Abstract
Parotoplana rosignana sp. nov., collected at Marina di Cecina, Rosignano Solvay, Castiglioncello and Quercianella (Leghorn, Ligurian Sea), shows a body length of 0.8–1.6 mm. Behind the brain on both sides, two longitudinal rows of medium–large testes reach, in a single line, two germaries at about one‐third of body length. Immediately after the small ovaries two rows of vitellaries are present on both sides; these run along the ‘kragenförmig’ pharynx to the penis papilla opening. The male copulatory organ has 12 spines of variable shape and length (79–125 µm) and a central funnel‐shaped sting (80 µm long). The first description of the post‐embryonal development of otoplanids is given in this flatworm, and it appears of proterogynic type, but the maturation of the female and male gametes in the adult is contemporaneous.
Introduction
The family Otoplanidae (Plathelminthes, Rhabditophora, Proseriata) is represented by a group of typically marine flatworms inhabiting the sandy‐breaker zone of sea coasts known as the ‘Otoplanen‐Zone’ of Remane (Citation1933).
Four subfamilies, differentiated by peculiar features used for their taxonomic classification, are recognized:
Archotoplaninae, characterized by a completely ciliate body and a long cylindrical pharynx, situated horizontally along the ventral zone. | |||||
Bulbotoplaninae, characterized by a partially ciliate body, a ciliate sole or ‘Kriechsohle’ and a ventrally located bulbous pharynx. | |||||
Otoplaninae, characterized by a partially ciliate body, a ciliate creeping sole and a long cylindrical pharynx situated horizontally along the ventral zone. Some genera (Otoplana and Kata) possess male genital pores for the discharge of surplus spermatozoa. | |||||
Parotoplaninae, characterized by a partially ciliate body, a ciliate creeping sole and a transversally located short collar‐shaped pharynx. | |||||
Otoplanidae species have been collected in different globe zones:
Seawater: | |||||
| |||||
Brackish and fresh water: | |||||
| |||||
The new species Parotoplana rosignana is attributed to the subfamily Parotoplaninae essentially on account of the collar‐shaped pharynx, perpendicularly positioned to ventral surface.
Material and methods
The specimens were collected in four localities of the Leghorn coast (Table ). Each organism was first anaesthetized with an acqueous solution of MgCl2 7%. Subsequently, at least 80 specimens were studied in vivo by slight squeezing under the coverslip, in order to draw the habitus with the aid of the Camera Lucida. Finally, by compressing the coverslip more forcefully, the spines of the sclerotic apparatus were examined.
Table I. List of sampling localities.
For histological procedures, five specimens were fixed in Stieve solution. The sections were stained with Heidenhain's hematoxylin, using eosin as counterstain.
A graphical elaboration was used to support the microscopic study.
Taxonomic accounts
Phylum PLATHELMINTHES Schneider, Citation1873
Class RHABDITOPHORA Ehlers, Citation1985
Superorder SERIATA Bresslau, 1928–Citation33
Order PROSERIATA Meixner, Citation1938
Suborder LITOPHORA Steinböck, Citation1925
Family OTOPLANIDAE Hallez, Citation1892
Subfamily PAROTOPLANINAE Ax, Citation1956
Genus Parotoplana Meixner, Citation1938
Parotoplana rosignana sp. nov.
Material examined
At least 30 mature and 50 immature specimens were studied in vivo.
Holotype: one sagittally sectioned specimen is deposited in the Electron Microscopy Laboratory Collection of the Dipartimento di Biologia, Unità di Etologia (Università di Pisa).
Description
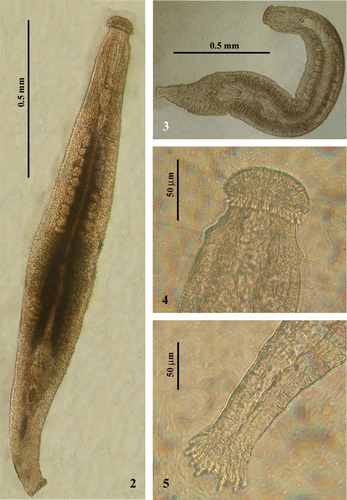
The body length of sexually mature organisms varies from 0.8 to 1.6 mm.
The anterior end is characterized by a well‐marked and swollen ‘Köpfchen’. Tactile hairs are present on the frontal and lateral sides in variable number and dimensions (Figures –).
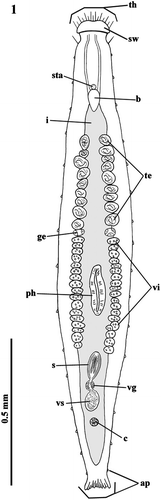
Figure 1 Habitus of Parotoplana rosignana sp. nov.: th = tactile hairs, sw = cephalic swelling, sta = statocyst, b = brain, i = intestine, te = testes, ge = germaries, vi = vitellaries, ph = pharynx, s = sclerotic apparatus, vg = vesicula granulorum, vs = vesicula seminalis, c = cocoon and ap = adesive papillae.
The small brain, located at the back of the statocyst, is ovoidal (Figures –).
The testes, placed behind the brain and anteriorly to the pharynx, consist of two rather close series of follicles along the longitudinal axis. They are not numerous (9–10 per side), are medium‐large in size and reach, in a single line, the two germaries at about one‐third of body length (Figures and ).
Immediately behind the small globoid ovaries, two rows of vitellaries are present on both sides, running along the pharynx up to opening of the penis papilla (Figure ).
The pharynx, situated in the posterior half of the body, shows the so‐called collar‐shaped or ‘kragenförmig’ organization, typical of the genus (Figure ).
The sacciform intestine is a caecum at both ends (Figure ).
In the postpharyngeal zone, the vesicula seminalis and the vesicula granulorum show the usual localization.
Behind the vesicula granulorum, in the genital atrium, an amber‐coloured cocoon can rarely be found (Figures and ).
The caudal, fan‐shaped plate is well‐developed and has numerous adhesive papillae (Figures , , and ).
Figures 2–5 Photographs ofParotoplana rosignana sp. nov. in vivo: 2–3, living animals; 4, Anterior end; 5, Posterior end.
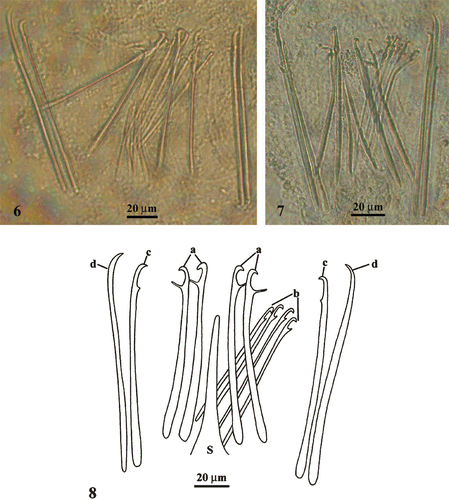
The male copulatory organ is characterized by a sclerotic apparatus with spines of variable shape and length (79–125 µm) and a central funnel‐shaped sting (Figures ):
a ‘Trichterrohr’ or funnel‐shaped sting (S), 80 µm long, is situated in the centre of the sclerotic complex; | |||||
2 pairs of slightly curved spines (a), 93–100 µm long, placed at both sides of the central sting with a large rounded proximal end, a distal hooked tip and a fine sub‐terminal prominence on the concave side; | |||||
a group of 4 thin spines (b) placed ventrally at one side of the (a), 79–85 µm long, with a hooked distal end and a pointed sub‐terminal prominence; | |||||
1 pair of spines (c), 115 µm long, arranged externally at both sides of the (b) with a hooked distal end and a small cuneiform sub‐terminal prominence; | |||||
2 spines (d), 125 µm long, the outermost ones at both sides, with a proximal end rounded and a tip hooked, slightly curved on the concave side. | |||||
Post‐embryonal development
At least 50 specimens were examined at various juvenile stages, which are described and numbered according to their temporal progression (Figure ).
Figure 9 Representation by drawings and photos of the life cycle ofParotoplana rosignana sp. nov.: sw = cephalic swelling, sta = statocyst, b = brain, i = intestine, te = testes, ge = germaries, ph = pharynx, vi = vitellaries, s = sclerotic apparatus, vg = vesicula granulorum, vs = vesicula seminalis, c = cocoon, ap = adhesive papillae, vc = vitellocytes, iesg = internal egg‐shell granules, eesg = external egg‐shell granules, c = cocoon, dc = digestive cells and gz = growth zone.
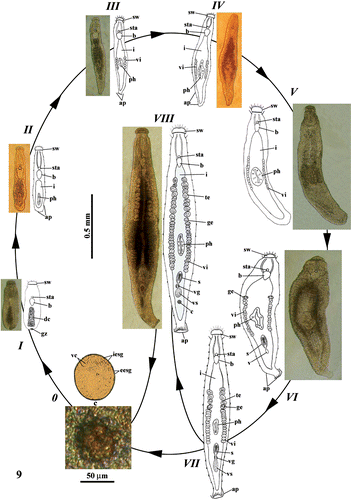
Stage 0
The globose and amber‐coloured cocoon, located in the genital atrium, contains ovocytes and vitellocytes surrounded by a capsule (shell or cocoon).
Stage I
Upon hatching, the juvenile is about 0.45 mm long, with a completely formed anterior end, similar to that of the adult. The cephalic swelling, the frontal and lateral tango‐receptors, the adhesive papillae, the statocyst and the ovoidal brain are present. The subsequent region is devoid of pharynx and intestine, but shows a mass of presumptive digestive cells. Male and female reproductive apparatuses are absent. The caudal end possesses a cluster of putative totipotent cells constituting the growth zone.
Stage II
When the young organism reaches a length of about 0.55 mm, the second body half has a small collar‐shaped pharynx and an Anlage of sacciform intestine. The male and female reproductive apparatuses are not yet formed. Bi‐glandular adhesive papillae are clearly visible on caudal end.
Stage III
When the juvenile is 0.65 mm long, two rows of vitellaries, including 5–6 follicles per side, are present laterally to the pharynx. The caudal end shows a small fan‐shaped plate with adhesive papillae.
Stage IV
When the body length reaches about 0.75 mm, the vitellaries are more numerous (11–12 follicles per side).
Stage V
The juvenile, about 0.9 mm long, presents an increased number of vitelline follicles (15–16 per side), which extend anteriorly to the pharynx.
Stage VI
The juvenile (about 1 mm long) shows two early germaries located just anteriorly to the yolk follicles. In the post‐pharyngeal zone, the sclerotic apparatus appears with spines and Anlage of vesicula seminalis.
Stage VII
The individual (about 1.3 mm long) is nearly complete. The female reproductive apparatus shows the typical adult organization, whereas the male one is slightly less developed, with vesicula seminalis, vesicula granulorum, sclerotic apparatus and only a few testicular follicles (3–5 per side).
Stage VIII
The mature animal reaches a body length of about 1.6 mm. The testes are numerous (9–12 per side).
Conclusive remarks
With the description of the new species, P. rosignana, the taxon Parotoplana now comprises a total of 17 known valid species: P. capitata Meixner, Citation1938 (Ax, Citation1956) found in France and Denmark (Atlantic Ocean and North Sea), P. moya Marcus, Citation1949 sampled in Brazil (Atlantic Ocean), P. multispinosa Ax, Citation1956 collected in France (East Pyrenees), P. papii Ax, Citation1956 discovered in France and Denmark (respectively, Atlantic Ocean and North Sea), P. primitiva Ax, Citation1956 collected in France (Atlantic Ocean), P. procerostyla Ax, Citation1956 sampled in France (East Pyrenees), P. renatae Ax, Citation1956 sampled in France (East Pyrenees), P. pacifica Ax & Ax, Citation1967 sampled at Reid Rock (Pacific Ocean), P. turgida Ax & Ax, Citation1974 discovered in Galapagos Islands (Pacific Ocean), P. bicupa Sopott‐Ehlers, Citation1976 sampled in Archachon (France, Atlantic Ocean), P. macrostyla Lanfranchi, Citation1978 collected at Castiglioncello (Leghorn, Ligurian Sea), P. uncinata Lanfranchi, Citation1978 collected in Secche della Meloria (Leghorn, Ligurian Sea), P. bermudensis Ax & Sopott‐Ehlers, Citation1987 collected in Bermuda Islands (Atlantic Ocean), P. lata Ax & Sopott‐Ehlers, Citation1987 sampled at North Rock (Bermuda Islands, Atlantic Ocean), P. mollis Ax & Sopott‐Ehlers, Citation1987 discovered in Bermuda Islands (Atlantic Ocean), P. subtilis Ax & Sopott‐Ehlers, Citation1987 collected at Windsor Bay (Bermuda Islands, Atlantic Ocean).
The study of our species habitus evidenced a body length (0.8–1.6 mm) similar to or not much lower than that of the following species: P. capitata (1.5–2 mm), P. mollis (1.5 mm), P. multispinosa (1.5–2 mm), P. papii (1–1.2 mm), P. renatae (1.5–2 mm) and P. subtilis (1.5–2 mm). The remaining species present a greater body length.
The appearance of the anterior swelling is reminiscent of that observed in P. macrostyla, but it is more marked. The distribution of tango‐receptors corresponds to that of P. lata, P. macrosyla, P. moya, P. pacifica and P. uncinata.
The small statocyst is spaced from the anterior end similarly to that of P. papii.
The path of the testes is typical of the genus, but their position and distribution in two rather close rows is present only in P. mollis. The number of follicles is similar to that found in P. bermudensis, P. capitata, P. lata, P. macrostyla, P. mollis, P. primitiva, P. renatae, and P. uncinata; their dimensions are comparable to those of P. bicupa, P. renatae and P. uncinata.
The two germaries are situated immediately behind the testes as in P. bicupa, P. multispinosa, P. pacifica, P. papii and P. subtilis. Their medium–large dimensions are near to those of P. bermudensis, P. papii and P. turgida.
The more internal path of the double row of yolk follicles is a peculiarity of the present species. Their small extension is more similar to P. bicupa, P. macrostyla, P. mollis, P. turgida and P. uncinata. Their dimensions are near to those measured in P. bicupa, P. macrostyla, P. mollis, P. renatae and P. uncinata.
The pharynx shows the typical characteristics observed in all the genera of the subfamily.
The sacciform intestine is similar to that of all the species of the genus.
The locations of the vesicula seminalis, vesicula granulorum and penis papilla appear to coincide with that of P. bicupa, P. primitiva, P. renatae and P. turgida.
The caudal end recollects that of P. macrostyla and P. uncinata.
The male copulatory organ possesses a ‘Trichterrohr’ or stylet as in P. macrostyla, P. pacifica, P. papii, P. procerostyla, P. renatae and P. uncinata. The central position of this funnel‐shaped structure is similar to that described in P. procerostyla, its length (80 µm) is in accordance with that of P. macrosyla, whereas the other species present a larger stylet (up to 120 µm).
The spines surrounding the stylet, in the new species, form four groups as in P. procerostyla, although they are of smaller dimensions (respectively, 93–100 µm and 105–110 µm). Both pairs have a hooked and pointed distal end like in P. procerostyla, although the hook of new species is more marked and turned downwards, and moreover the spine proximal end is larger. The sub‐terminal prominence is long and fine, whereas that of P. procerostyla is shorter and has an underlying swelling.
The group of four spines differs in number, position and form from the corresponding six of P. procerostyla. Their hooked and pointed distal end and the orientation of the marked sub‐terminal prominence appear different, as well as being shorter: 79–85 µm in comparison with 100 µm of P. procerostyla.
The exterior pairs differ from each other, whilst in P. procerostyla they are identical. The pair on the proximal end is larger and has a small cuneiform sub‐terminal prominence. The other pair is similar to the corresponding one of P. procerostyla, although wider on the proximal end. Their length in P. rosignana is 115–125 µm, whereas in the other species it reaches 125–130 µm.
On the basis of the data presented, we conclude that our species differs from the previously described species in body dimensions and, above all, the characteristics of the sclerotic apparatus. The most similar species is P. procerostyla.
Post‐embryonal development
Only a few data are available on development and growth of ‘turbellarians’. They deal with egg development (van Beneden 1870), embryogenesis (Hallez Citation1887) and fertilization (Schockaert Citation1905). Copulation and egg laying of the acoel Polychoerus carmelensis were described more recently (Costello & Costello Citation1938, Citation1939); descriptions of turbellarian reproduction and embryogenesis are present in Henley (Citation1974) and Giesa (Citation1966). None of these studies explain the modalities of the post‐embryonal development. Hallez (Citation1909) proposed a short and incomplete account of immature specimens closely related to otoplanids.
The cocoon of P. rosignana is present in the genital atrium before the deposition, and contains oocytes and vitellocytes. Only in P. multispinosa, a ‘grosses Ei’ is found in the genital atrium (Ax Citation1956, p. 730). This corresponds with the cocoon of P. rosignana. The eggshell appears constituted of two layers: externally, the ‘Schalendrüsen’ or shell glands form the exterior envelope, and inside, the granules of vitellocytes and oocytes produce the interior envelope. At stage I, the newly hatched juvenile possesses statocyst, brain and a rudiment of digestive system, without pharynx or intestine. The caudal extremity shows a cell mass, presumably constituted of neoblasts. The trend of growth process is clearly antero‐posterior. At stage II, the juvenile is longer with a small pharynx and a short intestine sack instead of the above‐mentioned cell cluster. The appearance of the vitellaries on either side of the pharynx indicates stage III. Stages IV and V are characterized by further growth and the development of pre‐ and post‐pharyngeal extensions of vitellaries. At stage VI, the germaries appear just in front of vitellaries, and the sclerotic apparatus appears in the post‐pharyngeal zone. Stage VII shows two lateral rows of testes anteriorly to the germaries. The male copulatory apparatus is completed with the testicular follicles reaching the post‐cerebral region and the vesicula seminalis provided with numerous sperm (stage VIII). At this point the organism reaches the maximum length (1.6 mm) and presents 9–12 testis follicles per side and a complete penis papilla.
Detailed study of the juveniles of P. rosignana evidenced the early outbreak of the female elements, contrary to our expectations. The proterogyny of the otoplanids is confirmed in Kata sp. by Noreña et al. (Citation2005). The cocoons and the juveniles are present in deep layers of the sandy breaker zone, which is only rarely sampled. For this reason, they are found only infrequently. The above‐described development type has been confirmed through our collection of juveniles of Parotoplanella sp., Postbursoplana sp., Orthoplana sp. and Otoplana sp.
References
- An der Lan , H. 1964 . Zur Turbellarien‐Fauna der Donau. . Archiv Hydrobiologie , 27 (supplement) : 3 – 27 .
- Ax , P. 1951 . Die Turbellarien des Eulitorals der Kieler Bucht. . Zoologische Jahrbucher Abteilung für Systematik , 80 : 277 – 378 .
- Ax , P. 1956 . Monographie der Otoplanidae (Turbellaria): Morphologie und Systematik. . Akademie der Wissenschaften und der Literatur Abhandlungen der mathematisch‐naturwissenschaftlichen Klasse , 13 : 159 – 278 .
- Ax , P. 1959 . Zur Systematik, Ökologie und Tiergeographie der Turbellarienfauna in den Ponto‐kaspichen Brackwassermeeren. . Zoologische Jahrbücher Abteilung für Systematik, Ökologie und Geographie der Tiere , 87 : 71 – 89 .
- Ax , P. and Armonies , W. 1990 . Brackish water Plathelminthes from Alaska as evidence for the existence of boreal brackish water community with circumpolar distribution. . Microfauna Marina , 6 : 7 – 109 .
- Ax , P. and Ax , R. 1967 . Turbellaria Proseriata von der Pazifikküste der USA (Washington). . Zoologische Morphologie Tiere , 61 : 215 – 254 .
- Ax , P. and Ax , R. 1974 . Interstitielle fauna von Galapagos V. Otoplanidae (Turbellaria, Proseriata). . Mikrofauna des Meeresbodens , 27 : 573 – 598 .
- Ax , P. and Sopott‐Ehlers , B. 1987 . Otoplanidae (Plathelminthes, Proseriata) von Bermuda. . Microfauna Marina , 3 : 261 – 281 .
- Ax , P. , Sopott‐Ehlers , B. and Weidemann , E. 1978 . Zur morphologie sublitoraler Otoplanidae (Turbellaria, Proseriata) von Helgoland und Neapel. . Zoomorphologie , 90 : 113 – 133 .
- Beneden van , E. 1870 . Recherches sur la composition et la signification de l'oeuf. . Mémoires Couronnés pour l'Acadèmie Royale de Belgique, Bruxelles , 34 : 61 – 69 .
- Bresslau , E. 1928‐33 . “ Turbellaria. ” . In Handbuch der Zoologie , Edited by: Kükenthal , W and Krumbach , T . Vol. 2 , 52 – 304 . Berlin : Walter de Gruyter .
- Calandruccio , S. 1897 . Anatomia e sistematica di due specie nuove di Turbellaria. . Atti dell'Accademia Gioenia di Scienze Naturali di Catania Ser. 4 , 10 : 1 – 18 .
- Costello , H. M. and Costello , D. P. 1938 . Copulation in the Acoelous Turbellarian Polichoerus carmelensis. . Biological Bulletin , 75 : 85 – 98 .
- Costello , H. M. and Costello , D. P. 1939 . Egg laying in the Acoelous Turbellarian Polichoerus carmelensis. . Biological Bulletin , 76 : 80 – 89 .
- Du Plessis , G. 1889 . Note sur l'Otoplana intermedia. . Zoologische Anzeiger , 12 : 339 – 342 .
- Ehlers , U. 1985 . Das Phylogenetische System der Plathelminthes , Stuttgart : Fischer .
- Giard , M. A. 1904 . Sur une faunule caractéristique des sables à Diatomées d'Ambleteuse (Pas‐de‐Calalis) III. Les Gastrotriches aberrants. Comptes Rendus de Sciences. . Société de Biologie , 56 : 1063 – 1065 .
- Gieysztor , M. 1938 . Über einige Turbellarien aus dem Süβwasserpsammon. . Archiv Hydrobiologie und Ichtyologie , 11 : 364 – 382 .
- Giesa , S. 1966 . Die Embryonalentwicklung von Monocelis fusca Örsted (Turbellaria: Proseriata). . Tiere Zeitschrift für Morphologie und Ökologie der Tiere , 57 : 137 – 230 .
- Graff von , L. 1913 . Turbellaria II. Rhabdocoelida. . Tierreich , 35 : 449 – 452 .
- Hallez , P. 1887 . Embryogénie des Dendrocoeles d'eau douce. . Separatabruck aus: Mémoires de la Sociéte des Sciences de Lille. Sér. 4 , 16 : 1 – 107 .
- Hallez , P. 1892 . Classification des Triclades. . Bulletin de la Société Zoologique de France , 17 : 106 – 109 .
- Hallez , P. 1909 . Cycle biologique d'une forme voisine des Otoplana. . Comptes Rendus de Académie des Sciences Paris , 8 : 1 – 3 .
- Hallez , P. 1910 . Un nouveau type d'Alloiocoele (Bothriomolus constrictus n. g. n. sp.). . Archive de Zoologie Expérimental et Général , 3 : 611 – 664 .
- Henley , C. 1974 . “ Platyhelminthes (Turbellaria). ” . In Reproduction of marine invertebrates, vol. 1. Acoelomates and pseucoelomate metazoans , Edited by: Giese , A. C and Pearse , J. S . 267 – 343 . New York : Academic Press .
- Karling , T. G. 1964 . Marine Turbellaria from the Pacific coast of North America (III) Otoplanidae. . Arkiv för Zoologi , 16 : 527 – 541 .
- Karling , T. G. 1973 . Anatomy and taxonomy of a new Otoplanid (Turbellaria, Proseriata) from south Georgia. . Mikrofauna des Meeresbodens , 16 : 362 – 269 .
- Lanfranchi , A. 1969 . Nuovi Otoplanidi (Turbellaria, Proseriata) delle coste della Liguria e della Toscana. . Bollettino di Zoologia , 36 : 167 – 188 .
- Lanfranchi , A. 1978 . Morphology and taxonomy of two new Otoplanids (Turbellaria, Proseriata) from the Ligurian Sea. . Zoologica Scripta , 7 : 249 – 254 .
- Lanfranchi , A. and Melai , M. 2007 . Morphology and taxonomy of a new species of Otoplanid (Plathelminthes, Rhabditophora, Proseriata) from the Ligurian Sea. . Italian Journal of Zoology , 74 : 209 – 214 .
- Luther , A. 1960 . Die Turbellarien Ostfennoskandiens. I. Acoela, Catenulida, Macrostomida, Lecithoepiteliata, Prolecithophora und Proseriata. . Fauna Fennica , 7 : 1 – 155 .
- Marcus , E. 1949 . Turbellaria Brasileiros (7). Boletins de Faculdade de Filosofia, Ciências e Letras. Universidade de Sâo Paulo. . Zoologia , 14 : 7 – 156 .
- Marcus , E. 1950 . Turbellaria Brasileiros (8). Boletins de Faculdade de Filosofia, Ciências e Letras. Universidade de Sâo Paulo. . Zoologia , 15 : 5 – 192 .
- Marcus , E. 1952 . Turbellaria Brasileiros (10). Boletins de Faculdade de Filosofia, Ciências e Letras. Universidade de Sâo Paulo. . Zoologia , 17 : 5 – 188 .
- Martens , P. M. and Schockaert , E. R. 1981 . Sand dwelling Turbellaria from the Netherlands Delta area. . Hydrobiologia , 84 : 113 – 127 .
- Meixner , J. 1938 . “ Turbellaria (Strudelwürmer) I. ” . In Die Tierwelt der Nord‐ und Ostsee, Teil IVb , Edited by: Grimpe , G and Wagler , E . 1 – 146 . Leipzig : Akademische Verlagsgesellschaft .
- Noreña , C. , Damborenea , C. and Brusa , F. 2005 . New freshwater interstitial Otoplanidae (Platyhelminthes: Proseriata) from the Paraná and Uruguay rivers, South America. . Journal of Natural History , 39 : 1457 – 1468 .
- Remane , A. 1933 . Verteilung und Organisation der benthonischen Mikrofauna der Kieler Bucht. . Wissenschaftlich Meeresuntersuchungen (N.F.), Abteilung Kiel , 21 : 161 – 221 .
- Riemann , F. 1965 . Turbellaria Proseriata mariner Herkunft aus Sanden der Flubsohle im limnischen Bereich der Elbe. . Zoologische Anzeiger , 174 : 299 – 312 .
- Schneider , A. 1873 . Untersuchungen über Plathelminthen. Separatabdruck aus d. 14. . Jahresbericht Oberhessen Gesellschaft für Natur‐ und Heilkunde Giessen , 14 : 1 – 78 . Tables 3–7
- Schockaert , R. 1905 . La fécondation et la segmentation chez le Thysanozoon brocchi. . La Cellule , 27 : 5 – 37 . Tables 1–3
- Sopott‐Ehlers , B. 1972 . Systematik und Ökologie von Proseriaten (Turbellaria) der deutschen Nordseekuste. . Mikrofauna des Meeresbodens , 13 : 221 – 229 .
- Sopott‐Ehlers , B. 1976 . Interstitielle Macrostomida und Proseriata (Turbellaria) von der französichen Atlantikkuste und den kanarischen Inseln. . Mikrofauna des Meeresbodens , 60 : 558 – 568 .
- Sopott‐Ehlers , B. 1985 . Zwei neue Proseriata (Platyhelminthes) von der französischen Atlantikküste. . Microfauna Marina , 2 : 380 – 396 .
- Sopott‐Ehlers , B. and Ehlers , U. 1980 . Zur Systematik und geographischen Verbreitung interstieller Turbellarien der kanarischen Inseln. . Mikrofauna des Meeresbodens , 80 : 585
- Steinböck , O. 1925 . Zur Systematik der Turbellaria metamerata, zugleich ein Beitrag zur Morphologie des Tricladen‐Nervensystems. . Zoologische Anzeiger , 64 : 165 – 192 .
- Steinböck , O. 1931 . Marine Turbellaria. . Zoology of the Faroe , 8 : 1 – 26 .
- Steinböck , O. 1932 . Die Turbellarien des arktischen Gebietes. . Fauna arctica , 6 : S295 – 342 .
- Tajika , K. I. 1983a . Zwei neue interstitielle Turbellarien der Gattung Archotoplana (Proseriata, Otoplanidae) aus Hokkaido, Japan. . Journal of the Faculty of Sciences Hokkaido University Series 6 Zoology , 23 : 179 – 194 .
- Tajika , K. I. 1983b . Zwei neue Otoplaniden (Turbellaria, Proseriata) aus Hokkaido, Japan. . Annotationes Zoologicae Japonenses , 56 : 100 – 110 .
- Tajika , K. I. 1983c . Zur Kenntnis Gattung Notocaryoplana Steinböck, 1935 (Turbellaria, Proseriata, Otoplanidae). . Bulletin of the National Science Museum Series A (Zoology) , 9 : 97 – 104 .
- Tajika , K. I. 1984 . Eine neue Art der Gattung Itaspiella Ax, 1956 (Turbellaria, Proseriata, Otoplanidae) aus Hokkaido, Japan. . Bulletin of the Liberal Arts and Science Course Nimon University School of Medicine , 12 : 25 – 33 .