Abstract
A new species of mesopsammic polyclad, Theama mediterranea sp. nov., from lower intertidal habitats of the Mediterranean Sea, is described. The species differs from congeneres found in the tropical Pacific and the Atlantic coast of South America, in terms of size, structure of the male copulatory organ and number of eyes. T. mediterranea sp. nov. has a short pelagic juvenile phase, while adults are strictly interstitial; the life cycle is annual. Based on observation of the type material of Theama evelinae Marcus, Citation1949, the genus Eutheama Faubel, Citation1983 is synonymized with Theama Marcus, Citation1949.
Introduction
Polyclads (Platyhelminthes: Polycladida) are best known for their epibenthic representatives, which can be large and brightly pigmented (see, inter alia, Newman & Cannon Citation2003). Members of the order may, however, also occur in sandy interstitial habitats, mostly as juveniles. On the contrary, very few fully interstitial polyclads are known. Exploitation by polyclads of the mesopsammic niche has been reported so far only for tropical habitats (see, for example, Sopott‐Ehlers & Schmidt Citation1975). Members of the family Theamatidae Marcus, Citation1949 are among the most characteristic mesopsammic polyclads and show marked adaptations to an interstitial life (such as reduced size, very elongate body, lack of tentacles; Bulnes & Faubel Citation2003). The few species of the family are known from the Atlantic (Brazil) and the Pacific oceans (Galapagos; northern Australia; Marcus Citation1949; Sopott‐Ehlers & Schmidt Citation1975; Bulnes & Faubel Citation2003). Research in intertidal habitats of the Mediterranean has revealed the presence of an abundant and widespread representative of the Theamatidae, which is new to science.
Materials and methods
Specimens were collected from sandy habitats by scooping the superficial layer of sediment. Extraction of the animals from the sediment was through MgCl2 decantation (see Martens Citation1984). For microscopic analysis, specimens were fixed in Bouin's fluid, embedded in 56° paraplast, and serial sections were cut at 4 μm, stained with Mayer's haematoxylin and eosin, and mounted in Depex. Whole mounts were prepared with Faure, lactophenol, or stained with paracarmine (after Mazzi Citation1977), and mounted in Eukitt. In the species description, single measurements are based on the holotype, while the range given is based on ten sectioned specimens, from various localities.
Karyological techniques were as described by Curini‐Galletti et al. (Citation1989). Relative lengths (r.l. = length of chromosome×100/total length of haploid genome) and centromeric indices (c.i. = length of short arm×100/length of entire chromosome) were obtained from measurements of camera lucida drawings of five metaphase plates, obtained from juvenile specimens. Karyometrical data (means and standard deviation) are presented in the karyotype formula as follows: haploid genome absolute length (in µm); relative length and centromeric index of each chromosome; chromosome nomenclature between parentheses (m = metacentric; sm = submetacentric).
The original material of Theama evelinae Marcus, Citation1949 was loaned by the Swedish Museum of Natural History (SMNH), where the type material of the new species is deposited. Paratypes of Theama occidua Sopott‐Ehlers and Schmidt, Citation1975 were loaned by the Anthropology and Zoological Museum, Göttingen (AZM). Voucher material of the new species is stored in the Collections of the Zoological Museum of the Dipartimento di Zoologia e Genetica Evoluzionistica, University of Sassari (Italy) (CZMSS).
Abbreviations used in figures
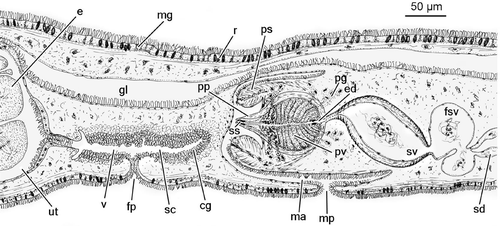
cg, cement glands; e, egg; ed, ejaculatory duct; fp, female pore; fsv, false seminal vesicle; gl, gut lumen; m, mouth; ma, male antrum; mg, mucous gland; mp, male pore; pb, penis bulb; pg, prostate glands; pp, penis papilla; ps, penis sheath; pv, prostate vesicle; r, rhabdite; ss, sclerotized sheath; sc, “shell chamber”; sd, sperm duct; sv, seminal vesicle; ut, uterus; v, vagina.
Results
Order POLYCLADIDA Lang, 1884
Suborder ACOTYLEA Lang, 1884
Family Theamatidae Marcus, Citation1949
Genus Theama Marcus, Citation1949
Theama mediterranea sp. nov.
(Figures , )
Type material
Holotype: a specimen sagittally sectioned, mounted on three slides (SMNH‐6659); Valledoria, (northern Sardinia, Italy), loc. La Ciaccia (40°54′57″ N, 8°46′03″ E), lower intertidal in coarse sand, leg. 30 March 1996. Paratype: sagittally sectioned, mounted on two slides (SMNH‐6660), Costa Paradiso (northern Sardinia, Italy), loc. Li Cossi (41°04′58″ N, 8°58′48″ E), lower intertidal in coarse sand, leg. 30 March 1996.
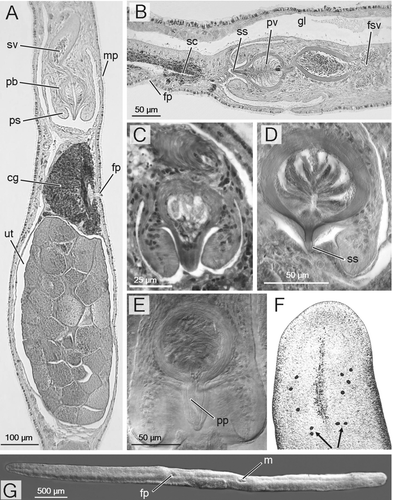
Figure 1. A, B, F, G,Theama mediterranea sp. nov. A, sagittal section of holotype (SMNH‐6659), ventral side to the right; B, paratype (SMNH‐6660); F, head of a living specimen, Le Brusc, France; arrows point to the cerebral eyes (photo courtesy of B. Sopott Ehlers); G, SEM picture of an adult specimen from the type locality, ventral view; head to the right. C, Theama occidua: paratype AZM‐P378, copulatory organ. D, E, Theama evelinae, copulatory organ of SMNH‐5076 b, sagittal section (D) and SMNH‐5076 e, whole mount (E).
Additional material: same data as holotype: seven specimens sagittally sectioned, and mounted, respectively, on one slide (CZMSS‐30); two slides (CZMSS‐31,32,33); three slides (CZMSS‐34, 35); four slides (CZMSS‐36); three whole mounts (Paracarmine: CZMSS‐37,38; Faure: CZMSS‐39); two specimens used for karyology, one specimen processed for SEM. Same data as paratype: one specimen sagittally sectioned, mounted on two slides (CZMSS‐40).
Italy: Capo Caccia, Dragunara cove, north‐western Sardinia, two specimens sagittally sectioned and mounted respectively on two slides (CZMSS‐41) and three slides (CZMSS‐42), leg. April 12, 1996; Porto Cesareo (Apulia): one specimen sagittally sectioned, mounted on two slides (CZMSS‐43), leg. 28 June 2005.
Greece: Gouvià (Corfu Is.), a whole mount (lactophenol) (CZMSS‐44), leg. April 1993; Piraeus, loc Glyfada, one specimen sagittally sectioned, mounted on the same slide as the previous, leg. April 1993.
Israel: Akko, one specimen sagittally sectioned and mounted on one slide (CZMSS‐45); two specimens used for karyology, leg. October 1997.
Specimens, attributed to the new species on the basis of general morphology as observed in semi‐squashed living specimens, were found in the following localities:
France: Le Brusc (September 1989; Sopott‐Ehlers, personal communication).
Italy: Sardinia: Porto Torres (April 1998); Tuscany: Castiglione della Pescaia (May 2006), Castiglioncello (May 2006); Latium: Santa Marinella (March 1992); Sicily: Giardini Naxos (September 2004); Friuli: Miramare, Trieste (July 2006).
Croazia: Omis (June, 2001); Cres (July, 2006).
Greece: Francocastelo, Crete (April 1990); Nea Mechaniona (May 1987); Cassandra peninsula (February 1991).
Israel: Shiqmona, Haifa (April 1988), Atlit (April, 1988); Caesarea (April 1988).
In all instances, specimens came from coarse‐grained (coarse sand to gravel), lower intertidal areas.
Comparative material examined
Theama occidua Sopott‐Ehlers & Schmidt, Citation1975: five paratypes, sagittal sections: AZM‐P372; P377–379, from James Bay, Galapagos Is.; AZM‐P373; from Caleta Black, Galapagos Is.
Theama evelinae Marcus Citation1949, original material. SMNH‐5076 a, c, d, e: whole mounts; SMNH‐5076 b: sagittally sectioned; all from Ilha do Sâo Sebastiao; Ilhabela (Sâo Paulo, Brazil), leg. E Marcus.
Etymology
The specific epithet is coined after the geographical area of distribution
Diagnosis
Theama with few marginal eyes (2–4 per side). With a pyriform penis bulb (97–115 µm long; 53–85 µm thick) provided with a 22–60 µm long penis papilla, and with a sclerotized inner lining of the distalmost portion of the ejaculatory duct. The penis bulb is surrounded by a tube‐shaped penis sheath, that is 95–130 µm long and 85–140 µm thick. A comparatively large uterus contains up to 35 mature oocytes in the median section.
Description
Living worms appear greyish, due to gut content and internal organs. When crawling, an adult specimen may extend to 15–16 mm in length, and to about 2.5 mm in width. The holotype, a fixed, contracted specimen, is about 8 mm long. The body is extremely flattened and the cephalic tentacles absent. The frontal end is obtusely truncated in large specimens, the distal end is more acutely pointed (Figure ). In juveniles, both ends appear roundish.
With two cerebral eyes at each side of the brain. Members of the pair lie very close to each other, and point in different directions. The marginal eyes form two irregular rows in front of the brain, ranging from 2 to 4 at each side of the head (Figure ).
Epidermis ciliated; cilia about 10 µm long, slightly longer dorsally than ventrally. Dorsally, the epidermis is columnar, with numerous, elongated rhabdites, their longest axis measuring 8–10 µm, and provided with mucous glands. The ventral epithelium is more cubical, with fewer and narrower rhabdites (about 6 µm long) and fewer mucous glands. Epidermal cells overlie a thick basement membrane. A thin layer of circular muscle fibres lies immediately beneath the basement membrane; the inner subepidermal longitudinal musculature is very strongly developed ventrally, weaker dorsally.
The ruffled, non‐ciliated pharynx lies in the mid‐body. It is about 700 µm wide, and is held vertically. The pharyngeal chamber is lined with a flat, non‐ciliated epithelium. The mouth lies ventrally, and opens at a position slightly anterior to the centre of the body. The main gut is median, and extends forwards to the cerebral area, and backwards to the level of the ovaries, with numerous lateral, comparatively very short, branches throughout its length. The gastrodermis is partly ciliated, particularly in its ventral side (Figure ).
Male genital organs
The numerous follicular testes are placed dorsally and laterally, and form two lateral rows from just behind the brain to the level of the seminal vesicle. Each row consists of up to 80 testes, about 1/5 of which lie posterior to the pharynx. Two vasa deferentia run ventrally and to the posterior from the testes. Distally, each duct forms a large, very convoluted false seminal vesicle, with thin walls. These false seminal vesicles fuse distally into a wide, transverse sperm reservoir, which opens into the proximal area of the (true) seminal vesicle directly through a broad pore, or, in a few specimens, through a narrower canal, 20–40 µm long. The seminal vesicle is ovoid (130 µm long; 50 µm high) (ranging 80–185 µm; 50–80 µm, respectively). In most specimens, its major axis is tilted at a 45° angle from the horizontal plane. The seminal vesicle is lined with a flat, nucleated epithelium, and surrounded by a very strong coat of muscle fibres, about 12 µm thick (range 10–20 µm), with outer circular and inner longitudinal muscles. Distally, the seminal vesicle opens into an ovoid prostatic vesicle, through a 20–30 µm long ejaculatory duct which is lined with a glandular epithelium, surrounded by a thin coat of muscular fibres.
The inner lining of the prostatic vesicle is formed by a tall, glandular epithelium, provided with radial folds. Two distinct kinds of gland are present, producing a fine granular secretion in the proximal 2/3, and a much coarser, distinctly eosinophilous secretion, in the distal third. Nuclei of at least a few of the prostatic glands are located outside the vesicle, and their ducts pierce the strong muscular wall (10–12 µm thick; range 7–20 µm) which surrounds the prostatic vesicle and forms the penis bulb. This bulb is pyriform, 107 µm long and 70 µm thick (range 97–115 µm and 53–85 µm, respectively), including a conical penis papilla, which protrudes into the male antrum for about 30 µm (range 22–60 µm).
The inner, most distal, portion of the ejaculatory duct within the penis papilla is lined with a sclerotized, cylindrical sheath, up to 1.5 µm thick and 12 µm long in the holotype (ranging 10–20 µm). The degree of sclerotization varies amongst the specimens examined, and in cases is markedly asymmetrical between the ventral and the dorsal side of the duct. The penis papilla is provided with an outer sheath of longitudinal musculature, and with an inner, much thinner layer of circular fibres. The longitudinal component disappears at the distal tip of the papilla, which is thus thinly lined with a flat epithelium and a few circular fibres. In quite a few specimens, the penis papilla appears distorted, presumably due to contraction of the musculature during fixation.
The penis bulb is housed within a tube‐shaped penis sheath, that is 114 µm long and 110 µm thick (ranging 95–130 µm and 85–140 µm). The sheath is fused with the penis bulb for about 2/3 of its length; the non‐fused, cylindrical distal rim surrounds the penis papilla. The penis sheath is ciliated on its outer side, and pierced by a few eosinophilous glands which discharge their contents at its tip. The sheath, and in particular its distal portion, is very muscular, with a strong radial component. A few longitudinal fibres of the sheath are attached to the basis of the penis bulb.
The male antrum is lined with a flat epithelium, non‐ciliated apart from the area close to the male pore, which lies about 1.2 mm posterior to the mouth.
Female genital organs
The ovary is located in the posterior third of the body and extends to near the posterior end of the body. The oviducts run laterally and from the anterior; at the level of the female gonopore, they bend sharply towards the median axis of the body, and enter the anterior end of an elongate uterine vesicle. The size of the uterus varies greatly amongst the specimens examined, depending on the degree of maturity and the number of oocytes contained. In mature specimens, the uterus, filled with eggs, protrudes from the body surface as a distinct bulge. The largest uterus to be measured, up to 550 µm long and 250 µm high, with up to 35 oocytes in the median sagittal section, belongs to the holotype (Figure ). The smallest observed uterus was 100 µm long and 145 µm high, with nine oocytes in the median section. The uterus is lined with a non‐ciliated epithelium, except for a small ciliated area close to the vagina. This latter runs anteriorly and ventrally and opens to the exterior through the female pore, located 300 µm to the posterior of the male pore. At least both the most proximal and distal portions of the vagina are ciliated; central areas appear to be unciliated. The vagina shows at least one distinct branch, which may act as a shell chamber. However, the massive presence of cement glands, which surround the vagina throughout its length, impedes more detailed observation.
Karyology
Chromosome number: 2n = 20. Most chromosomes are metacentric, slightly differing in size: the smallest pair of the set is about half the length of the largest. Karyotype formula: 14±0.1; Chrom. I: 13.31±0.74; 46.33±2.78 (m); Chrom. II: 12.22±0.57; 43.56±3.59 (m); Chrom. III: 11.2±0.9; 34.58±1.61 (sm); Chrom. IV: 10.98±0.68; 47.41±1.65 (m); Chrom. V: 10.6±0.61; 44.71±2.73 (m); Chrom. VI: 9.82±0.9; 36.01±3.10 (sm); Chrom. VII: 9.24±0.44; 39.97±2.66 (m); Chrom. VIII: 8.23±0.83; 45.48±2.09 (m); Chrom. IX: 7.82±1.11; 29.55±2.77 (sm); Chrom. X: 6.98±0.65; 39.17±4.13 (m).
Notes
Specimens of the new species were commonly, in cases abundantly, found throughout the central and eastern Mediterranean, in coarse grained, lower intertidal sediments. Conversely, extensive sampling performed in recent years by the senior author in southern Spain (Alboran Sea) and the neighbouring Atlantic Ocean (north to Galicia) failed to reveal the presence of the species, which may thus be considered a Mediterranean endemism.
The life cycle of the new species is presumably annual: mature specimens were only found in late spring to early summer (March–July); by September, only juveniles were found.
Specimens kept in the laboratory showed no attempt to feed on any of the species found in the same sediments or on crushed mussels, and all died within a few days. Prior to their decease, mature specimens collected at the end of April 1999 laid numerous cup‐shaped cocoons. These cocoons were attached to valves of the mussel Mytilaster minimus (Poli, 1795), and contained 12–20 eggs. A week after deposition, juveniles began to emerge; these were ovoid and flattened, about 140 µm long, with two long sensory cilia arranged symmetrically at the anterior and posterior end of the body, one median, anterior eye, and a large mouth in the ventral median line behind the middle of the body. Newly hatched juveniles were agile, and spent most of their time swimming at the surface. After a few days, they were mostly seen crawling along the bottom. Attempts to feed the juveniles with commercial food for planktivores, mesopsammic copepods, and nauplii of Artemia salina Leach, 1819, were unsuccessful, and all died after a week at most.
Discussion
The family Theamatidae Marcus, Citation1949 includes interstitial acotylean polyclads, with long, thread‐like bodies, without tentacles, and provided with a short, nearly median pharynx. The male reproductive system is characterized by a strongly muscular seminal vesicle and a posteriorly oriented penis bulb, which contains the interpolated, radially chambered prostatic vesicle, and is surrounded by a penis sheath. The female system is posterior, without a Lang's vesicle, but with an uterine sac leading to the short vagina (Faubel Citation1983; Prudhoe Citation1985).
The family is comprised of two genera:
Theama Marcus, Citation1949, with a sclerotized stylet tipping the penis papilla. Type and only species: Theama evelinae Marcus, Citation1949; | |||||
Eutheama Faubel, Citation1983, with an unarmed penis papilla. Type species: Eutheama occidua (Sopott‐Ehlers & Schmidt, Citation1975). Other species: Eutheama forrestensis Bulnes & Faubel, Citation2003. | |||||
Faubel (Citation1983) introduced a third genus, Dicteros Jacubowa, Citation1906, to the Theamatidae. However, there is no consensus on the taxonomic placement of the genus. Prudhoe (Citation1985) and Cannon (Citation1986), due to the presence of a ventral sucker and of marginal tentacles (Jacubowa Citation1906), place Dicteros in the Cotylea Lang, 1884, close to the genus Pseudoceros Lang, 1884, from which it mainly differs for the lack of the prostatic organ.
Apart from the obvious differences with the Dicteros species, the generic placement of the new species is nonetheless problematic. According to present genus diagnoses, the new species, which is not provided with an external stylet, should pertain to the genus Eutheama. However, examination of the original material of T. evelinae, stored at the SMNH, casts doubts on the tenets of the present taxonomy of the family. The material available for T. evelinae, the type of which was never designated (cf. Marcus Citation1949), includes four whole mounts and a single sectioned specimen (SMNH‐5076 b). This latter corresponds, down to the finest details, to the sagittal reconstruction drawn by Marcus (Citation1949: fig. 106), and could be safely assumed to be the specimen on which the species description was largely based. It is therefore unfortunate that the poor fixation and sectioning of this specimen has resulted in the damage and removal of portions of the epithelial lining, both of the body surface and of the male antrum. Indeed, what Marcus interpreted as a stylet in fact corresponds to a sclerotized inner lining of the ejaculatory duct, with the outer tissues of the penis papilla that are badly preserved and in some areas clearly detached (Figure ). The overall poor quality of the mount is reflected by the position of the male pore, which Marcus (Citation1949) described as located at the extreme anterior area of the male antrum. In reality, this is the place of a breach of the thin tissue layers surrounding the antrum, and the real pore, which is more posterior, can be (albeit with some difficulty) seen in the following sections. On the other hand, in the whole mounts (i.e. SMNH‐5076e, Figure ), by means of interferential contrast, it is easy to appreciate the absence of any external stylet, as well as the presence of a tissue sheath lining the entire length of the penis papilla, similarly to the situation described above for T. mediterranea sp. nov. The presence of an external stylet topping the penis papilla is thus to be excluded for T. evelinae. Furthermore, observations on the type species of the genus Eutheama, E. occidua, has also revealed the presence of a small sclerotized area of the inner ejaculatory duct. On these bases, we consider the genus Eutheama as an objective junior synonym of Theama, the definition of which should include the statement ‘with penis papilla unarmed; with or without a sclerotized inner portion of the ejaculatory duct’. The species formerly attributed to Eutheama are hence transferred to Theama, which thus includes four species: T. evelinae (type species), T. occidua, T. forrestensis, and T. mediterranea sp. nov.
The new species is easily distinguished from the eastern Australian T. forrestensis (Bulnes & Faubel Citation2003), as the latter is a very minute species (the holotype, a fixed mature specimen, is 1 mm long), characterized by the presence of a common (male+female) genital pore. The species is described without any sclerotization of the inner lining of the ejaculatory duct, and the penis papilla, which is bulky and massively muscular, is indeed quite different from that of the other species of the genus, in which it is far more slender and elongate. Furthermore, due to its minute size, the production of eggs is limited, and the uteri of all specimens studied contained at most one single egg (Bulnes & Faubel, Citation2003).
The other three species are similar in general morphology. T. evelinae, described from southern Brazil (Marcus Citation1949), has a copulatory organ that is 95–110 µm long and 75–85 µm thick, and thus comparable in size to the new species. However, its prostate vesicle is more rounded, and the penis papilla markedly pointed, with a more evident sclerotized inner lining (Figure ). The unfused, distal rim of the penis sheath of T. evelinae shows an evident inner, angular fold, which is absent in T. mediterranea n. sp. Furthermore, T. evelinae is a smaller species, averaging 5–7 mm in length, and its marginal eyes are more numerous, ranging 4–7 per side (Marcus Citation1949). T. occidua, from the Galapagos Is., has a smaller copulatory organ, 61–77 µm long and 41–51 µm thick. The fusion between the muscular lining of the prostate vesicle and the penis sheath is more proximal than in the other species of the genus (Figure ). This results in a comparatively longer and more mobile penis papilla, the appearance of which varies strongly with fixation. The penis sheath as a whole is smaller, being 43–58 µm long and 60–65 µm thick. Furthermore, in all specimens of T. occidua examined, dorsal cilia were shorter than ventral cilia, to the contrary of the new species.
Information on the karyology of polyclads is limited, and present data are the first on a species of the family Theamatidae. In the Acotylea, the haploid chromosome numbers range from 8 to 10, with most species showing n = 10, and isobrachial chromosomes (Galleni & Puccinelli Citation1984, Citation1985). Karyotypes with n = 10, with mostly meta‐submetacentric chromosomes, are also found in the Cotylea (Galleni & Puccinelli Citation1981; Curini‐Galletti & Campus Citation2007). The karyotype of T. mediterranea thus confirms the prevalence of sets with n = 10 and isobrachial chromosomes in the Polycladida, which appear particularly conservative with this regard.
Assessment of the phylogenetical relationships of the new species has yet to be attempted. In fact, not only are very few morphological characters available for phylogenetical analysis at present in the family, but the existence of two, often conflicting, taxonomical systems of the Polycladida (Faubel Citation1983, Citation1984; Prudhoe Citation1985), which place the family Theamatidae in two different superfamilies (Leptoplanoidea Faubel, Citation1984 and Planoceroidea Poche, 1925, respectively), makes the mere choice of an outgroup problematical.
Finally, it is somehow surprising that such an abundant, widespread, predatory species, extremely large for a mesopsammic organism, has not already been described. This fact clearly testifies to the poor state of our knowledge of interstitial marine organisms in general, and of their ecological role and overall contribution to marine biodiversity.
Acknowledgements
Gratitude is due to Dr. Bella Galil National (Institute of Oceanography, Shiqmona), Prof. Dr. Eviatar Nevo and the staff of the Institute of Evolution (University of Haifa) for their generous hospitality, and the use of facilities at their laboratories. Gavino Oggiano and Francesco Mura dealt with most of the histological preparation. Ulrich and Beate Ehlers are thanked for loaning the paratypes of Theama occidua. A particular mention is due to the staff of the Swedish Museum of Natural History, who patiently extended the loan of reference and type material well beyond any reasonable time. This research project benefited from a grant from the Italian Ministry for Research (MIUR Prin‐2004).
References
- Bulnes , V. N. and Faubel , A. 2003 . Eutheama forrestensis n.sp. (Acotylea, Polycladida, Plathelminthes) from Australia. . Zootaxa , 220 : 1 – 8 .
- Cannon , L. R. G. 1986 . Turbellaria of the world. A guide to families and genera , Brisbane : Queensland Museum .
- Curini‐Galletti , M. and Campus , P. 2007 . Boninia neotethydis n. sp. (Platyhelminthes: Polycladida: Cotylea)—the first lessepsian flatworm. . Journal of the Marine Biological Association of the United Kingdom , 87 : 1 – 8 .
- Curini‐Galletti , M. , Puccinelli , I. and Martens , P. M. 1989 . Karyometrical analysis of ten species of the subfamily Monocelidinae (Proseriata, Platyhelminthes) with remarks on the karyological evolution of the Monocelididae. . Genetica , 78 : 169 – 178 .
- Faubel , A. 1983 . The Polycladida, Turbellaria. Proposal and establishment of a new system. Part I. The Acotylea. . Mitteilungen des hamburgischen zoologischen Museums und Instituts , 80 : 17 – 121 .
- Faubel , A. 1984 . The Polycladida, Turbellaria. Proposal and establishment of a new system. Part II. The Cotylea. . Mitteilungen des hamburgischen zoologischen Museums und Instituts , 81 : 189 – 259 .
- Galleni , L. and Puccinelli , I. 1981 . Karyological observations on Polyclads. . Hydrobiologia , 84 : 31 – 44 .
- Galleni , L. and Puccinelli , I. 1984 . Karyology of five species of Turbellaria from the Øresund, Denmark. . Ophelia , 23 : 141 – 148 .
- Galleni , L. and Puccinelli , I. 1985 . Karyology of Yungia aurantiaca (Turbellaria: Polycladida). . Transactions of the American Microscopic Society , 104 : 122 – 128 .
- Jacubowa , L. 1906 . Polycladen von Neu‐Britannien und Neu‐Caledonien. Jenaische Zeitschrift für die gesammten . Naturwissenschaften , 41 : 113 – 158 .
- Marcus , E. 1949 . Turbellaria Brasileiros (7). . Boletins da Faculdade de Filosofia, Ciências e Letras, Universidade de Sâo Paulo, Zoologia , 14 : 7 – 155 .
- Martens , P. M. 1984 . Comparison of three different extraction methods for Turbellaria. . Marine Ecology Progress Series , 14 : 229 – 234 .
- Mazzi , V. 1977 . Manuale di Tecniche Istologiche e Istochimiche , Padova : Piccin .
- Newman , L. and Cannon , L. R. G. 2003 . Marine flatworms: the world of Polyclads , Collingwood, Victoria : CSIRO publishing .
- Prudhoe , S. 1985 . A monograph on polyclad Turbellaria , Oxford : British Museum (Natural History), Oxford University Press .
- Sopott‐Ehlers , B. and Schmidt , P. 1975 . Interstitielle Fauna von Galapagos XIV. Polycladida (Turbellaria). . Mikrofauna des Meeresbodens , 54 : 193 – 222 .