Abstract
A study was carried out on the population structure and spatial niche of Speleomantes italicus (Dunn, 1923) in a subterranean system of 10 caves in Umbria (Italy). The studied populations revealed a peak of activity from April to July. The relative abundance of salamanders varied throughout the year and the observed frequencies of individuals differed significantly from expected, confirming a seasonal pattern of activity. Both external and internal temperatures positively influenced the abundance of salamanders inside the caves. This relationship may principally be indirect, and presumably salamander cave activity follows prey temporal distribution patterns, which in turn are also linked to temperature. The presence of salamanders was negatively affected by high levels of air moisture both outside and inside the cave. In summer, salamanders find refuge inside caves because the air moisture outside reaches values incompatible with the physiological requirements of the species. Adults showed a significant tendency to use areas inside the cave that were closer to the entrance when the external climate conditions come in the vicinity of those suitable for the species (increased external relative humidity and decreased external temperature), and the external influence on internal micro‐climate is reduced. Comparisons to other Speleomantes species are also provided.
Introduction
Amphibian adaptations to cave habitat count different degree of specialization from a simple trogloxeny to a very specialised troglobian life, particularly evident in phreatobian species of the families Proteidae and Plethodontidae. In the Palaeartic fauna, except the troglobitic Proteus Laurenti, 1768 species, a well developed troglophily is observed in the salamander genera Paradactylodon Risch, 1984 (Hynobiidae), Lyciasalamandra Veith and Steinfartz, 2004, Euproctus Gené, 1838 (Salamandridae) and Speleomantes Dubois, 1984 (Plethodontidae), which exploit cave habitat for reproduction, feeding and recovery (see Bologna Citation1982 for a review).
The Italian cave salamander, Speleomantes italicus (Dunn, 1923), is a Plethodontidae (Caudata) distributed north‐west of Emilia (Province of Reggio Emilia), in Tuscany (Province of Lucca), and in the south down to Abruzzi (on the south‐eastern slope of the Gran Sasso Massif, Province of Pescara) (Lanza et al. Citation1995, Citation2006). It is a eurizonal (80–1600 m a.s.l) and meso‐hygrophylic species, usually related to forest ecosystems or derived habitats, inhabiting clefts but also caves and artificial cavities (Lanza et al. Citation2006). Scarce information is available on the ecology of this species (Pastorelli et al. Citation2001; Casali et al. Citation2005; Pastorelli et al. Citation2005; Vignoli et al. Citation2006). In particular, only a few studies have addressed the population structure and spatial niche of S. italicus, and all these concern populations from the Northern Apennines (Pastorelli et al. Citation2001, Citation2002, Citation2005).
Studies on the population structure and spatial distribution of the other two mainland Italian Speleomantes species have been conducted by Salvidio et al. (Citation1994), Cimmaruta et al. (Citation1999), Pastorelli et al. (Citation2001), Salvidio and Pastorino (Citation2002) and Forti et al. (Citation2005).
The aim of this paper is to examine the population structure and the spatial use of Central Apennine populations of S. italicus, and to compare these aspects to those of S. strinatii and S. ambrosii (Lanza, 1955) critically reviewing extant literature.
Material and methods
Study area
The research was carried out on Central Appennine hypogean populations of S. italicus in two limestone zones: (a) the Monte Cucco Regional Park (Province of Perugia, Umbria Region) on Monte Cucco itself (12°44′02.7″ N, 43°22′28.9″ E) (maximum elevation 1566 m a.s.l.); (b) at the base of Monte Ingino (12°35′10.7″ N, 43°21′33.04″ E) near Gubbio in the same province and region. The climate is subcontinental–temperate at the valley bottom and subcontinental in the mountain areas. Annual average temperatures vary from 6°C to 11°C (Menichetti 1987) and rainfall from 900 to 1440 mm/year (1900 at high altitudes). For a detailed description of the study area and the caves that were investigated, see Vignoli et al. (Citation2006).
Seven natural caves were sampled, five of them situated on Monte Cucco (AN, BN, CN, DN and EN), two on Monte Ingino (FN and GN); three additional artificial cavities (AA, AA2 and BA) were examined in the Monte Cucco Regional Park. Cave AA2 has a blocked entrance, hence to study it we passed via cave AA which is connected to it by a 100‐m long artificial passage; for this reason AA and AA2 populations were considered as single one. Cave DN has a narrow artificial entrance that limits the influence of external weather conditions on the cave environment. The remaining caves had similar entrance morphology and micro‐climatic features.
General methods
Caves were sampled monthly from January to December 2004 except when adverse weather conditions occurred. Inside the caves, salamanders were sampled by means of Visual Encounter Surveys (VES) (Heyer et al. Citation1994), caught by hand and then sexed. Individuals smaller than the smallest captured male with evident mental gland (typical of reproductive males) were considered juveniles (Vignoli et al. Citation2006). As far as the use of habitat is concerned, the elevation from the ground (H) (measured to the nearest 0.01 m as the length of the projection on ground of the specimens at the first sight) and the distance from cave entrance (E) (measured to nearest 0.01 m as a straight line from the entrance to the projection on ground of the salamander position at the first sight) for each specimen were recorded. Relative humidity (%) and temperature (°C) inside and outside the caves (RHint, RHext and Tint, Text, respectively) were recorded using a hair hygrometer (Fischer) and a digital thermometer, respectively.
Digital photos of the dorsal and ventral colour patterns were taken to allow individual identification (Salvidio et al. Citation1994; Laghi et al. Citation2005; Vignoli et al. Citation2006). To avoid data pseudoreplication biases (Hurlbert Citation1984), data on recaptures were excluded from the analyses. Because in most caves the number of observed adults was low, we took in account only the caves with n>15 (AA2 and EN) for estimating the sex ratio. The salamander foraging activity observed during the same period for all the sampled caves (Vignoli et al. Citation2006), led us to considered all the caves as suitable for salamanders and hence usable in the analyses of the spatial use, despite the low number of sampled individuals. Salamander monthly activity pattern (A) was evaluated only for Cave EN, where we observed a consistent number of individuals throughout the year.
Heterogeneity G‐test was performed to test the null hypothesis that the replicates did not differ from each other significantly and consequently could be pooled and treated as a single sample (Caves AA and BN were not considered because the sampling effort and the salamander sample, respectively, were too small for the analysis).
To assess the monthly activity of the cave salamanders, we followed the statistical procedures of Rugiero and Luiselli (Citation2006). We first determined the relative sampling effort per month by dividing the number of minutes spent in the caves searching for salamanders each month by the total number of minutes spent into the caves during the entire research period. Using a null hypothesis of equal activity among months, we then generated the expected number of salamanders that were active each month by multiplying the total number of active specimens found during the study by the relative sampling effort for each month. Thus, instead of using the Chi‐square test as indicated by Rugiero and Luiselli (Citation2006), the observed and expected values for each cave were compared using G‐test of goodness‐of‐fit, considering each cave as a single replicate.
All the variables were normally distributed or were log‐transformed to achieve normality (Kolmogorov–Smirnov test) and were then analysed using parametric tests. t‐Tests were performed to evaluate differences in terms of (E) and (H) between sexes and between adults and juveniles. Linear regressions were used on all cave data pooled to assess relationships between (A), (E) and (H) (response variables) and the physical parameters RHint, RHext, Tint and Text (predictor variable), and between (E) (response variable) and (H) (predictor variable); Caves AA2 and DN were excluded from this analysis because the internal RH and T are slightly influenced by external weather conditions due to their peculiar morphology (see study area section). Statistical analyses, all tests being two‐tailed and with alpha set at 5%, were made using Statistica (StatSoft, Inc. 2001, Version 6).
Results
A total of 152 cave salamanders (5 adults of which were not sexed) were sampled during the study period, 32 were recaptured at least once, for a total of 192 captures. The month‐by‐month observed occurrence of males, females and juveniles in the various caves and artificial cavities is provided in Table . In January and February, only one juvenile specimen was observed. The overall sex ratio (males/females) ranged from 9 to 0.14, (n males = 73; n females = 63; mean = 2.16±2.80 SD). In the caves AA2 and EN (both with n>15) on average, males were slightly more abundant than females (n males = 40; n females = 36; mean ratio = 1.31±0.33) and the sex ratio underwent seasonal variations. In the same caves the monthly relative abundance pattern evaluated for 10 months was similar between sexes (Chi‐square test. χ2 = 9.228, df = 9, P = 0.416) but significantly different among adults and juveniles (Chi‐square test. χ2 = 335.691, df = 9, P<0.0001) (relative abundance: meanmales = 3.67±3.73; meanfemales = 3.00±3.98; meanjuveniles = 2.25±2.09). In the study area the populations showed a peak of activity from April to July (57% of the entire sample), whereas in winter, the observed activity was significantly reduced (4%; in January and February one single juvenile was observed), indicating a marked seasonal variation in the activity pattern of this species (Figure ).
Table I. Month–by–month observed occurrence of males, females and juveniles in the sampled caves.
Figure 1. Month‐by‐month observed frequencies of occurrence and sex ratio of cave salamanders in AA2 and EN caves. Symbols: white columns = total sampled specimens; black and white diagonal striped columns = males; black columns = females; grey columns = juveniles; black line = sex ratio (males/females).
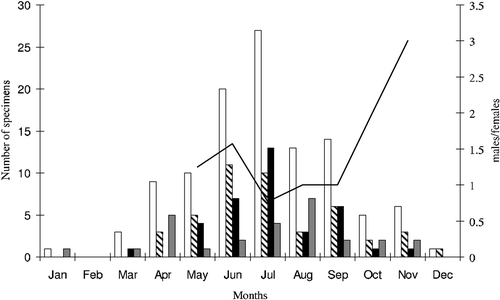
The caves differed significantly from each other in terms of observed and expected specimens frequency (Heterogeneity G‐test. GH = 100.13, df = 75, P<0.05), and consequently the data could not be pooled and analysed as a whole. Apart from AA2 (G‐test of goodness‐of‐fit. G = 6.39, df = 6, P = 0.47), the observed frequencies of the specimens throughout the year differed significantly from expected in all the examined caves (AN: G = 37.78, df = 11; BA: G = 24.53, df = 10; CN: G = 33.17, df = 8; DN: G = 37.03, df = 8; EN: G = 58.80, df = 11; FN: G = 39.87, df = 9; GN: G = 30.08, df = 8; HN: G = 13.66, df = 4; in all cases P<0.01).
In EN (n = 71), the presence and abundance of individuals inside the cave was strongly related to all considered physical parameters, being positively related to both internal and external temperature, and negatively related to relative humidity inside and outside the cave (Figure ; Table ).
Figure 2. Month‐by‐month observed specimens and physical measured parameters in cave EN. Symbols: black columns = total sampled specimens; white columns = temperature inside the cave; black and white diagonal striped columns = temperature outside the cave; dot line and empty circles = relative humidity inside the cave; continuous line and black circles = relative humidity outside the cave.
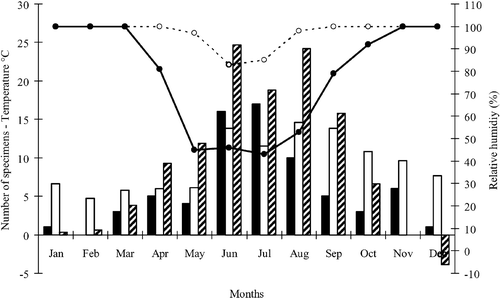
Table II. Relationships between the monthly number of observed salamanders (A) and physical parameters throughout the year evaluated by using Linear regression (analyses referred to cave EN).
As regards to the use of space, salamanders were found from just outside the caves to 18.30 m from the entrance, and from 0.75 m under the cave floor to 2.85 m height from the ground (mean = 0.995, SD = 0.727). There were no significant discrepancies between sexes and age classes in exploiting different areas of the cave in terms of distance from entrance (E) and height from the ground (H) (Esex: meanmales = 57 = 8.116±4.262 m, meanfemales = 48 = 7.799±4.129, t = −0.385, df = 103, P = 0.701. Hsex: meanmales = 57 = 1.091±0.812 m; meanfemales = 48 = 1.006±0.626 m, t = 0.588, df = 103, P = 0.558. Eage: meanadults = 105 = 7.971±4.184 m, meanjuveniles = 48 = 6.929±3.541 m, t = 1.423, df = 145, P = 0.157. Hage: meanadults = 105 = 1.044±0.731 m, meanjuveniles = 42 = 0.871±0.711 m, t = 1.309, df = 145, P = 0.192). There was a statistically significant relationship between (E) and external temperature (positive) and relative humidity (negative) (Table ), but not for the same parameters inside the cave; (H) was marginally related to (E) (Linear regression. R = 0.181, F 1,96 = 3.248, P = 0.075). The same analyses performed on males, females and juveniles separately, highlighted a significant tendency in adults to occupy areas of the cave closer to the cave entrance in relation to increased external relative humidity and decreased external temperature (Table ). Juveniles showed a negative relation between (H) and internal temperature (R = −0.390, F = 5.222, P<0.05), whereas the position on the cave walls of the adults of both sexes was independent of physical parameters (for all tests P>0.05). Only males showed a behavioural response to increased distance from the cave entrance in relation to height from the ground (Linear regression. E vs. H; R = 0.377, F 1,32 = 5.300, P<0.05).
Table III. Relationships between distance from entrance (E) and physical parameters evaluated by using linear regression.
Discussion
Although physical conditions vary in the investigated hypogean sites about three (temperature) or four (air moisture) times less than in the adjacent external areas, the studied S. italicus populations exhibited a clear seasonal‐linked phenology. Lack of data in winter confirms that the observed activity inside the caves only partly represents the entire life cycle of this species which inhabits the external environment at least during the coldest periods of the year (Lanza Citation1946).
S. italicus populations inhabiting the study area seem to be represented by an exiguous number of individuals (personal observations) if compared with those of Northern Apennines (i.e. Pastorelli et al. Citation2001). Salamanders relative abundance varied throughout the year and the observed frequencies of individuals differed significantly from expected based on sampling effort, highlighting a specific temporal pattern of activity. Moreover, this pattern of activity is peculiar for each cave, confirming the influence of several cave characteristics that were not taken into consideration in this study (size, altitude, exposure of the entrance, etc.). The seasonal numeric variation of individuals in the studied populations, with one activity peak in summer, is analogous to that of a population of S. strinatii studied by Salvidio et al. (Citation1994). Other populations of co‐generic species showed two activity peaks, in early summer and in autumn: e.g. the S. strinatii population (quoted as ambrosii) studied by Salvidio (Citation1993) and S. ambrosii and S. strinatii populations studied by Forti et al. (Citation2005). This comparable activity pattern may be due to the simultaneous increase of dipterans density (particularly Limnobiidae) inside the caves in late spring and summer, which constitute the main prey in the diet spectrum of the investigated populations (Forti et al. Citation2005; Vignoli et al. Citation2006). Conversely, two cave populations of S. ambrosii studied by Salvidio et al. (Citation2002) were characterised by two activity peaks in spring and autumn, which seem to follow the rainfall temporal distribution pattern, that is related more to rock‐face populations (Salvidio Citation1991, Citation1993) than to those inhabiting caves.
The observed average sex ratio (males/females) was close to the unity. In a single population of S. strinatii (quoted as ambrosii), Salvidio et al. (Citation1994) highlighted a slightly larger number of females. The authors tried to explain the female‐biased sex ratio by the fact that females have to spend much more time foraging than males in order to store up trophic resources for parental care activity. Our data on two populations (AA2 and EN) revealed seasonal variations in the sex ratio alternating the female‐ and male‐biased ratio. For this reason, we wish to refrain from discussing our results more in depth due to difficulties in interpretation.
The presence of salamanders inside the cave (evaluated only in Cave EN) was positively influenced by external and internal temperature. This relationship between salamander activity and temperature may well be indirect and may be due principally to the strong correlation between potential prey life cycles and temperature; as it is likely that salamander cave activity follows prey temporal distribution patterns (Forti et al. Citation2005; Vignoli et al. Citation2006). The positive relationship between the abundance of individuals and temperature inside and outside the cave, was also highlighted by Salvidio et al. (Citation1994) for S. strinatii (quoted as ambrosii). Moreover, our results revealed that the presence of salamanders was negatively affected by high levels of air moisture both outside and inside the cave. Similar results were reported by Forti et al. (Citation2005) for S. ambrosii and S. strinatii.
Our findings can be subjected to two different interpretations that are not mutually exclusive: (a) the effect of temperature is indirect and salamander activity could chiefly be influenced by prey abundance and unaffected by internal relative humidity which, although decreases in June and July, remains within the suitable range for the species (Lanza Citation1946; Bruno Citation1973 for a review); (b) internal and external relative humidity are strongly related, though in the hottest months of the year air moisture outside the cave reaches values likely incompatible with the physiological requirements of the species, which thus finds refuge inside the cave.
As regards to the utilisation of space (distance from the cave entrance and height from the ground) by salamanders, no difference was found between sexes and age classes. A clear relationship was shown between distance from entrance and external physical parameters, positive for temperature and negative for relative humidity; whereas no correlation was found for the same parameters inside the caves. In particular, adults showed a significant tendency to use areas of the cave that were closer to the entrance, in relation to increased external relative humidity and the decreased external temperature. Salamanders tend to occupy areas closer to the cavity entrance when the external climate conditions approach those suitable for the species (Lanza, Citation1946; Bruno, Citation1973 for a review). Males were observed at higher positions on the wall with respect to the ground, in relation to increased distance from entrance. This behavioural response to a decrease in the external climate influence could be due to different causes that are difficult to interpret in lack of further investigations, i.e. searching for the optimal conditions of air moisture and temperature and/or following prey distribution in foraging activity. As just pointed out for the males, also the negative relation between internal temperatures and the position on the wall (H) of juveniles is hard to comment on. Differently from studies on other Speleomantes species (Salvidio et al. Citation1994; Salvidio, Citation1996), no tendency was shown by juvenile specimens in space occupancy pattern inside the cavities. Previous investigations showed that juveniles of S. strinatii prefer the zones near the cave entrance, presumably owing to competition and risk of cannibalism carried out by adults (Salvidio et al. Citation1994).
Acknowledgements
The Italian Ministry for the Environment issued capture permits (DPN/2D/2006/10441). We wish to thank the speleologists of Costacciaro, Perugia, who helped us in the first phase of the field research.
References
- Bologna , M. A. 1982 . Anfibi cavernicoli con particolare riguardo alle specie italiane. . Lavori della Società Italiana di Biogeografia (N.S.) , 7 (1978) : 451 – 463 .
- Bruno , S. 1973 . Anfibi d’Italia: Caudata (Studi sulla fauna erpetologica italiana—XVII). . Natura, Milano , 64 : 209 – 450 .
- Casali , S. , Suzzi Valli , A. , Busignani , G. and Tedaldi , G. 2005 . I costumi arboricoli di Speleomantes italicus (Dunn, 1923) nella Repubblica di San Marino (Amphibia, Plethodontidae). . Annali del Museo Civico di Storia Naturale ‘Giacomo Doria’, Genova , 97 : 145 – 152 .
- Cimmaruta , R. , Forti , G. , Nascetti , G. and Bullini , L. 1999 . Spatial distribution and competition in two parapatric sibling species of European plethodontid salamanders. . Ethology Ecology and Evolution , 11 : 383 – 398 .
- Forti , G. , Cimmaruta , R. and Nascetti , G. 2005 . Behavioural responses to seasonal variation of autoecological parameters in populations of Speleomantes strinatii (Aellen, 1958) and S. ambrosii (Lanza, 1955) (Amphibia, Plethodontidae). . Annali del Museo Civico di Storia Naturale ‘Giacomo Doria’, Genova , 97 : 179 – 192 .
- Heyer , W. R. , Donnelly , M. A. , McDiarmid , R. W. , Hayek , L. C. and Foster , M. S. 1994 . Measuring and monitoring biological diversity: Standard methods for amphibians , Washington : Smithsonian Institution Press .
- Hurlbert , S. H. 1984 . Pseudoreplication and the design of ecological field experiments. . Ecological Monographs , 54 : 187 – 211 .
- Laghi , P. , Pastorelli , C. and Scaravelli , C. 2005 . Individual pattern recognition of Speleomantes italicus (Dunn, 1923) (Amphibia, Plethodontidae). . Annali del Museo Civico di Storia Naturale ‘Giacomo Doria’, Genova , 97 : 153 – 160 .
- Lanza , B. 1946 . L’Hydromantes Gistel in Toscana e notizie sui suoi costumi (Amphibia; Caudata; Plethodontidae). . Archivio Zoologico Italiano , 31 : 219 – 237 .
- Lanza , B. , Caputo , V. , Nascetti , G. and Bullini , L. 1995 . Morphologic and genetic studies of the European plethodontid salamanders: Taxonomic inferences (genus Hydromantes). . Monografie del Museo Regionale di Scienze Naturali, Torino , 16 : 1 – 366 .
- Lanza , B. , Vanni , S. and Nistri , A. 2006 . “ Speleomantes italicus (Dunn, 1923). ” . In Atlante degli Anfibi e dei Rettili d’Italia/Atlas of Italian Amphibians and Reptiles. Societas Herpetologica Italica , Edited by: Sindaco , R , Doria , G , Razzetti , E and Bernini , F . 252 – 257 . Firenze : Edizioni Polistampa .
- Pastorelli , C. , Laghi , P. and Scaravelli , D. 2001 . Studi preliminari sull’ecologia di Speloemantes italicus (Dunn, 1923) nell’Appennino tosco‐romagnolo (Caudata: Plethodontidae). . Pianura , 13 : 347 – 351 .
- Pastorelli , C. , Laghi , P. and Scaravelli , D. 2002 . Seasonal activity and spatial distribution of a Speleomantes italicus population in a natural cave. . Biota , 3 : 119 – 126 .
- Pastorelli , C. , Laghi , P. and Scaravelli , D. 2005 . Spacing of Speleomantes italicus (Dunn, 1923): Application of a Geographic Information System (G.I.S.) (Amphibia, Plethodontidae). . Annali del Museo Civico di Storia Naturale ‘Giacomo Doria’, Genova , 97 : 169 – 177 .
- Rugiero , L. and Luiselli , L. 2006 . Ecological modelling of habitat use and the annual activity patterns in an urban population of the tortoise, Testudo hermanni. . Italian Journal of Zoology , 73 : 219 – 225 .
- Salvidio , S. 1991 . Habitat ed attività stagionale delle popolazioni interstiziali di Speleomantes ambrosii nell’alta Val Bisagno (Liguria Centrale) (Amphibia, Plethodontidae). . Rivista Piemontese di Storia Naturale , 12 : 69 – 74 .
- Salvidio , S. 1993 . Life history of the European plethodontid salamander Speleomantes ambrosii. . Herpetological Journal , 3 : 55 – 59 .
- Salvidio , S. 1996 . L’ecologia dei Pletodontidi europei: stato delle ricerche sul geotritone Speleomantes ambrosii. . Studi Trentini di Scienze Naturali—Acta Biologica , 71 : 133 – 136 .
- Salvidio , S. , Lattes , A. , Tavano , M. , Melodia , F. and Pastorino , M. V. 1994 . Ecology of a Speleomantes ambrosii population inhabiting an artificial tunnel. . Amphibia–Reptilia , 15 : 35 – 45 .
- Salvidio , S. , Alario , G. , Pastorino , M. V. and Ferretti , M. 2002 . Seasonal activity and abundance of Speleomantes ambrosii in cave habitats. . Biota , 3 : 149 – 153 .
- Salvidio , S. and Pastorino , M. V. 2002 . Spatial segregation in the European plethodontid Speleomantes strinatii in relation to age and sex. . Amphibia–Reptilia , 23 : 505 – 510 .
- Vignoli , L. , Caldera , F. and Bologna , M. A. 2006 . Trophic niche of cave populations of Speleomantes italicus. . Journal of Natural History , 40 : 1841 – 1850 .