, Balanus amphitrite, and its role as a juvenile hormone. . Insect Biochemistry and Molecular Biology , 30 : 85 – 890 .
- Yamamoto , H. , Tachibana , A. , Kawaii , S. , Matsamura , K. and Fusetani , N. 1996 . Serotonin involvement in larval settlement of the barnacle, Balanus amphitrite. . Journal of Experimental Zoology , 275 : 339 – 345 .
- Yamamoto , H. , Tachibana , A. , Matsumura , K. and Fusetani , N. 1995 . Protein kinase C (PKC) signal transduction system involved in larval metamorphosis of the barnacle, Balanus amphitrite. . Zoological Science , 12 : 391 – 396 .
- Yamamoto , H. , Shimizu , K. , Tachibana , A. and Fusetani , N. 1999 . Roles of dopamine and serotonin in larval attachment of the barnacle, Balanus amphitrite. . Journal of Experimental Zoology , 284 : 746 – 758 .
- Zega , G. , Pennati , R. , Groppelli , S. , Sotgia , C. and De Bernardi , F. 2005 . Dopamine and serotonin modulate the onset of metamorphosis in the ascidian Phallusia mammillata. . Developmental Biology , 282 : 249 – 256 .