Abstract
An ultrastructural examination was carried out on the pleuropodia of three species of Orthoptera Acrididae collected in four different stages of embryonal development in which the pleuropodia, due to their particular morphology, seem to have a greater activity. From the results obtained it was seen that the pleuropodia of the species examined appear as trilobed estroflexions, made up of a monolayer epithelium, of ectodermic origin, having secretory activity that appeared particularly marked in correspondence to stage 8 of development during which the embryo extends throughout the egg. In correspondence to stage 4, which precedes katatrepsis, the secretory activity mainly consists in the production of electron‐dense granules; in the other stages of development, instead, other than the granules there were also vesicles having not very electron‐dense contents, which are liberated among the microvilli. As regards the possible role of the pleuropodia, it has been hypothesized that they are active in the production of enzymes necessary for hatching. The ultrastructural aspect found in correspondence to the poles of the pleuropodial cells, suggests that these formations also have a role in hydro‐ionic transport, mechanisms that could, for example, involve an exchange of material between the periembryonal space and the hemocele, without excluding, however, other possible roles inherent in the metabolism of the embryos.
Introduction
In many insect embryos the first abdominal segment has similar organs, called pleuropodia by Wheeler (Citation1890), that gradually grow during embryonal development and successively detach at the moment of hatching from the egg.
Previous investigations conducted on pleuropodia of species belonging to various Orders of insects (Slifer Citation1937, Citation1938; Hagan Citation1951; Engelmann Citation1957; Louvet & Marcel Citation1969; Ando Citation1971; Louvet Citation1973, Citation1983; Viscuso et al. Citation1975; Viscuso & Germenia Citation1979; Lambiase et al. Citation2003; Rost et al. Citation2004) showed a variety of shapes and structures of the pleuropodia, thus hypothesizing for these formations a variety of meanings and functions in relationship both to the characteristics of embryonal development that these animals encounter and to their metabolic needs.
In particular, in the Orthoptera only the morphological organization of these organs during embryonal development is known (Slifer Citation1938; Viscuso et al. Citation1975; Viscuso & Germenia Citation1979), whereas, as regards the ultrastructural organization of the pleuropodia, only a late embryonal stage of the Locusta migratoria is known (Louvet Citation1975), a detailed ultrastructural analysis on the embryonal development progress of the pleuropodia is lacking. This analysis would allow a better understanding of these structures; this is particularly important given the recent observations on the morphological evolution of pleuropodia (Viscuso & Germenia Citation1979), which showed particular modifications in relationship to the developmental stage that could correspond to a different role of these structures during embryogenesis. Based on these observations and other previous investigations, it thus seemed of great interest to carry out these investigations examining the pleuropodia of three species of Orthoptera Acrididae, i.e. Eyprepocnemis plorans (Charp.), Aiolopus thalassinus (Fabr.) and Aiolopus strepens (Latreille), collected in four different stages of embryonal development, according to the classification performed by De Luca and Viscuso (Citation1972) in which the pleuropodia, due to their particular morphology, seem to show the greatest activity (Viscuso & Germenia Citation1979).
Materials and methods
The investigations were carried out on pleuropodia of the Orthoptera Acrididae E. plorans, A. thalassinus and A. strepens. These species were kept under controlled temperature (30±1°C), humidity (70%±5%) and photoperiod (12 h light/12 h dark). Under such conditions the embryonal development of E. plorans is about 28 days, while in A. thalassinus and A. strepens it is about 14 days.
Eggs from the ootheca were treated with sodium hypochlorite, placed in Petri capsules containing humid sand, and kept in a climatic chamber at a temperature of 30±1°C and with 70%±5% relative humidity, i.e. in the same conditions that exist in the animal breeding area. The embryos corresponding to the stages of development taken into consideration were collected from the eggs in Ringer's solution and treated on the basis of the methodologies used. Pleuropodia were taken under stereo‐microscope.
Aside from the duration of the embryonal development, pleuropodia of the three species were taken at the same development stage. In particular, the embryonal stages that we examined are: stage 4, phase c, which precedes the katatrepsis, the eye is already pigmented and the third pair of legs has the typical N position; stage 6, when the last blastokinesis movements have been carried out, the embryo occupies half of the egg and is still open dorsally on the yolk; stage 8, phases a and b, when the embryo occupies all the egg, the dorsal opening appears closed and the cuticle is not pigmented; and finally, stage 9, phases a and b, which precede the hatching of the embryo, during which there is the gradual pigmentation of the cuticle (De Luca & Viscuso Citation1972).
SEM
The embryos were fixed in 1% glutaraldehyde in Sörensen buffer pH 7.4, 0.1 M for 1 h at room temperature, dehydrated in ethanol, put into HMDS (hexamethyldisilazane) (Nation Citation1983), air‐dried at room temperature and mounted on copper stubs. After carbon and gold splattering, the samples were observed under the scanning electron microscope Hitachi S‐4000.
TEM
The pleuropodia removed from the embryos in the examined stages of development were fixed in 2.5% glutaraldehyde Sörensen buffer pH 7.4, 0.1 M for 4 h at room temperature. After repeated washing in the same buffer, they were post‐fixed in 1% osmium tetroxide in the same buffer for 1 h at room temperature, dehydrated in graded alcohol series and transferred to propylene oxide prior to embedding in Embed 812. Ultra‐thin sections were mounted on copper–rhodium grids of 200 or 300 mesh, stained with uranyl acetate and lead citrate (Reynolds Citation1963) and observed under the electron transmission microscope Philips CM10.
To identify the presence of lysosomes, we used the method for the ultrastructural localization of acid‐phosphatase based on the use of cerium (Robinson & Karnowsky Citation1983).
Results
In the Acrididae the evaginated pleuropodia presented characteristics that were generally similar: in all stages of development examined, they appeared as small lateral evaginations located at the first abdominal segment, whose size gradually increased reaching a maximum at stage 8 of development. In particular, as already described by Viscuso and Germenia (Citation1979), at stage 4, phase c, pleuropodia cells are cubic with a vesicular nucleus; the basophil cytoplasm contains secretion granules, which accumulate at the apical region. At stage 6, after katatrepsis is completed, cells are cylindric, with big nuclei, positioned a little more towards the basal region; cytoplasm contains vacuoles even in the perinuclear region. The cell membrane in the apical region presents a striated border. At stage 8, phase b, cytoplasm presents more vacuoles both around the nucleus and the apical region. It contains highly basophil areas and secretion granules. The connection peduncle is much reduced.
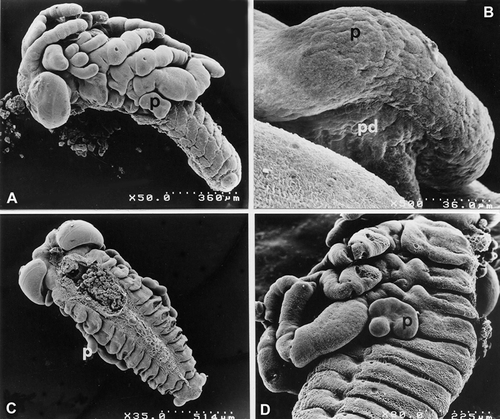
From the SEM observations, it was seen that at stage 4 phase c, they have irregular margins and are connected to the wall of the embryo by a peduncle that is both wide and short (Figure ); at stage 6, instead, the pleuropodia, which are more voluminous with respect to the previous stage, appear to be made up of small lobes, separated by a indentation, which is deep in A. thalassinus, turned towards the caudal extremity of the embryo and by a larger lobe, though less marked, turned towards the cephalic extremity (Figure ). In the following stages, up to stage 8 phase b, the pleuropodia gradually increase in dimension but do not modify their shape; also the peduncle gradually lengthens and progressively shrinks until it completely reduces at the end of development so that the pleuropodia can be released by the embryo at hatching.
Figure 1. SEM micrographs ofE. plorans and A. thalassinus embryos at various stages of development. A,B, E. plorans stage 4, phase c. A, 50×; B, 500×. C, E. plorans stage 6 (35×). D, A. thalassinus stage 6 (80×). Abbreviations: p, pleuropodium; pd, conecting peduncle.
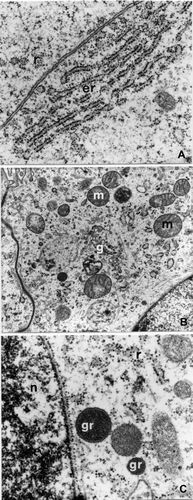
TEM observations showed that at stage 4 the pleuropodia are composed of epithelial cells, generally cubic in shape, in a single layer with a vesicular and euchromatic nucleus; the cytoplasm is rich in free ribosomes, the rough endoplasmic reticulum is not very extended, localized in proximity to the nucleus, and is made up of parallel flattened cisterns (Figure ), and of loosely dilated cisterns having finely granular contents: the latter extend to the apical region of the cells.
Figure 2. E. plorans stage 4, phase c: epithelial cells. A, Flattened cisternae of granular endoplasmic reticulum in parallel (er; 28,500×). B, Golgi apparatus (g) localized near the nucleus (15,500×). C, Electron dense granules (gr) near the nucleus (n; 39,000×). Abbreviations: m, mitochondria; r, free ribosomes.
The dictyosomes, in the supra‐nuclear position, are numerous and appear to be made up of few small piled up sacs that appear to be detached from the vesicles, containing a material that is weakly electron‐dense (Figure ); electron‐dense granules of various dimensions are present both in proximity to the nucleus (Figure ) and apically, both outside the cell among the microvilli; the latter, in particular, appear associated with tubular structures with dimensions that are similar to the cytoplasmatic microtubules (Figure ). The microtubules inside the cytoplasm are numerous and are isolated from, and in parallel to, the cellular axis.
Figure 3. E. plorans stage 4, phase c: apical region of epithelial cells. A, Electron dense granule (gr) among the microvilli (mv) associated to the microtubular‐like structures (arrows; 27,500×). B, Coated pit (cp; 39,000×). C, Smooth endoplasmic reticulum (el; 21,000×). D, Above the apical microvilli there is a cuticle (c) composed of a very thin electron‐dense lamina (73,000×).
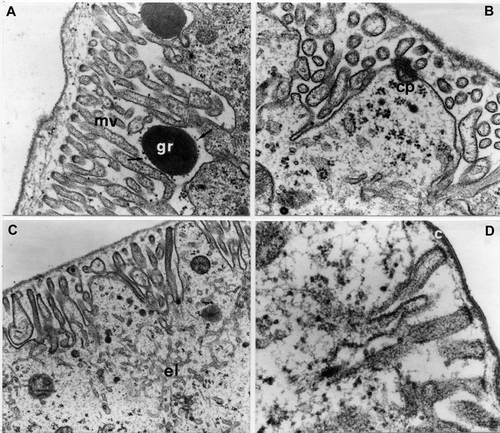
The apical surface of the cells has densely packed microvilli that are about 1 µm in length, among which are large cellular expansions, more or less marked, often with coated pits (Figure ); beneath the microvilli there are numerous mitochondria and a smooth endoplasmatic reticulum made up of interconnected tubules, which also penetrate the cellular expansions (Figure ).
The apical microvilli have a cytoplasm rich in fibrils parallel to the major axis and above them there is a cuticle composed of a very thin electron‐dense lamina about 20 nm thick (Figure ); the structural organization of the cuticle (at this stage), unlike that of the embryo, does not undergo any modifications until the end of embryonal development.
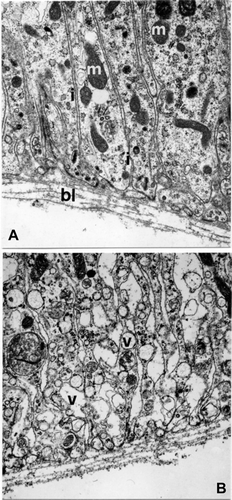
In the basal region of the cells, there are deep folds of the basal membrane inside which there is a thin basal lamina with a fibril appearance.
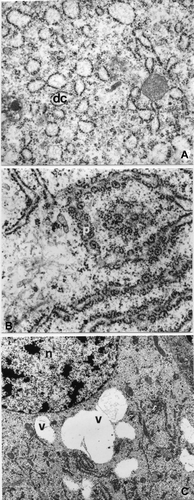
In correspondence to stage 6, the secretory activity of the pleuropodia cells appears more intense with respect to the previous stage, as can be seen from a greater extension of the organelles involved in this process. The rough endoplasmic reticulum, in fact, occupies a large part of the cytoplasm and appears to be made up of both parallel flattened cisterns and piled up dilated cisterns (Figure ); there are numerous polyribosomes adhering to the rough endoplasmic reticulum (Figure ).
Figure 4. A. strepens stage 6: epithelial cells. A, The RER is made up of piled dilated cisterns (dc; 39,000×). B, Polyribosomes (p) adherent to the RER (6600×). C, Secretory vesicles (v) containing an electron transparent material near the nucleus (n; 10,000×).
The secretion elaborated inside the cells, prevalently present in a vesicle form, often confluent among them, with a electron‐transparent content, prevalently near the nucleus (Figure ); in proximity of some vesicles there are ample cytoplasmatic areas containing glycogen granules (Figure ).
Figure 5. E. plorans stage 6. A, Near the vesicles there is a thickening of glycogen granules (gl; 15,500×). B, Electron transparent vesicles (v) which open among the microvilli (mv; 21,000×). C, Microtubules (mt) converging on an attachment plaque (p; 15,500×). Abbreviations: g, electron dense granules between microvilli and cuticle; m, mitochondria.
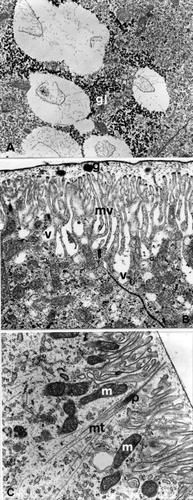
In correspondence to the apical regions of the cells, the microvilli, about 2 µm long, are tightly close; electron‐dense granules of various dimensions are present between the microvilli and the cuticle (Figure ). In the underlying cytoplasm the mitochondria are more numerous and the electron‐transparent vesicles seem to open among the microvilli (Figure ); the microtubules converge on the attachment plaques (Figure ).
In the basal region of the cells there are numerous and deep enfoldings of the plasmatic membrane among which are a large number of mitochondria.
Stage 8 is the stage of development in correspondence to which the pleuropodia reach their greatest dimension; the cells appear cylindrical with large nuclei in the basal position.
In phase a, the cells manifest marked secretory characteristics and ultrastructural characteristics that are similar to the cells of the previous stage: the RER, however, prevalently made up of parallel flattened cisterns with numerous and extensive polyribosomes, is more dense near the nucleus; the mitochondria are particularly numerous both in the apical region, below the microvilli (Figure ) and in the basal region among the deep enfolding of the plasmatic membrane where there are evident areas containing glycogen. The cytoplasm contains electron‐dense granules, compact in appearance, and vesicles, of various dimensions, with an electron‐transparent content; the latter are more numerous in the apical region where they seem to open among the microvilli. The microtubules are isolated and run parallel to the cellular axis, as in the previous stages, bundles of microtubules converge on the attachment plaques.
Figure 6. E. plorans stage 8. A, Phase a: numerous mitochondria (m) under the microvilli (mv; 13,000×). B, Phase b: cells of the second cytotype: electron transparent vesicles (v; 5,200×). C, Phase b: cells of the second cytotype: electron transparent vesicles (v) that merge at the apical extremity (arrow) giving rise to ample cavities between the microvilli (8900×). Abbreviations: n, nucleus with peripheral indentations.
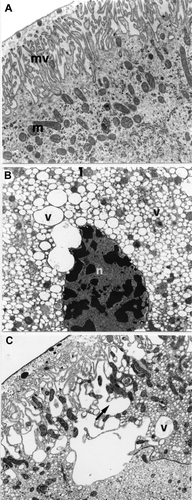
In phase b, instead, within the epithelium there are two types of cells, irregularly alternated with differentiated ultrastructural characteristics: the first type has structural aspects similar to those of the cells of the previous phase; the second cytotype, instead, is characterized by the presence of an irregular shaped nucleus, sometimes with marked peripheral indentations, containing large chromatin granules and a cytoplasm with numerous small electron‐transparent vesicles (Figure ). The latter extend to the apical extremity of the cells where they merge, opening among the microvilli, thus giving rise to the formation of large cavities that give this cellular region a ‘spongy’ appearance (Figure ).
In correspondence to stage 9, phase a, there are also two types of cells, as seen in the previous stage; the cells of the first type have nuclei, with peripheral indents, rich in chromatin granules (Figure ); in the ergastoplasmic areas there are numerous dilated cisterns containing, in their centre electron‐dense granules with irregular margins and electron‐transparent vesicles (Figure ). There are many lysosomes in these cells as was seen by the specific technique (Figure ) (Robinson & Karnowsky Citation1983), as well as lamellar bodies (Figure ). In the second cytotype the nucleus has large chromatin granules and the cytoplasm containing numerous small vesicles, with an electron‐transparent content.
Figure 7. A. thalassinus stage 9, phase a. Epithelial cells of first type. A, Nucleus (n) with peripheral indentations (2950×). B, Rough endoplasmic reticulum (er) with dilated cisternae containing an electron dense granule (f) and electron transparent vesicles (v; 21,000×). C, Lysosomes (l; 28,500×). D, Multilamellar body (mb; 28,500×).
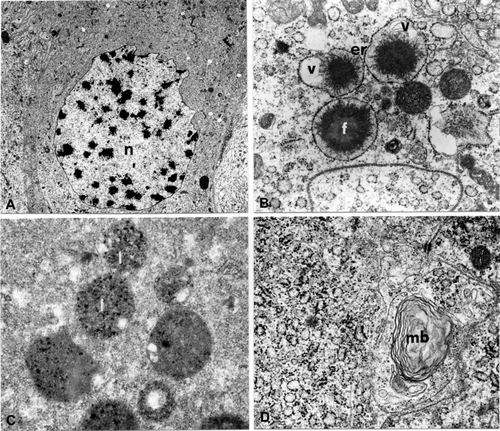
The observations carried out in correspondence to the basal region of the cells showed the presence of deep plasmatic membrane infolding and an increase in the number of mitochondria in the cells of the first type (Figure ); in the second cytotype the cytoplasm is rich in electron transparent vesicles (Figure ).
Figure 8. E. plorans stage 9, phase a. A, Basal region of first epithelial cells (15,500×). B, Basal region of second epithelial cells (15,500×). Abbreviations: bl, basal lamina; i, enfoldings of cell membrane; m, mitochondria; v, vesicles.
In phase b of this stage, all the cells show marked degenerative aspects: the nucleus is picnotic and the cytoplasm is rich in lysosomes and endocellular organelles that are not clearly defined. In proximity to the apex of the cells, furthermore, the spaces between the microvilli become more ample and numerous lamellar bodies are found in the apical region. Marked degenerative aspects are also found in the cells of the peduncle that are particularly flattened in this stage of development.
Discussion
From the results obtained, compared to those found in literature, it was seen that the pleuropodia of E. plorans, A. thalassinus and A. strepens have generally similar characteristics: in all the stages of development examined, these structures appear as trilobed extroflexions, whose dimensions increase during embryonal development reaching a maximum at stage 8. The pleuropodia are connected to the body of the embryo by means of a short peduncle, which tends to progressively reduce until it disappears in correspondence to stage 9 of development, as these structures are abandoned by the embryo at hatching.
In all the stages that we considered, the pleuropodia are always made up of a monolayer epithelium, of ectodermic origin, lying on a thin fibrillar basal lamina, and overlaid by a thin cuticular intima, whose structure, unlike that of the cuticle of the embryo, does not change during embryonal development.
In agreement with Slifer (Citation1938), Louvet and Marcel (Citation1969), and Louvet (Citation1973, Citation1975), the pleuropodia are organs having a glandular activity that progressively increases from stage 4 to stage 8. This is shown by the gradual increase of the cytoplasmic organelles involved in this process, among which the rough endoplasmic reticulum with its associated polyribosomes, and by the increase of the RNA content, during these embryonal stages, as seen in E. plorans (Viscuso et al. Citation1975). At the end of stage 8, in phase b of development, the degenerative processes of the pleuropodia begin to be evidenced by the presence of strongly vaculated cytoplasm of the cells and pyknotic nucleus, both by the presence of lysosomes and lamellar bodies in these cells; this aspect will involve all the cells of the pleuropodia at stage 9 of development and will also involve, at the same time, the cells of the peduncle such as to permit the detachment of these formations from the wall of the body of the animal.
A particular finding was that the elaborated secretion seemed to diversify in relationship to the stage of development considered: in all the species examined, in fact, in correspondence to stage 4, the secretory activity is prevalently for the formation of electron‐dense granules, which are then released by means of apical dilatations of the cells; from stage 6, instead, the secretory activity leads to the formation both of granules, morphologically similar to those of stage 4, and of vesicles, with a not very electron‐dense content, which are liberated among the microvilli; this process increases during the successive stages of development given rise to that more dilated cavities that give a highly irregular and spongy appearence to this region of the cells.
Even though the investigations carried out do not allow the definition of a specific role of the elaborated material, we believe that it is probable that it can be involved in the production of enzymes that are necessary for hatching, as was suggested by Slifer (Citation1937), Louvet (Citation1973) and Rost et al. (Citation2004).
In the light of these findings, however, we cannot exclude the possibility that the secretion can have diversified functions in relationship to the stage of development considered. It emerged from the observation of stage 4 of development that there is a secretion with a granular aspect and in the successive stages of development there is also a secretion with a vesicular aspect; only in correspondence to stage 4 of development is there a smooth endoplasmic reticulum in the cytoplasm of the pleuropodial cells, which was not seen in a significative way in the other stages of development considered. Stage 4 of development, from the structural point of view, is different with respect to the other stages examined; it thus appears logical to suppose that this diversity is also reflected in its function.
The constant presence of microvillus structures on the free surface of the cells, associated with endocytosis processes and to a large number of mitochondria, and of deep enfolding of the membrane in correspondence to the basal region always associated with numerous mitochondria, allows us to hypothesize that, other than glandular activity, the pleuropodia play an active role in the transport of liquids and ions. It is known, in fact, that this structural aspect is a typical characteristic of organs of which a osmoregulatory and excretion function has been shown, ie the malpighian tubules (Berridge & Oschman Citation1969; Taylor Citation1971) and the calyciform cells of the middle intestine of some Lepidoptera. This characteristic, also previously found in the pleuropodia of Carausius and Rhizotrogus (Louvet Citation1973, Citation1983), could represent a mechanism that transports the enzyme for hatching, not excluding, however, other possible roles inherent in the metabolism of the embryo, such as the exchange of material between the embryonal space and the hemocele.
It is difficult to interpret the presence of large cytoplasmic areas containing glycogen that also remain in the terminal stages of development when the cells of the pleuropodia show degenerative phenomena. Even if there are no data concerning this aspect, it cannot be excluded that the accumulation of this material in the species that we examined, already seen in the pleuropodial cells of Blabera craniifer (Bullière Citation1970), of Locusta migratoria (Louvet Citation1975) and of Rhizotrogus majalis razoum (Louvet Citation1983), could depend on a slowing down of the cellular metabolism following the progressive reduction of the peduncle.
A particular structural aspect is the presence of extracellular tubular structures, often associated with electron‐dense granules, similar to the cytoplasmic microtubules, at least in size, from which they could derive during secretory activity.
The presence of extracellular microtubules, also if already hypothesized in other cases (Heyn Citation1972; Kurz‐Isler & Wilburg Citation1978; Reuter et al. Citation1987), certainly represents an exceptional event; as regards insects, in particular, their presence has been found in the extracellular lumen of the testicle of Phagmatobia fuliginosa (Wolf Citation1993) and in the oviduct of Phasmida Baculum thaii (Viscuso et al. Citation1997). In the last case the tubular structures were associated with secretion granules and their presence was placed in relationship to the fragmentation of the same granules.
In this case, in particular, the structural situation appears similar that already seen for Baculum, it cannot thus be excluded that their presence could, in some way, be in relationship to the fragmentation of the granules in such a way as to facilitate their more rapid use; however, given the peculiarity of the finding and the few data in our possession, we believe that this subject needs further investigation.
References
- Ando , H. 1971 . Studies on the pleuropodia of an ovoviviparus cockroack, Opistoplatia orientalis Burmeister (Blattaria: Epilampridae). . Bulletin of the Sugadaira Biological Laboratory of Tokyo , 4 : 59 – 71 .
- Berridge , M. J. and Oschman , J. L. 1969 . A structural basis for fluid secretion by Malpighian tubules. . Tissue & Cell , 1 : 247 – 272 .
- Bullière , F. 1970 . L'évolution des pleuropodes au cours du développement embryonnaire de Blabera craniifer (Insecte, Dictyoptère). . Archives d'Anatomie Microscopique et de Morphologie Expérimentale , 59 : 201 – 220 .
- De Luca , V. and Viscuso , R. 1972 . Morfologia dell'embrione di Eyprepocnemis plorans (Charp.) (Orth. Acrid.) nei vari stadi di sviluppo. . Redia , 53 : 123 – 138 .
- Engelmann , F. 1957 . Bau und Funktion des weiblichen Geschlechtsapparates bei der ovoviviparen Schabe Leucophaea maderae (Fabr.) (Orthoptera) und einige Beobachtungen über die Entwicklung. . Biologisches Zentralblatt , 76 : 722 – 740 .
- Hagan , H. R. 1951 . Embryology of the viviparous Insects , New York : Ronald Press co .
- Heyn , A. N. J. 1972 . Intra‐ and extracytoplasmic microtubules in coleoptiles of Avena. . Journal of Ultrastructure Research , 40 : 433 – 457 .
- Kurz‐Isler , G. and Wilburg , H. 1978 . Extracellular microtubule‐like structures in the retina of the rainbow trout. Development, intercellular connectivity and reaction to vincristine. . Cell & Tissue Research , 191 : 15 – 26 .
- Lambiase , S. , Grigolo , A. and Morbini , P. 2003 . Ontogenesis of pleuropodia in different species of Blattaria (Insecta): A comparative study. . Italian Journal of Zoology , 70 : 205 – 212 .
- Louvet , J. P. 1973 . L'ultrastructure du pleuropode et son ontogenèse, chez l'embryon du phasme Carausius morosus Br. I. Etude du pleuropode de l'embryon âgé. . Annales des Sciences Naturelles – Zoologie et Biologie Animale , 15 : 525 – 594 .
- Louvet , J. P. 1975 . Premières observations sur l'ultrastructure du pleuropode chez le criquet migrateur. . Comptes Rendus de l'Académie des Sciences Paris , 280D : 1301 – 1304 .
- Louvet , J. P. 1983 . Ultrastructure du pleuropode chez l'embryon du Hanneton Rhizotrogus majalis razoum (Coleoptera: Melolonthidae). . International Journal of Insect Morphology & Embryology , 12 : 97 – 117 .
- Louvet , J. P. and Marcel , S. 1969 . Etude infrastructurale du pleuropode chez l'embryon du Phasme Carausius morsus Br. . Comptes Rendus de l'Académie des Sciences Paris , 269D : 351 – 354 .
- Nation , J. L. 1983 . A new method using hexamethyldisilazane for preparation of soft insect tissues for scanning electron microscopy. . Stain Technology , 58 : 347 – 351 .
- Reuter , P. , Dorn , A. , Batel , R. , Schröder , H. C. and Müller , W. E. G. 1987 . Evidence for the existence of microtubule protein in the extracellular space of marine sponges. . Tissue & Cell , 19 : 773 – 782 .
- Reynolds , E. S. 1963 . The use of lead citrate at high pH as an electron opaque stain in electron microscopy. . Journal of Cell Biology , 17 : 208 – 212 .
- Robinson , J. M. and Karnowsky , M. J. 1983 . Ultrastructural localization of several phosphatases with cerium. . Journal of Histochemistry & Cytochemistry , 31 : 1197 – 1208 .
- Rost , M. M. , Poprawa , I. and Klag , J. 2004 . Ultrastructure of the Pleuropodium in 8‐d‐old Embryos of Thermobia domestica (Packard) (Insecta, Zygentoma). . Annals of the Entomological Society of America , 97 : 541 – 547 .
- Slifer , E. H. 1937 . The origin and fate of the membranes surrounding the grasshopper egg: Together with some experiments on the source of the hatching enzyme. . Quarterly Journal of Microscopical Science , 79 : 493 – 506 .
- Slifer , E. H. 1938 . A cytological study of the pleuropodia of Melanoplus differentialis (Orthoptera, Acrididae) which furnishes new evidence that they produce the hatching enzyme. . Journal of Morphology , 63 : 181 – 206 .
- Taylor , H. H. 1971 . Water and solute transport by the malpighian tubules of the stick‐insect, Carausius morosus. The normal ultrastructure of the type 1 cells. . Zoologische Zellforsch , 118 : 333 – 368 .
- Viscuso , R. , De Luca , V. , Vanella , A. and Cozzolino , A. 1975 . Osservazioni sui pleuropodi di Eyprepocnemis plorans (Charp.) (Orth Acrid.). I. Dati morfologici e biochimici. . Redia , 56 : 1 – 12 .
- Viscuso , R. and Germenia , G. 1979 . Morfologia dei pleuropodi dell'embrione di Eyprepocnemis plorans (Charp.) (Orth. Acrid.) nei vari stadi di sviluppo. . Bollettino di Zoologia , 46 (suppl.) : 305
- Viscuso , R. , Narcisi , L. , Sottile , L. and Giuffrida , A. 1997 . Secretory product of the lateral oviducts of Baculum thaii Haus. (Phasmida: Phasmatidae) and its change during egg transit. . International Journal of Insect Morphology & Embryology , 25 : 369 – 379 .
- Wheeler , W. M. 1890 . On the appendages of the first abdominal segment of embryo insects. . Transactions of the Wisconsin Academy of Sciences, Arts and Letters , 8 : 87 – 140 .
- Wolf , K. W. 1993 . Extracellular tubular elements within testes of Phragmatobia fuliginosa L. (Arctiidae: Lepidoptera). . Invertebrate Development , 24 : 137 – 142 .