Abstract
The geographic variation of seven southern European populations of Ursus arctos was investigated through multivariate morphometrics of the skull. Univariate and multivariate analyses were run on 63 cranial and dental measurements recorded on 50 specimens from the Apennines, the Alps, and the Transcaucasus, and on 14 specimens of the fossil Ursus spelaeus. Detailed analyses of sexual dimorphism, age structure, and ranges of variation have been carried out on the Apennines population, which morphological variation has been little explored. Subsets of characters were selected to allow comparison with data derived from literature for populations from the Pyrenees, the Rhodopi‐Rila‐Pirin, the Balkans, and the Caucasus. Analyses clearly indicate that the Apennine bear is morphologically distinct from both a western (Alps, Pyrenees, Balkans, and Rhodopi) and an eastern contingent (Caucasus and Transcaucasus), therefore suggesting that the Apennine population should be reconsidered as a separate taxon, namely Ursus arctos marsicanus. These preliminary results suggest caution in restocking conservation actions.
Introduction
The brown bear Ursus arctos Linné, 1758 is the most widespread species of the family Ursidae occurring across the Holartic. Like other large carnivores, this species still occurs in Europe, but it is forced to live in highly fragmented and human‐dominated landscapes. Today, surviving brown bear populations are low in numbers and highly fragmented in southern, central and western Europe. More specifically, the range of western European populations is limited to isolated mountain areas (Cowan Citation1972; Corbet Citation1978; Servheen Citation1990; Soerensen Citation1991; Kudaktin and Chestin Citation1993), and the survival of the majority of them is at risk. One important priority in conservation is the release of animals to restock non‐viable populations and to favor connections with other populations (Swenson et al. Citation2000). However, any transfer of individuals should only operate in a clear systematic and genetic framework (Taberlet & Buvet Citation1994; Felizola Diniz‐Filho & Pires De Campos Telles Citation2002).
The fragmentation of the formal range and the high variability of the brown bear led in fact to the description of many subspecies (see, for example, Miller Citation1912; Hall Citation1981; Ellerman & Morrison‐Scott Citation1966, as reported by Pasitschniak‐Arts Citation1993), but in the most recent checklists all subspecies are considered synonyms (see for example Wozencraft Citation1993). Genetic variation throughout the distribution range of the species has been recently investigated by different authors, mainly through nuclear and mitochondrial DNA sequence comparisons (Goldman et al. Citation1989; Taberlet & Buvet Citation1992, Citation1994; Hartl & Hell Citation1994; Randi et al. Citation1994; Kohn et al. Citation1995; Chestin & Mikeshina Citation1998). The morphological variation of Central Asian and eastern European populations has been described by Kudaktin and Chestin (Citation1983), Rösler (Citation1984), and by Chestin and Mikeshina (Citation1998), while there is a very little information regarding the western European populations, i.e. Alpine, Apennine, and Pyrenean. In fact, the last extensive work was carried out by Couturier (Citation1954).
The Apennine brown bear occurs in a small isolated mountain area of the Italian Central Apennines. It is a critically endangered population and its relationships with the other European populations of U. arctos have been a continuous cause of scientific debate. Altobello (Citation1921) described the Apennine brown bear as a distinct subspecies, i.e. U. a. marsicanus. But the description was based on few characters examined on one female and two juveniles, and all subsequent authors have since rejected it, or at least considered it as doubtful (Toschi Citation1965). Only Conti (Citation1954), who examined the skull of one adult male, suggested that the Apennine population should be reconsidered as a distinct taxon. This hypothesis was reinforced through the analysis of a wider sample by one of the authors (Vigna Taglianti et al. Citation1984; Vigna Taglianti Citation1988, Citation2003). Therefore, the aim of this study was to better clarify the morphological relationships of the Apennine bears with the Alpine and other southern European populations through a multivariate morphometric approach.
Materials and methods
A total of 63 measurements were recorded by means of a precision caliper (accurate to the nearest 0.1 mm) on the skull, the teeth and the mandible following Von den Driesch (Citation1976). Details on characters are reported in Figure and Table . Measurements were recorded on 50 specimens of U. arctos, including 35 specimens (15 males, 20 females) from the Apennines, 8 specimens (4 males, 4 females) from the Alps and 7 specimens (4 males, 3 females) from the Transcaucasus. The same measurements were also recorded on 14 specimens of Ursus spelaeus Rosenmüller and Heinroth, 1784, from Central Italy (Grotta di Equi, Tuscany), considered as a reference group. Data on another 83 specimens from the Pyrenees (n = 16), Caucasus (n = 12); Rhodopi Mountains (n = 26), and Balkans (n = 29) were taken from literature (Couturier Citation1954; Vanev Citation1990).
Figure 1. Characters recorded on the skull, the mandible and the teeth of each specimen. See Table for details.
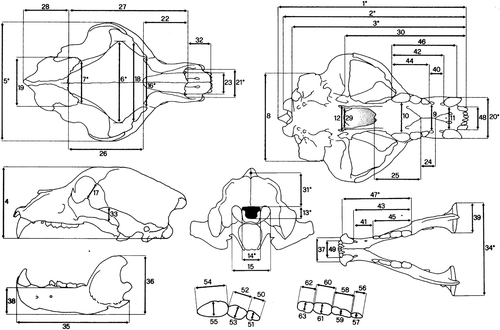
Table I. Description of the 63 characters measured. Asterisks indicate measurements in common with both Couturier (Citation1954) and Vanev (Citation1990), used for comparisons among all samples. Those in parentheses were exclusively recorded by the authors on the populations from the Alps and the Apennines (see text).
Localities, sample sizes and source of materials are reported in Figure and Table .
Figure 2. Samples location superimposed to the distribution ofUrsus arctos in Europe. ALP: Alps; APP: Apennines; BAL: Balkans; CAU: Caucasus; PYR: Pyrenees; RHO: Rhodopi, Rila, and Pyrin Mts; TRC: Transcaucasus; U.s.: Ursus spelaeus from Grotta degli Equi (Tuscany, Italy). See Table for details.
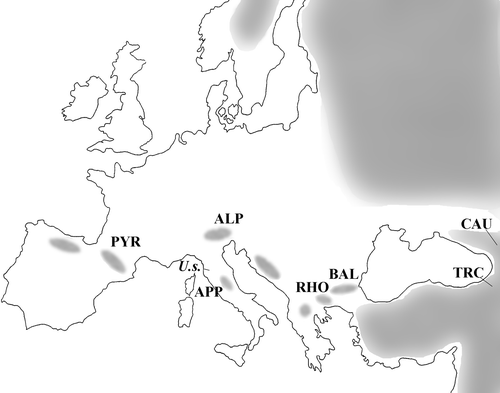
Table II. List of samples.
Specimens were ascribed to three age classes: adult (over 6 years old, according to Rausch Citation1963), subadult (2–6 years old) and juvenile (less then 2 years old), following the indications reported on the collection labels.
A square scatter matrix of all measurements was first produced using the STATISTICA program (StatSoft, Inc., version 6.0, 1984–2001) in order to detect measurement errors and misclassifications of sex and age rapidly. Univariate and multivariate analyses of variance were run on the Apennine data set to evaluate the influence of age and sex on morphological variation. Principal component scores were evaluated to detect any size factor and to correct the data following the method described by Burnaby (Citation1966, as discussed in Rohlf & Bookstein Citation1988), Thorpe (Citation1983), and Marcus (Citation1990). A multivariate canonical analysis was performed on the ‘size‐independent’ matrix for comparisons among the western European samples, i.e. from the Alps, the Apennines and the Pyrenees. Pairwise comparisons through the Tukey LSD test were performed for characters showing the largest differences among groups. A geometric morphometric data set (Bookstein Citation1991; Rohlf & Marcus Citation1993) was then created to visualize the main shape differences between the Apennine and the Alpine skulls. Cartesian coordinates of 31 landmarks were recorded on the dorsal projection of the digital image of an adult male from the Alps and and adult male from the Apennines, by using the program tpsDig (Rohlf Citation2002b). Coordinates were then translated, rotated, scaled to unit centroid size and superimposed through the General Procustes Analysis (Rohlf & Slice Citation1990), by using the program tpsSuper (Rohlf Citation2002b). A thin plate spline deformation grid was produced with the same program in order to visualize the differences in shape between the two skulls.
Finally, a subset of 13 common characters (see Table ) was selected for comparison with data reported from literature on other mountain populations from southern Europe, i.e. Balkans, Rila‐Rhodopi, and Caucasus (Vanev Citation1990).
Analyses were performed only considering the adult males. Mahalanobis distances resulting from canonical variate analysis run on all extant samples and the fossil U. spelaeus were used to produce a UPGMA dendrogram of relationships among populations (Sneath & Sokal Citation2001), using the NTSYS program (Rohlf Citation2002a).
Results
Variation of the Apennine population
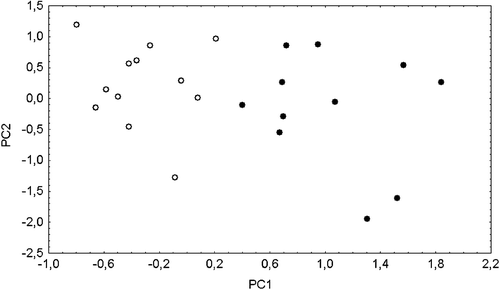
MANOVA for the whole set of characters computed on adult specimens shows significant differences between the two sexes (Wilks' Lambda F = 320.8, P = 0.043). The univariate analyses reveals that males are significantly larger than females in 41 out of the 63 measurements considered (F statistics always significant at P<0.05). The only exception were the dental features (characters 50–63), among which only the length of M1 was found to be significantly larger in males (variable 52: F = 6.5, P = 0.020). The scatter plot of the first and second principal component scores (Figure ) shows that males and females are clearly distinct along the first principal component axis (61.87% variance explained), whose coefficients are all of the same sign and magnitude, confirming that size is the main factor affecting sexual dimorphism. As a matter of fact, significance of canonical variate analysis between sexes is lost when PC1 scores are excluded (F = 0.334, P<0.93, vs. F = 5.348, P<0.002 when PC1 is included). It is worth noting that the same results were obtained for the Alpine and the Pyrenean samples, thus allowing the pooling of males and females in successive ‘size‐independent’ comparisons between the western European samples.
Figure 3. Scatter plot of the first two principal component scores computed on the adult specimens of the Apennine sample; open circles = males; filled circles = females.
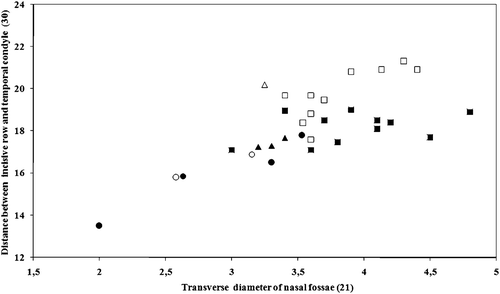
Multivariate analysis of variance run separately for males and females did not show any significant difference between age classes (Wilks' Lambda F females = 7.34, P = 0.126; F males = 7.34, P = 0.126). Nevertheless, univariate analyses run separately for each variable for the female sample, including 5 juveniles, 3 sub adults, and 12 adults revealed that 7 characters differ significantly among age classes. These characters include the transverse diameter of nasal fossae (variable 21, F = 5.36, P = 0.046), the distance between the margin of the incisive row and the inferior boundary of the temporal condyle (variable 30, F = 5.63, P = 0.021), the length of the diastema between canines and premolars (variables 40 and 41, F = 7.16, P = 0.006, and F = 6.55, P = 0.011, respectively) and the distance between the the inferior canine and M3 (variable 47, F = 4.40, P = 0.037). Variation of these most significant characters is directly related to age, with mean values always increasing from juveniles to adults (Multiple R = 0.571, P = 0.032). Figure shows variation of the two most correlated variables (variables 21 and 30, r = 0.868) for the three age classes in males and females.
Figure 4. Scatter plot of characters 21(transverse diameter of nasal fossae) and 30 (distance between the margin of the incisive row and the edge of the temporal condyle) for the Apennine sample. Males and females are shown as open and filled symbols, respectively; circles = juveniles; triangles = sub adults; squares = adults.
Relationships among the western European populations
Figure shows the scatter plot of the first and the second canonical variate scores (79.9% and 20% of total variance explained, respectively) for the samples from the Alps, the Apennines and the Pyrenees (for which data are derived from Couturier Citation1954), obtained from the pooled (males and females) ‘size‐independent’ matrix of (n−1) principal component scores. The three populations are well differentiated (Wilks' Lambda F = 15.183, P<0.0001), with the Alpine and the Pyrenean populations partially overlapping along the first canonical axis. Results from MANOVA run on all characters to evaluate the effect of sample locality on morphological variation indicate that the differences among the western European populations are mainly related to the variation of the orbital region (variables 6, 7, 16, 17, 18 and 24), the occipital bones (variables 14 and 15) and the mandible (variable 36). The dental features which show significant differences between the Alpine and the Apennine bears (these data are not available for the Pyrenean sample) include the diastema between the upper and lower canines and Pm, (variables 40 and 41), and the size of Pm4 (variables 50 and 51). More specifically, the characters most relevant to the separation of the Apennine population from the Alps‐Pyrenees cluster are the ecto‐orbital apophyses (variable 6), the post‐orbitary constriction width (variable 7), and the ecto‐orbital constriction (variable 16). The large Pm4 is also typical. The variation ranges of these most diagnostic characters in males and females of Appennine bears are reported in Table . The Apennine bears are characterized by a greater distance between the ecto‐orbital apophyses and by larger ecto‐orbital and post‐orbital constrictions (variables 7 and 16). Furthermore the PM4 (variables 50 and 51) is much larger in the Apennine than in the Alpine bears (data on the dental features are lacking for the Pyrenean population). Differences in shape between the Alpine and Apennine skulls are summarized and visualized through the deformation grid in Figure . The deformation grid confirms that the main shape differences are located in the middle region of the skull, with an expansion of the ecto‐orbital and the postorbital apophyses in the Apennine bear along with a slight shortening of the rostrum and a minor narrowing and elongation of the occipital area.
Figure 5. Scatter plot of the first and the second canonical variate scores for the three Western European populations, i.e Alps, Pyrenees, and Apennines, derived from the‘size‐independent’ matrix (see text).
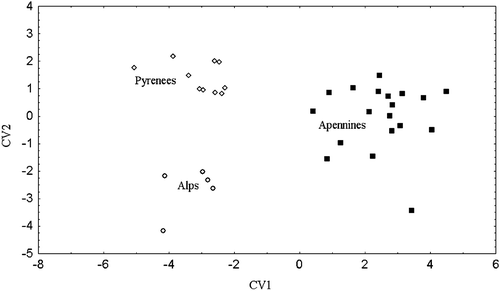
Figure 6. Above: pictures of the dorsal skull of an Alpine (left) and Apennine (right) adult male with landmarks (black dots) used to produce the aligned configurations for a geometric superimposition through GPA (Rohlf & Slice, Citation1990). Below: thin plate spline deformation grid derived from the superimposition of the two configurations through tpsSplin (Rohlf Citation2002). Regions of deformations indicate the main shape differences between the two skulls.
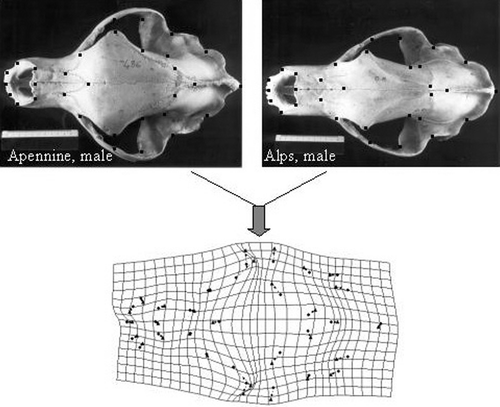
Table III. Mean, standard deviation, maximum and minimum values, of the most diagnostic characters of the Apennine bears. Data are reported for males (M, n = 12) and females (F, n = 12) separately. Character codes are those reported in table and Figure .
Relationships between western and eastern populations
As sexual dimorphism in the eastern populations revealed to be not only determined by a size factor, pooling of sexes through the exclusion of the first principal component scores was not possible. Therefore multivariate comparisons among the western and eastern populations were performed on the adult males only. Mahalanobis distances and F statistics computed from canonical variate scores on the subset of 13 variables common to both the eastern and the western samples (see Table ) indicate that the Apennine population is significantly different from all other European populations, while the Alpine do not differ significantly from any other than the Apennine bears (Table ). Relationships among all extant populations are summarized in the UPGMA dendrogram shown in Figure . The dendrogram was derived from Mahalanobis distances on a sample including the fossil U. spelaeus as the reference group. The phenogram confirms the isolation of the Apennine bears, while the other European samples are grouped into two major clusters. One cluster comprises all the central southern European samples, i.e. the Alps, Pyrenees, Rhodopi, and Balkans, while the second includes the eastern populations from the Caucasus and Transcaucasus. This eastern contigent is mostly differentiated along the second canonical axis, whose coefficients vector indicate a relevant influence of the width of the rostrum (var 20 – distance between canine alveoles), and the height of the occipital region (var 31 – distance between the external protuberance of the occipital and superior edge of the foramen occipitalis). More specifically, the skull of the eastern European bears shows a slender rostrum and a higher occipital region compared to the western European bears.
Figure 7. UPGMA dendrogram derived from Mahalanobis distances on canonical variate scores of all samples, includingUrsus spalaeus as the reference group.
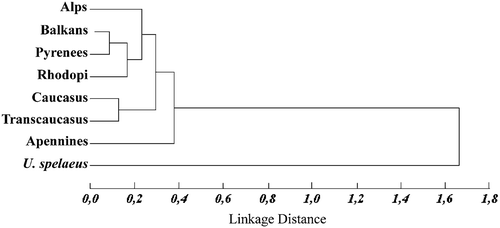
Table IV. Mahalanobis distances (below diagonal), and F statistics (above diagonal) computed on canonical variate scores on the seven populations analyzed. * = P<0.01; ** = P<0.001; *** = P<0.0001. For populations codes see Table .
Discussion
This first morphometric comparison among seven southern European populations of Ursus arctos suggests a closer relationship between the eastern European bears from Caucasus and Transcaucasus compared to a western European contingent, including the bears from the Alps, the Pyrenees, the Balkans and the Rhodopi‐Rila mountains. The populations included in this latter cluster show a small degree of morphological divergence, which do not support any separation at subspecific level.
Among the western European bears, size represents an important aspect of non‐geographic variation, and it appears to be an important factor in both sexual dimorphism and age growth. By contrast, the eastern bears show more integrated size and shape patterns related to age and sexual dimorphism. These differences could reflect a deviation in allometric patterns which are likely to be due to an early isolation of the western from the eastern European stocks. However, allometric growth patterns need to be further investigated to elucidate the relationships among and within these groups. Furthermore, the eastern European bears show some peculiarities in cranial shape, such as a slender rostrum and a higher occipital region. But the high variability found in the brown bears from the Caucasus by Chestin and Mikeshina (Citation1998) suggests caution in any inference regarding the morphological differentiation in the cranial features of a western and an eastern European cluster. In fact, future studies should be carried out on a much wider sample.
The Italian population from the Apennines represents the only highly diverging population, as it is morphometrically isolated when compared to both the western and the eastern contingent, including the Italian population from the Alps. Interestingly, the morphometric differentiation of the Apennine bears is mainly related to differences in the shape of the skull which cannot be considered as a simple variation in size.
The uniqueness of the Apennine bears has already been noticed by Altobello (Citation1921), who suggested considering it a separate subspecies, namely U. arctos marsicanus. Our results clearly indicate that the Apennine population represents a differentiated taxon, characterized by a unique and characteristic skull among the European brown bears. The diagnostic features of this subspecies include the expansion of the ecto‐orbital apophyses, the narrow postorbital constriction, the short rostrum, and the short diastema between canines and molars. The large Pm4 is also typical. Recent analyses of mtDNA variation have revealed a common origin of all the western European populations of U. arctos, including the Apennine bears (Randi et al. Citation1994; Taberlet & Buvet Citation1994). We suggest that despite this common origin, the Apennine brown bear underwent a strong morphological divergence, which was possibly due to an early isolation of a small population, or as a consequence of a bottleneck. An absence of gene flow with the neighboring populations from the Alps and genetic drive may have played a role in this rapid morphological divergence, which was probably amplified by adaptations to local conditions. As a consequence we suggest that the subspecies U. a. marsicanus should be revalued following Altobello (Citation1921), Conti (Citation1954), and Vigna Taglianti (Citation2003).
The reconsideration and acceptance of the Appennine population as a distinct taxon will have a strong effect on any action to be undertaken for the conservation of the species in Italy. As was also recently stressed by Randi (Citation2003), there should be distinct conservation management for the Alpine and Apennine brown bear populations, and the Apennine brown bears should be managed as an Evolutionary Significant Unit (ESU, Conner & Hartl Citation2004).
Acknowledgments
All the Apennine brown bear specimens were provided by the Servizio Scientifico of the Parco Nazionale d'Abruzzo, Lazio e Molise. We are indebted with the responsible Cinzia Sulli and with rangers and researcher of the park for their helpful collaboration. Paolo Mazza and Marco Rustioni provided access to the collection of Ursus spelaeus in the Museo di Geologia e Paleontologia dell'Università di Firenze. A special acknowledgment goes to Nicolò Falchi for the original drawings of the skulls. Ettore Randi kindly revised the manuscript and anticipated part of his unpublished results on genetic variation. We are also indebted to Marco Corti and an Sandro Lovari for their valuable and helpful suggestions which greatly contributed to the improvement of the manuscript. Financial support was provided by the Ministero della Ricerca Scientifica (40% and 60% projects) and by the Consiglio Nazionale delle Ricerche, project N. 94.02937.04. This work is dedicated to the memory of Marco Corti (1950–2007), unforgettable theriologist, morphometrician and friend, who contributed with invaluable comments to the improvement of this manuscript.
References
- Altobello , G. 1921 . “ Mammiferi. IV. Carnivori, Carnivora. ” . In Fauna dell'Abruzzo e del Molise , Campobasso : Edizioni Colitti e Figlio .
- Bookstein , L. F. 1991 . Morphometric tools for landmark data—geometry and biology , New York : Cambridge University Press .
- Burnaby , T. P. 1966 . Growth‐invariant discriminant functions and generalised distances. . Biometrics , 22 : 96 – 110 .
- Chestin , I. E. and Mikeshina , N. G. 1998 . Variation of the brown bears in Caucasus, Russia. . Journal of Mammalogy , 79 : 118 – 130 .
- Conner , J. and Hartl , D. 2004 . A Primer of Ecological Genetics , Sunderland : Sinauer Associates, Inc. .
- Conti , S. 1954 . Morfologia comparata craniale ed encefalica degli orsi pleistocenici della Liguria. Correlazioni con alcune forme attuali (U. arctos, U. marsicanus, U. horribilis). . Mem. Mus. civ. Stor. nat. “G. Doria”, Genova , 1 : 1 – 68 .
- Corbet , G. B. 1978 . The mammals of the Paleartic region—a taxonomic review , London : British Museum of Natural History .
- Couturier , M. A. J. 1954 . L'ours brun, Ursus arctos L Grenoble, published privately
- Cowan , I. M. 1972 . The status and conservation of bears, Ursidae of the world 1970. . International Conference on Bear Research and Management , 2 : 343 – 367 .
- Ellerman , J. R. and Morrison‐Scott , T. C. S. 1966 . Checklist of Palaeartic and Indian mammals 1758 to 1946 , London : British Museum of Natural History . 2nd ed
- Felizola Diniz‐Filho , J. A. and Pires De Campos Telles , M. 2002 . Spatial autocorrelation analysis and the identification of operational units for conservation in continuous populations. . Conservation Biology , 16 : 924 – 935 .
- Goldman , D. , Rathna , G. P. and O'Brien , S. J. 1989 . Molecular genetic distance estimates among Ursidae as indicated by one‐ and two‐dimensional protein electrophoresis. . Evolution , 43 : 282 – 295 .
- Hall , E. R. 1981 . The mammals of North America , New York : John Wiley and Sons . 2nd ed
- Hartl , G. B. and Hell , P. 1994 . Maintenance of high levels of allelic variation in spite of a severe bottleneck in population size. The brown bear, Ursus arctos in the Western Carpathians. . Biodiversity Conservation , 3 : 546 – 554 .
- Kohn , M. , Knauer , F. , Stoffella , A. , Schroeder , W. and Paabo , S. 1995 . Conservation genetics of the European brown bear—a study using excremental PCR of nuclear and mitochondrial sequences. . Molecular Ecology , 4 : 95 – 103 .
- Kudaktin , A. and Chestin , I. 1983 . “ Phenotypic peculiarities of brown bear in the West Caucasus. ” . In Physiological population ecology , Edited by: Bolshakov , V. N . 145 – 147 . Moscow : Saratov .
- Kudaktin , A. and Chestin , I. 1993 . “ The Caucasus. ” . In Bears. distribution, ecology, use and protection , Edited by: Vaisfeld , M and Chestin , I . 136 – 169 . Moscow : Nauka .
- Marcus , L. F. 1990 . “ Traditional morphometrics. ” . In Proceedings of the Michigan morphometrics workshop , Edited by: Rohlf , F. J and Bookstein , F. L . 77 – 122 . Ann Arbour : The University of Michigan Museum of Zoology . Special Publication 2
- Miller , G. S. 1912 . Catalogue of the mammals of Western Europe, Europe exclusive of Russia in the collection of the British Museum , London : British Museum of Natural History .
- Pasitschniak‐Arts , M. 1993 . Ursus arctos. . Mammalian Species , 439 : 1 – 10 .
- Randi , E. 2003 . Conservation genetics of carnivores in Italy. . Comptes Rendus Biologies , 326 (suppl 1) : 54 – 60 .
- Randi , E. , Gentile , L. , Boscagli , G. , Huber , D. and Roth , H. U. 1994 . Mitochondrial DNA sequence divergence among some west European brown bear, Ursus arctos L. populations. . Heredity , 73 : 480 – 489 .
- Rausch , R. 1963 . Geographic variation in size in North American brown bears, Ursus arctos L., as indicated by condylobasal length. . Canadian Journal of Zoology , 41 : 33 – 45 .
- Rohlf , F. J. 2002a . NTSYS‐pc, version 2.10z , New York, Setauket : Exeter Software .
- Rohlf FJ. 2002b. Tps series. New York: Department of Ecology and Evolution, State University of New at Stony Brook. Available online at: http//life.bio.sunysb/edu/morph .
- Rohlf , F. J. and Bookstein , F. L. 1988 . A comment on shearing as a method for ‘size correction’. . Systematic Zoology , 36 : 356 – 367 .
- Rohlf , F. J. and Marcus , L. F. 1993 . A revolution in morphometrics. . Trends in Ecology and Evolution , 8 : 29 – 132 .
- Rohlf , F. J. and Slice , D. E. 1990 . Extensions of the Procrustes method for the optimal superimposition of landmarks. . Systematic Zoology , 39 : 40 – 59 .
- Rösler , R. 1984 . Beiträge zur Kenntnis des Braunbären,Ursus arctos L. der Rumänischen Karpaten 1. Naturwissenschaftliche Forschungen über Siebenbürgen. II. . Herausgegeben von Heinz Heltmann , 8 : 233 – 293 .
- Servheen , C. 1990 . The status and conservation of the bears of the world. . International Conference on Bear Research and Management, Monograph series , 2 : 1 – 32 .
- Sneath , P. H. and Sokal , R. R. 2001 . Numerical taxonomy—the principles and practice of statistics in biological research , San Francisco : W. H. Freeman and Company . 3rd ed
- Soerensen , O. J. 1991 . The brown bear in Europe in the mid 1980s. . Aquilo, Serie Zoologica , 27 : 3 – 16 .
- Swenson , J. E. , Gerstl , N. , Dahle , B. and Zedrosser , A. 2000 . Action Plan for the conservation of the Brown Bear in Europe (Ursus arctos). 114 Nature and Environment, Council of Europe Publishing
- Taberlet , P. and Bouvet , J. 1992 . Génétique de l'ours brun des Pyréneées,Ursus arctos—premieres résultats. . Comptes Rendus de l'Academie des Sciences de Paris , 314 : 15 – 21 .
- Taberlet , P. and Bouvet , J. 1994 . Mitochondrial DNA polymorphism, phylogeography, and conservation genetics of the brown bear, Ursus arctos in Europe. . Proceedings of the Royal Society of London , B 255 : 195 – 200 .
- Thorpe , R. S. 1983 . A biometric study of the effects of growth on the analysis of geographic variation—tooth number in green geckos, Reptilia—Thelsuma. . Journal of Zoology, London , 201 : 13 – 26 .
- Toschi , A. 1965 . Mammalia–Lagomorpha–Rodentia–Carnivora–Ungulata–Cetacea. Fauna d'Italia XII , Bologna : Edizioni Calderini .
- Vanev , U. 1990 . Craniometricna charakteristica na kafjavata mecka (Ursus arctos L. 1758) v Balgaria [Dissertation thesis, University of Sophia]
- Vigna Taglianti , A. 1988 . Stato attuale delle conoscenze sulla biologia e la conservazione dei Carnivori in Italia. . Supplemento alle Ricerche di Biologia della Selvaggina , 14 : 401 – 417 .
- Vigna Taglianti , A. 2003 . “ Ursus arctos. ” . In Fauna d'Italia, Vol 3: Mammalia—Carnivori, Artiodattili , Edited by: Boitani , L , Lovari , S and Vigna Taglianti , A . 85 – 98 . Bologna : Edizioni Calderini .
- Vigna Taglianti , A. , Iacobone , G. and Loy , A. 1984 . Osservazioni sistematiche e zoogeografiche sull'orso bruno nell'Appennino Centrale. . Bollettino di Zoologia , 51 (suppl.) : 113
- Von den Driesch , A. 1976 . A guide to the measurement of animal bones from archeological sites. . Peabody Museum, Harvard University Bulletin , 1 : 46 – 62 .
- Wozencraft , W. C. 1993 . “ Order Carnivora. ” . In Mammal species of the world—a taxonomic and geographic reference , Edited by: Wilson , D. E and Reeder , D. M . 279 – 344 . Washington : Smithsonian Institution Press . 2nd ed