Abstract
Y‐box proteins are a highly conserved family of gene expression regulatory factors. During gametogenesis they may play a dual role, as both transcriptional activators of germ cell‐specific genes and as translational repressors of stored maternal transcripts. We report the identification of RlYB2, a Y‐box homolog gene specifically expressed in the germ cells of green frogs belonging to Rana esculenta complex, a model system characterized by a hybridogenetic gametogenesis. In developing germ cells of the hybrid R. esculenta, arisen by natural cross of the parental species R. ridibunda and R. lessonae, one set of the parental genomes is excluded and the remaining one, first endoreduplicates and then is transmitted to gametes. In situ hybridizations performed on gonadal tissues showed that the RlYB2 transcript was widely expressed in the ooplasm at early stages of oogenesis in both the parental species and hybrids. Interestingly, a hybridization signal, presumably related to RlYB2‐like nascent transcripts, was observed in nuclei of stage II oocytes. The presence of RlYB2 mRNA during early oogenesis suggests that this gene may be involved in regulating the transcription and/or translation of maternal mRNAs in this special vertebrate model system.
Introduction
In amphibians, large amounts of RNA molecules are synthesized during oogenesis. Many of these maternal mRNAs are stored in oocytes in a translationally masked form until the appropriate stage of development. Y‐box proteins are a family of regulatory proteins that are demonstrated to play a key role in both transcriptional and translational control in germ cells of Vertebrates and invertebrates (Wolffe Citation1994). All members of this family contain a nucleic acid binding cold shock domain (CSD), which is well conserved between prokaryotic and eukaryotic organisms (Sommerville Citation1999). During Drosophila oogenesis, mRNAs that are transported from nurse cells to the oocyte, are kept translationally silent in messenger ribonucleoproteins (mRNPs) by the Y‐box protein Yps (Ypsilon Schachtel) (Mansfield et al. Citation2002). Xenopus FRGY2 was originally identified as an oocyte‐specific transcription factor that associates with the regulatory element Y‐box found in the promoters of genes selectively actives in oocytes (Tafuri & Wolffe Citation1990; Sommerville Citation1992; Wolffe et al. Citation1992). More recently it has been proposed that FRGY2 cooperates with the DEAD‐box helicase Xp54 in translational silencing of mRNAs accumulated in mRNP particles (Nashchekin et al. Citation2006). MSY2, the murine homologue of FRGY2, is required for both transcription of testis‐specific genes and translational repression of paternal mRNAs (Yang et al. Citation2007). MSY2 is also expressed in oocytes, wherein it contributes to stabilization and translational regulation of maternal mRNAs during early embryonic stages (Gu et al. Citation1998; Yu et al. Citation2002). The absence of the DNA/RNA‐binding protein MSY2 results in male and female infertility (Yang et al. Citation2006).
In this study we used the western group of Palaearctic green frogs comprising Rana ridibunda, R. lessonae and the natural hybrid R. esculenta (Graf & Polls Pelaz Citation1989) as a model system. In the widespread population system, the hybridogenetic interspecific R. esculenta hybrid shows germ line‐specific loss of R. lessonae genome and produce haploid gametes that contain an intact R. ridibunda genome. The hybrid condition is recovered at the next generation by backcrossing with the parental species whose genome was eliminated (Tunner & Heppich‐Tunner Citation1991). In spite of the molecular mechanisms of hybridogenesis is still unknown, cytogenetic studies and morphological inspections demonstrated the presence of alterations in the gametogenesis of the hybridogenetic frogs (Bucci et al. Citation1990; Ogielska Citation1990). In addition, a delay in sexual maturation was observed in R. esculenta when compared to the parental species (Ogielska & Wagner Citation1993). In the present work, we identified in R. lessonae a germ cell‐specific Y‐box homolog gene, named RlYB2, and we analyzed its expression pattern during gametogenesis of both the parental species and the hybrid.
Materials and methods
Animals
Adults of R. ridibunda, R. lessonae and R. esculenta used in this study were collected (from wild mixed L‐E and R‐E populations) in the nearby of Wroclaw (Poland). The taxonomic status of each individual has been determined by morphometric and molecular analyses.
Preparation of germinal and somatic tissues
Both ovaries containing oocytes at different stages of development (Ogielska & Kotusz Citation2004) and somatic tissues were obtained from adult females, MS22‐anaestethized, of R. ridibunda, R. lessonae and R. esculenta. To obtain individual defolliculated oocytes, the ovarian tissue was incubated in 0.2% collagenase (type II, Sigma) in 0.1 M sodium phosphate pH 7.4.
Isolation of RlYB2
A partial cDNA clone was identified by RT‐PCR strategy from the total RNA extracted from ovary tissue of R. lessonae adult specimens. Degenerate primers (sense strand 5′‐GGWACWGTNAARTGGTTT‐3′, antisense strand‐1 5′‐AGTNACRTTNGCNGCYTC‐3′, antisense strand‐2 5′‐GTTRGCNAARTGYCGRCGC‐3′) were designed from the conserved cold shock domain (CSD) belonging to Y‐Box proteins and used in seminested reactions. PCR conditions were: 30 cycles at 94°C for 30 s, 47°C for 1 min, 72°C for 30 s. To obtain a complete sequence, 5′ and 3′ RACE reactions were performed using SMART 5′/3′ RACE cDNA amplification kit (BD) with following sequence‐specific oligonucleotides:
-
YBF 5′‐CCACCAGACAGCAATAAAGAGGAACAACCC‐3′
-
YBR 5′‐GCACCCTTCTCTCCTTCCACCACATCG‐3′
-
YBF2 5′‐CGATGTGGTGGAAGGAGAGAAGGGTGC‐3′
-
YBR2 5′‐GGTTGTTCCTCTTTATTGCTGTCTGGTGG‐3′
In order to isolate full‐length clone the following sequence‐specific primers, designed on the 5′ and 3′ UTR was used:
-
RYBU20 5′‐GGTAACAGGTGGGGAAGAACTGACGCC‐3′
-
RYBL1129 5′‐GTGCTCCAAAGATACGTGCCGCAATAAG‐3′
The PCR products were TA‐cloned into pGEM‐T easy vector (Promega) and sequenced by automated fluorescent cycle sequencing (ABI).
Sequence analysis
We used a BLAST search (Altschul et al. Citation1997) to identify related sequences. Sequences corresponding to the Cold Shock Domain were aligned with CLUSTALW 1.83 (Thompson et al. Citation1997). Evolutionary distances were calculated using Kimura's equation (Nei et al. Citation1983) and used for the phylogenetic tree construction by the Neighbor‐joining method using the MEGA 3.0 package (Kumar et al. Citation2004). Sequences were obtained from the EMBL/GenBank through the National Center for Biotechnology Information (NCBI) Web site (http://www.ncbi.nlm.nih.gov). The accession number of RlYB2 ( Rana lessonae Y‐box 2 ) is AM409315.
RNA extraction and RT‐PCR analysis
Total RNA was extracted from fresh or frozen different adult tissues and staged oocytes using Nucleospin RNA II kit (Macherey‐Nagel). First strand cDNA was sinthesized using Superscript II Reverse Transcriptase (Invitrogen), from 1 µg of total RNA. RT‐PCR analysis was performed using two different sets of primers, RYBU20 and YBR2, YBF and YBR. ß‐actin primers were used for standardization as described in Marracci et al. (Citation2007): forward primer CGCGTAGCACAAGATCAC, reverse primer CTTTGGGGTTAAGTGGAGC. Control reactions were performed in the absence of reverse transcriptase.
In situ hybridization
Whole‐mount in situ hybridizations were carried out on oocytes, at various maturing stages, of R. ridibunda, R. lessonae and R. esculenta, using digoxigenin‐labelled antisense and sense RNA probes generated from the full‐length RlYB2 or the partial cDNA clone (Ikenishi & Tanaka Citation2000). Paraffin sections of whole‐mount in situ hybridized oocytes were performed.
For in situ hybridization on cryostat sections (8–10 µm), testes and ovaries were fixed in 4% paraformaldehyde at room temperature for 2 h, cryoprotected with 30% sucrose in PBS o/n at 4°C, and stored at −70°C until cryosectioning. Both testis and ovary cryosections were hybridized as described in Marracci et al. (Citation2007).
Results
Isolation and sequence analysis of RlYB2
Using the RT‐PCR strategy with degenerate primers, we isolated in R. lessonae a partial cDNA sequence, 198 bp long, homolog to Y‐box gene family (Figure ). To extend this partial sequence we performed 5′ and 3′ RACE experiments with specific primers and we obtained a complete sequence of 1273 bp. The complete sequence included a 5′UTR region of 69 nt, an ORF encoding a predicted protein of 327 amino acids, and a 3′ UTR region of 223 nt (Figure ). A full‐length clone spanning from nt 20 to 1102 was amplified (Figure ). Sequence comparison revealed that this gene resembled most closely Y‐box2 subfamily and hence was called RlYB2 (Figure ). RlYB2 shared 63% amino acid identity with Xenopus FRGY2 and only 43% amino acid identity with Xenopus FRGY1 (Figure ) (Sommerville Citation1999). The predicted RlYB2 protein had the highly conserved N‐terminal CSD (from aa 37 to 85; Figure ) with motifs related to RNP‐1 and RNP‐2 (Auweter et al. Citation2006). Moreover the sequence contains thirteen putative serine/threonine phosphorylation sites (Yurkova & Murray Citation1997) and two putative nuclear localization signals (from amino acids 157 to 170; Figure ) (Nashchekin et al. Citation2006). An arginine–proline rich profile occurred from aa 114 to 317 (Figure ). A putative cytoplasmic polyadenylation element (CPE) is located in 3′ UTR region (Simon & Richter Citation1994) (Figure ).
Figure 1. Nucleotide and deduced amino acid sequence of cDNA encoding RlYB2. The region of the partial clone is in bold. The regions of the specific primers used in PCR reactions are underlined. The CPE element is in italic. The stop codon is marked with a black bar.
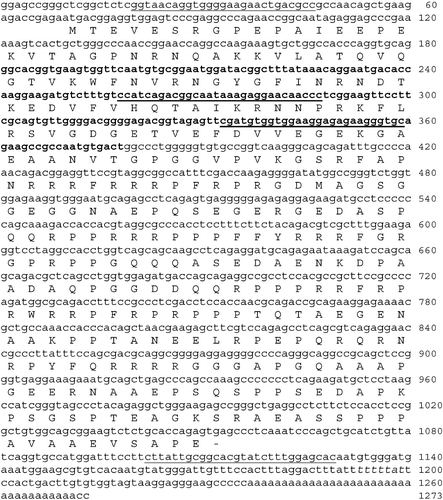
Figure 2. A, EMBL/GenBank accession numbers of RlYB2 homolog proteins used in the Y‐box family phylogenetic tree are: Homo sapiens YBOX1, YBOX2, CSDA (NP_004550, NP_057066, AAH15913); Mus musculus YBOX1, Csda1, Csda2, Csda3, YBOX2 (AAH13450, NP_058571, Ensemble predicted proteins Csda1, Csda2, Csda3); Rattus norvegicus YBOX1, YBOX2 (NP_113751, XP_220618); Canis familiaris YBOX1, YBOX2(XP_856109, XP_546585); Gallus gallus YBOX1, YBOX2 (BAA05380, XP_423576); Xenopus laevis FRGY1, FRGY2 (AAA49715, AAA49716); Rana lessonae RlYB2 (AM409315); Carassius auratus YBOX1, YBOX2 (BAA19849, BAA19850); Tetraodon nigroviridis YBOX1 (CAG03208); Oryzias latipes YBOX1 (BAC45236, CAC39436); Ciona intestinalis YboxA, YboxB, YboxC (Ensembl predicted proteins ENSCINP00000010636, ENSCINP00000010626, ENSCINP00000010634). B, The predicted amino acid sequence of RlYB2 compared with FRGY1 and FRGY2 of Xenopus. The identical residues are black‐shaded, the similar ones are grey‐shaded. Gaps have been introduced for maximizing the similarity. The CSD domain is boxed. The asterisks denote serine and threonine residues that represent potential sites of phosphorylation. RNP‐1 and RNP‐2 like motifs are remarked by black bars. NES sequences are highlighted by dotted bars.
Spatial expression analysis of RlYB2
RT‐PCR assays were performed in somatic and germinal adult tissues of R. ridibunda, R. lessonae, and R. esculenta. The results showed that RlYB2 mRNA was expressed in R. lessonae ovary (Figure ). A faint band was detected in R. lessonae testis while no RlYB2 transcripts were found in somatic tissues such as heart, liver, brain, intestine, and spleen (Figure ). A similar expression profile was detected in R. ridibunda and R. esculenta (data not shown).
Expression pattern of RlYB2 during oogenesis
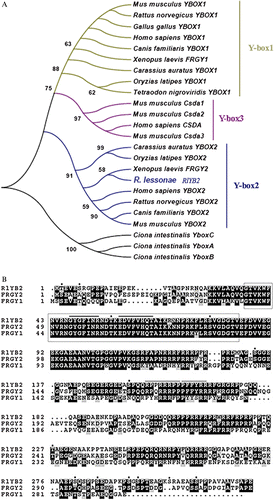
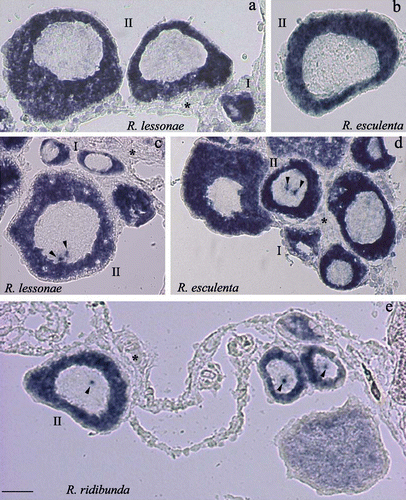
Whole‐mount in situ hybridizations performed by using the full‐length clone as a probe reveal that RlYB2 is expressed in stage I and II oocytes in both the parental species and hybrid (Figure ). No hybridization signal was detected in later stage oocytes (Figure ). This expression profile was confirmed by RT‐PCR analysis carried out on defolliculated oocytes of R. lessonae (Figure , bottom panel). A similar pattern was revealed in R. ridibunda and R. esculenta (data not shown). In situ hybridizations on ovary cryosections, carried out by using full‐length or partial clone as a probe, demonstrated that the RlYB2 transcript is thoroughly distributed in the cytoplasm of stage I and II oocytes of the parental species and hybrid (Figure ). In addition to the cytoplasmic expression signal, hybridization experiments performed by using the partial clone as a probe show one or two hybridization spots in the nuclei of stage II oocytes of R. ridibunda, R. lessonae, as well as R. esculenta (Figure ). The ovary somatic cells were not labeled (Figure ).
Figure 4. Top panel: whole‐mount in situ hybridizations showing the expression pattern of RlYB2 during oogenesis of R. lessonae (a), R. ridibunda (b), as well as R. esculenta (c). Some oocyte stages are indicated. Scale bars represent: (a) 500 µm (b)100 µm; (c) 500 µm. Bottom panel: RT‐PCR analysis in oocytes of R. lessonae at different stages (I–VI) of oogenesis.
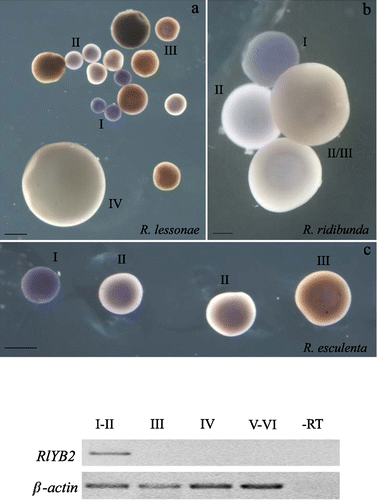
Figure 5. Cryostat sections of oocytes hybridized with the full length(a,b) or the partial clone (c,d,e) as a probe. The arrowheads point to the presence of spots of the hybridization signal in oocytes of R. lessonae (c), R. esculenta (d) and R. ridibunda (e). Representative stages of oocytes are shown. The asterisks indicate ovary somatic cells (a,c–e). Scale bar represent 500 µm.
In situ hybridizations on frozen sections of testes of R. ridibunda, R. lessonae, and R. esculenta did not reveal any RlYB2 expression signal. This apparent discrepancy with the results of RT‐PCR might depend on the larger sensitivity of the RT‐PCR method respect to the in situ hybridizations.
Discussion
The development of the germ cells in green frogs of the genus Rana has been thoroughly investigated by means of cytological, genetic and cytogenetic approaches (Uzzell et al. Citation1980; Bucci et al. Citation1990, Citation1999; Ogielska & Wagner Citation1993; Wagner & Ogielska Citation1993; Ragghianti et al. Citation1995; Ogielska & Kotusz Citation2004). Interesting information emerged from studies about Rana esculenta gametogenesis (Chieffi et al. Citation2000; Ferrara et al. Citation2004; Meccariello et al. Citation2004). Furthermore, results concerning the characterization of germ cell molecular markers during the hybridogenetic gametogenesis of these animals have been reported in Marracci et al. (Citation2007).
With the aim to study the genetic pathways that regulate the gametogenesis of the green frogs, we have identified and characterized in Rana esculenta complex a Y‐box homologue, named RlYB2. Protein sequence multialignment and phylogenetic analyses showed that it belonged to the Y‐box2 subfamily of genes encoding for cold‐shock domain proteins. RlYB2 shared with the Y‐box proteins the presence of a highly conserved CSD domain containing two RNP‐1 and RNP‐2 like motifs that are believed to be important for binding nucleic acids (Sommerville Citation1999). The presence of numerous residues target for serine/threonine kinases suggested that the RlYB2 activity may be regulated by phosphorylation (Yurkova & Murray Citation1997). It has been hypothesized that phosphorylation of the C‐terminal portion of Y‐box proteins facilitates mRNA–protein interactions, while dephosphorylation promotes the release of the bound mRNA (Matsumoto & Wolffe Citation1998). Like other Xenopus maternal mRNAs, RlYB2 contained in the 3′UTR region a CPE element potentially implicated in the control of the translation during early embryogenesis (Simon & Richter Citation1994).
As other members of the Y‐box2 subfamily, RlYB2 was specifically expressed in germ cells of both the parental species and hybrid. In particular, RlYB2 transcript is thoroughly distributed in the cytoplasm of previtellogenic stage I and II oocytes that are characterized by an extensive lampbrush chromosome phase occurring in meiotic prophase I. During lampbrush phase a read‐through massive transcription of maternal mRNAs is carried out. The detection of labelled spots within nuclei of stage II oocytes hybridized with a conserved portion of RlYB2 mRNA can suggest that in Rana, Y‐box genes are actively transcribed on lampbrush chromosomes, although further investigations will be necessary to better analyse this point. In Xenopus, FRGY2 protein was localized in both the cytoplasm and the nuclei of previtellogenic oocytes (Sommerville & Ladomery Citation1996). It has been proposed that FRGY2 may have a role in coupling the transcription and splicing of nuclear maternal pre‐mRNAs to their translation in the cytoplasm (Matsumoto & Wolffe Citation1998). The presence of a weak band in the testis revealed by RT‐PCR can suggest a lower level of RlYB2 transcription in the male germ line when compared to the female germ line.
In conclusion, RlYB2 expression pattern appeared largely shared in the real species R. ridibunda and R. lessonae, and the R. esculenta hybrid. Taking into account that in Xenopus the Y‐box proteins are massively expressed during oogenesis, the presence of the RlYB2 transcript in early phases of Rana oogenesis suggests a possible function of this gene in regulating both the female germ cell differentiation pathway and/or the translational control of stored mRNAs in oocytes. It will be important to carry on a further analysis on the transcriptional and translational pattern of RlYB2 in larval germ cells of hybrid frogs where hybridogenetic mechanisms of exclusion and endoreduplication take place.
Abbreviation list
R = A+G, Y = C+T, N = A+C+G+T, W = A+T.
nt = nucleotide
aa = amino acid
UTR = untranslated region
ORF = open reading frame
Acknowledgements
We would like to thank G. De Matienzo and M. Fabbri for technical assistance, and S. Di Maria for frog care. This work was supported by Pisa University grants.
References
- Altschul , S. F. , Madden , T. L. , Schaffer , A. A. , Zhang , J. , Zhang , Z. , Miller , W. and Lipman , D. J. 1997 . Gapped BLAST and PSI‐BLAST: A new generation of protein database search programs. . Nucleic Acids Research , 25 : 3389 – 3402 .
- Auweter , S. D. , Oberstrass , F. C. and Allain , F. H. 2006 . Sequence‐specific binding of single‐stranded RNA: Is there a code for recognition? . Nucleic Acids Research , 34 : 4943 – 4959 .
- Bucci , S. , Ragghianti , M. , Mancino , G. , Berger , L. , Hotz , H. and Uzzell , T. 1990 . Lampbrush and mitotic chromosomes of the hemiclonally reproducing hybrid Rana esculenta and its parental species. . Journal of Experimental Zoology , 255 : 37 – 56 .
- Bucci , S. , Ragghianti , M. , Mancino , G. , Petroni , G. , Guerrini , F. and Giampaoli , S. 1999 . Rana/Pol III: A family of SINE‐like sequences in the genomes of western Palearctic water frogs. . Genome , 42 : 504 – 511 .
- Chieffi , P. , Franco , R. , Fulgione , D. and Staibano , S. 2000 . PCNA in the testis of the frog, Rana esculenta: A molecular marker of the mitotic testicular epithelium proliferation. . General & Comparative Endocrinology , 119 : 11 – 16 .
- Ferrara , D. , Palmiero , C. , Branno , M. , Pierantoni , R. and Minucci , S. 2004 . Testicular activity of Mos in the frog, Rana esculenta: A new role in spermatogonial proliferation. . Biology of Reproduction , 70 : 1782 – 1789 .
- Graf , J. ‐D. and Polls Pelaz , M. 1989 . “ Evolutionary genetics of the Rana esculenta‐complex. ” . In Evolution and ecology of unisexual vertebrates , Edited by: Dawley , R. M and Bogart , J. B . 289 – 301 . Albany, NY : New York State Museum . Bulletin 466
- Gu , W. , Tekur , S. , Reinbold , R. , Eppig , J. J. , Choi , Y. C. , Zheng , J. Z. , Murray , M. T. and Hecht , N. B. 1998 . Mammalian male and female germ cells express a germ cell‐specific Y‐Box protein, MSY2. . Biology of Reproduction , 59 : 1266 – 1274 .
- Ikenishi , K. and Tanaka , T. S. 2000 . Spatio‐temporal expression of Xenopus vasa homolog, XVLG1, in oocytes and embryos: The presence of XVLG1 RNA in somatic cells as well as germline cells. . Development Growth & Differentiation , 42 : 95 – 103 .
- Kumar , S. , Tamura , K. and Nei , M. 2004 . MEGA3: Integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. . Briefings in Bioinformatics , 5 : 150 – 163 .
- Mansfield , J. H. , Wilhelm , J. E. and Hazelrigg , T. 2002 . Ypsilon Schachtel, a Drosophila Y‐box protein, acts antagonistically to Orb in the oskar mRNA localization and translation pathway. . Development , 129 : 197 – 209 .
- Marracci , S. , Casola , C. , Bucci , S. , Ragghianti , M. , Ogielska , M. and Mancino , G. 2007 . Differential expression of two vasa/PL10‐related genes during gametogenesis in the special model system Rana. . Development Genes Evolution , 217 : 395 – 402 .
- Matsumoto , K. and Wolffe , A. P. 1998 . Gene regulation by Y‐box proteins: Coupling control of transcription and translation. . Trends in Cell Biology , 8 : 318 – 323 .
- Meccariello , R. , Cobellis , G. , Scarpa , D. , Fienga , G. , Pierantoni , R. and Fasano , S. 2004 . Detection of msj‐1 gene expression in the frog, Rana esculenta testis, brain, and spinal cord. . Molecular Reproduction and Development , 68 : 149 – 158 .
- Nashchekin , D. , Zhao , J. , Visa , N. and Daneholt , B. 2006 . A novel Ded1‐like RNA helicase interacts with the Y‐box protein ctYB‐1 in nuclear mRNP particles and in polysomes. . Journal of Biological Chemistry , 281 : 14263 – 14272 .
- Nei , M. , Tajima , F. and Tateno , Y. 1983 . Accuracy of estimated phylogenetic trees from molecular data. II. Gene frequency data. . Journal of Molecular Evolution , 19 : 153 – 170 .
- Ogielska , M. 1990 . The fate of intramitochondrial paracrystalline inclusion bodies in germ line cells of water frogs (Amphibia, Anura). . Experientia , 46 : 98 – 101 .
- Ogielska , M. and Kotusz , A. 2004 . Pattern and rate of ovary differentiation with reference to somatic development in anuran amphibians. . Journal of Morphology , 259 : 41 – 54 .
- Ogielska , M. and Wagner , E. 1993 . Oogenesis and ovary development in natural hybridogenetic water frog, Rana esculenta L. 1. Tadpole stages until metamorphosis. . Zoologische Jahrbücher (Physiologie) , 97 : 349 – 368 .
- Ragghianti , M. , Guerrini , F. , Bucci , S. , Mancino , G. , Hotz , H. , Uzzell , T. and Guex , G. D. 1995 . Molecular characterization of a centromeric satellite DNA in the hemiclonal hybrid frog Rana esculenta and its parental species. . Chromosome Research , 3 : 497 – 506 .
- Simon , R. and Richter , J. D. 1994 . Further analysis of cytoplasmic polyadenylation in Xenopus embryos and identification of embryonic cytoplasmic polyadenylation element‐binding proteins. . Molecular and Cell Biology , 14 : 7867 – 7875 .
- Sommerville , J. 1992 . RNA‐binding proteins: Masking proteins revealed. . Bioessays , 14 : 337 – 339 .
- Sommerville , J. 1999 . Activities of cold‐shock domain proteins in translation control. . Bioessays , 21 : 319 – 325 .
- Sommerville , J. and Ladomery , M. 1996 . Transcription and masking of mRNA in germ cells: Involvement of Y‐box proteins. . Chromosoma , 104 : 469 – 478 .
- Tafuri , S. R. and Wolffe , A. P. 1990 . Xenopus Y‐box transcription factors: Molecular cloning, functional analysis and developmental regulation. . Proceedings of the National Academy of Sciences of the United States of America , 87 : 9028 – 9032 .
- Thompson , J. D. , Gibson , T. J. , Plewniak , F. , Jeanmougin , F. and Higgins , D. G. 1997 . The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. . Nucleic Acids Research , 25 : 4876 – 4882 .
- Tunner , H. and Heppich‐Tunner , S. 1991 . Genome exclusion and two strategies of chromosome duplication in oogenesis of a hybrid frog. . Naturwissenschaften , 78 : 32 – 34 .
- Uzzell , T. , Hotz , H. and Berger , L. 1980 . Genome exclusion in gametogenesis by an interspecific Rana hybrid: Evidence from electrophoresis of individual oocytes. . Journal of Experimental Zoology , 214 : 251 – 259 .
- Wagner , E. and Ogielska , M. 1993 . Oogenesis and ovary development in natural hybridogenetic water frog, Rana esculenta L. 2. After metamorphosis until adults. . Zoologische Jahrbücher (Physiologie) , 97 : 349 – 368 .
- Wolffe , A. P. 1994 . Structural and functional properties of the evolutionarily ancient Y‐box family of nucleic acid binding proteins. . Bioessays , 16 : 245 – 251 .
- Wolffe , A. P. , Tafuri , S. , Ranjan , M. and Familari , M. 1992 . The Y‐box factors: A family of nucleic acid binding proteins conserved from Escherichia coli to man. . The New Biologist , 4 : 290 – 298 .
- Yu , J. , Hecht , N. B. and Schultz , R. M. 2002 . RNA‐binding properties and translation repression in vitro by germ cell‐specific MSY2 protein. . Biology of Reproduction , 67 : 1093 – 1098 .
- Yang , J. , Medvedev , S. , Yu , J. , Schultz , R. M. and Hecht , N. B. 2006 . Deletion of the DNA/RNA‐binding protein MSY2 leads to post‐meiotic arrest. . Molecular and Cellular Endocrinology , 250 : 20 – 24 .
- Yang , J. , Morales , C. R. , Medvedev , S. , Schultz , R. M. and Hecht , N. B. 2007 . In the absence of the mouse DNA/RNA‐binding protein MSY2, messenger RNA instability leads to spermatogenic arrest. . Biology of Reproduction , 76 : 48 – 54 .
- Yurkova , M. S. and Murray , M. T. 1997 . A translation regulatory particle containing the Xenopus oocyte Y box protein mRNP3+4. . Journal of Biological Chemistry , 272 : 10870 – 10876 .
