Abstract
The present paper gives a comparative analysis of the calling songs of selected populations within species of the genus Cicada Linnaeus from the Greek and Turkish mainlands as well as from a number of representative Aegean islands, with a view to compare present cicada biogeography patterns with the palaeogeography of the area. Recordings of the male calling songs and analyses of selected acoustic variables have been carried out. The general trend in the species distribution and variation in the calling songs produced appeared to be consistent with most of the paleogeographical events in the eastern Mediterranean basin. Cicada species from this area appear to have derived from some stock present in the Miocene in the then united landmass of Aegaeis. Present distribution might have originated mostly by vicariance rather than by dispersal. Therefore, the palaeogeography of the Eastern Mediterranean basin area with emphasis on the Miocenic island groups offers a good explanation for the present distribution patterns and level of endemism in species of the genus Cicada.
Introduction
Species of the genus Cicada Linnaeus, 1758 are medium‐sized cicadas mostly associated with maquis vegetation along the Mediterranean area, and commonly occur in Olea europaea, Pinus spp. and Quercus spp., as well as in Eucalyptus spp. and Vitis vinifera (Patterson et al. Citation1997, Puissant & Sueur Citation2001, Sueur et al. Citation2004). The genus involves a complex of seven closely related species, all morphologically similar but easily distinguished by the acoustic signals produced by males (Quartau & Simões Citation2006).
Along the Mediterranean basin one of the most interesting hot spots for Cicada diversity is the Aegean Sea area. In fact, the Balkans and related regions constitute a high diversity area which offered a number of important refugia during the last cold period, and hence an area where several species within varied taxa might have evolved and from where they might have dispersed to other countries, namely in Europe (e.g. Oosterbroek Citation1994; Taberlet et al. Citation1998; Hewitt Citation1999; Çiplak Citation2004a, Citation2004b; Augustinos et al. Citation2005). According to Oosterbroek and Arntzen (Citation1992) and Oosterbroek (Citation1994), phylogenetically recent groups within different taxa became distributed over the entire Mediterranean but are predominantly of Balkan and West Asia Minor origin. Moreover, the Aegean Sea area has a diverse topography with numerous islands varying in area and distance from the mainland, differing in geological formation processes and with different rates and timing of island uplift. Therefore, this area has a complex colonization history that would have favoured differences in the distribution, divergence and speciation by isolation in several groups, including within the genus Cicada.
The present study has been accomplished with the following main purposes. First, to offer an updated overview of the distribution of the closely related species of the genus Cicada in the Aegean sea area and on which the authors have accumulated a large set of original data mostly of distributional, ecologic and acoustic level during the last decade (e.g. Quartau & Simões Citation2005, Citation2006; Simões et al. Citation2000, Citation2006). Second, by taking into account new acoustic data from further island populations recently investigated, to compare present cicada biogeography patterns with the palaeogeography of the area. In such a way, it is expected that the present analysis might enable one to identify some of the most relevant historical and biogeographic factors responsible for the present patterns of distribution and acoustic variation in the species of Cicada of the Aegean area.
Brief overview on the Aegean paleogeography
The following overview is a summary of the data from Welter‐Schultes (Citation2000), Çiplak (Citation2004b) and Garfunkel (Citation2004).
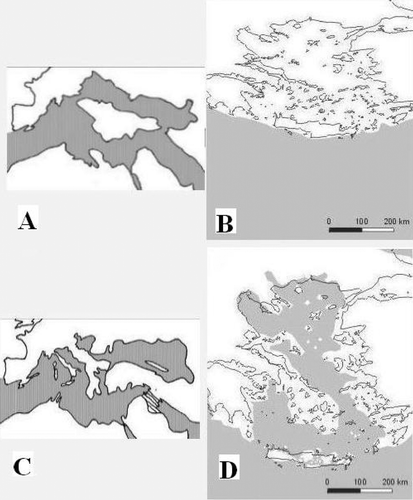
The Eastern Mediterranean basin is thought to be already present in the middle Jurassic, possibly a relict of the Mesozoic Tethys Ocean. Paleogeography of the Aegean Sea and related areas is the result of tectonic evolution of particular plates and blocks. It is assumed that such fragments formed the Aegean plate (the continuous landmass of the Aegaeis), originated in the Cretaceous with the detachment from north of Africa and the posterior movement north‐east in the Tethys Sea. Major tectonic changes around this plate are assumed to have taken place since the late Oligocene and the most important events have occurred during the Miocene (Figure ). In fact, during the late Miocene (Tortonian stage), the Aegean basin became a sea and the Aegean plate was divided in two different plates (Anatolia and Greece plus some parts of the Balkans) (Figure ). This was a very important event in evaluating biogeography of the area since it probably led to independent differentiation of vicariant Aegean stocks in Anatolia and Greece. However, during the Messinian salinity crisis, the desiccation of the Mediterranean provided biotic interchange between Africa, Asia, Europe and the former islands. Therefore, terrestrial connections appeared once again between Greece and Anatolia. Nevertheless, deep canyons in the dried basin with unfavourable climatic conditions must have acted as barriers to the dispersal of many organisms. It was during this period of time that the formation of the Hellenic island arc with several paleoislands occurred (Figures ). Later in the Pliocene the southern portion of the Hellenic arc was tectonically uplifted and the paleoislands were joined to form Crete, which became permanently isolated. Finally, with the opening of the Gibraltar barrier, the reflooding of the Mediterranean in the Pliocene resulted in the more‐or‐less present day land and sea configuration (Figure ), isolating the vicariant populations on the Aegean islands. Additionally, subsequent glacial events during the Pliocene and Pleistocene periods might have also had a great impact on the present faunal composition of Greece, Anatolia and related areas.
Figure 1 Paleogeography of the Eastern Mediterranean. Based on Welter‐Schultes (Citation2000) and Çiplak (Citation2004). A, Middle Miocene (15 Myr); B, Middle Miocene (15 Myr; Aegean area with contour corresponding to present day landmass areas); C, Late Miocene/Tortonian (8 Myr); D, Late Miocene/Messinian (5 Myr; Aegean area with contour corresponding to present day landmass areas). Landmass represented in white and sea represented in grey.
Material and methods
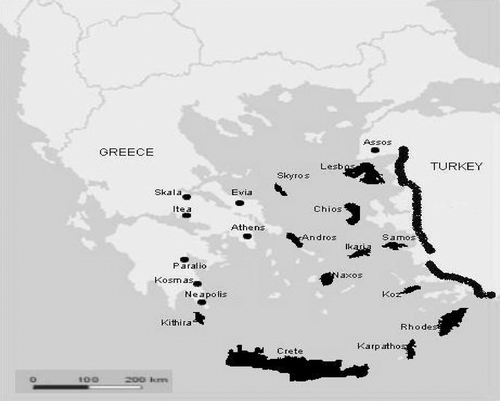
The present work is based on a composite set of data gathered during the last decade in the Aegean Sea area as a result of intensive field‐work in the mainland (Greece, Turkey and Egypt) as well as in 12 Greek islands (figure ; Table ). Recordings of the male calling songs and analyses of the selected acoustic variables (Table ) have been carried out as previously stated (e.g. Pinto‐Juma et al. Citation2005; Quartau & Simões Citation2005; Simões et al. Citation2006).
Table I. Populations of Cicada species sampled with number of individuals (N) used in the present study.
Table II. Description of the acoustic variables analysed.
Statistical tests were performed using STATISTICA 6.0 software (StatSoft, Tulsa). Variables were not normally distributed and, as such, nonparametric tests were used to compare several independent samples for each acoustic variable, with a significance level of α = 0.05 (Dytham Citation2003).
Reduction of dimensionality of the data matrix was made again through an R‐type principal component analysis and using the Kaiser criterion to retain only components with eigenvalues higher than one (e.g. Quartau & Simões Citation2005; Simões et al. Citation2006).
Finally, to compare the amount of variation in the acoustic signals of individual specimens, the coefficient of variation (CV) corrected for small samples (Sokal & Rohlf Citation1981) was applied: CV = 100×(1+1/4n)×SD/Average (n = sample size, SD = standard deviation). Therefore, CV is basically the standard deviation expressed as a percentage of the mean, allowing the comparison of variation between sets of data even when the averages differ greatly. As such, the coefficients of variation for each variable were compared at the intra‐individual (CVind), intra‐population (CVpop) and intra‐species (CVsp) levels.
Results
Altogether, the calling songs of 222 males were analysed (Figure , Table ), corresponding to the sounds of four of the seven known species of the genus Cicada: C. cretensis, C. lodosi, C. mordoganensis and C. orni.
The different species were always allopatric with the exception of C. lodosi and C. mordoganensis, whose populations proved to be sympatric in some pinewood areas in Turkey.
Species distribution
Complementing previous work (Quartau & Simões Citation2005, Citation2006), the present results incorporated additional data and gave a more complete and clearer trend in the distribution of the species under study. Cicada lodosi proved to be a species that occurs exclusively on the Turkish mainland but in respect to the other Cicada species their patterns of distribution are more complex than at first thought. Cicada orni occurs in the west and central Aegean side, extending northeast to Turkey (Figure , Table ) and C. mordoganensis appears only in the east of the Aegean Sea area along the islands of most of the Anatolian coast and on the mainland to the west and south of Turkey. Therefore, there is a clear separation in the distribution of this pair of closely related species.
Interestingly, C. orni and C. mordoganensis were not found in the islands from the Hellenic arc, since only the closely related C. cretensis occurs in Crete (Quartau & Simões Citation2005). The present results include also new specimens from the islands of Kithira and Karpathos (Table ) that are close to C. cretensis from Crete as shown in Figure , a scatter plot of the first two components of a PCA based on the 13 variables selected and for the 3 species analysed here. The first four extracted components accounted for 90.8% of the total variation. More than two‐thirds (68.8%) of the variation in the study was explained by the first two components. There is some separation between the three cicada species analysed when combining components 1 and 2, as can be seen in Figure . Most of the acoustic variables contributed to this pattern of distribution.
Figure 3 Bidimensional diagram of an R‐type principal component analysis applied to a total of 215 males (28 of Cicada cretensis, 103 of C. orni and 84 of C. mordoganensis), based on 13 acoustic characters (standardized data). C. cretensis specimens represented in open symbols, C. mordoganensis in grey symbols and C. orni in black symbols.
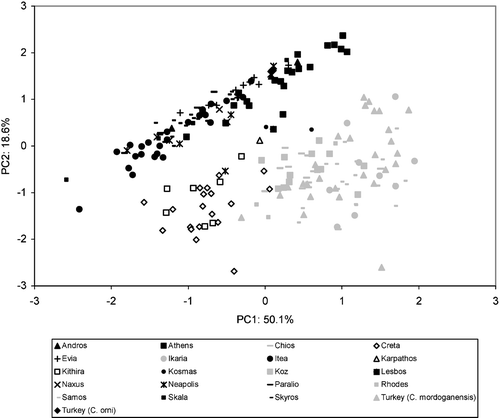
Table III. Descriptive statistics of 13 acoustic parameters in the sampled populations of Cicada cretensis. The time and frequency parameters are in seconds and in Hz, respectively.
Song structure variation
Figures – represent the results of a PCA applied to each of the previous Cicada species, showing no evident pattern of variation for any of them. Cicada lodosi was not included since it is the only species with a continuous calling song pattern and also because the sample was too small for a population analysis. As previously found by Simões et al. (Citation2006), specimens of C. orni appear very close together with no clear separation of different populations as can be seen in the bidimensional diagram of Figure of a PCA analysis, where more than half (64.9%) of the total variation was explained by the first two components.
Figure 4 Bidimensional diagram of relationships between 103 specimens ofCicada orni in a principal component analysis based on a correlation matrix between 13 acoustic characters (standardized data). Ate, Athens; Kosm, Kosmas; Neap, Neapolis; Paral, Paralio.
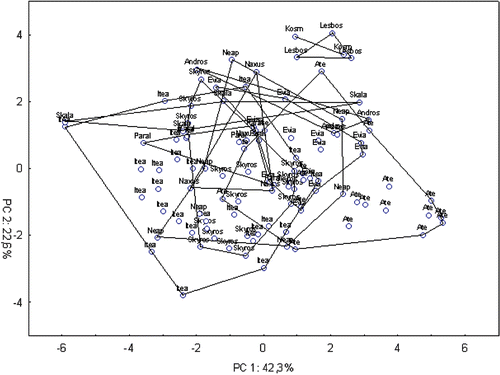
Figure 5 Bidimensional diagram of relationships between 84 specimens ofCicada mordoganensis in a principal component analysis based on a correlation matrix between 13 acoustic characters (standardized data).
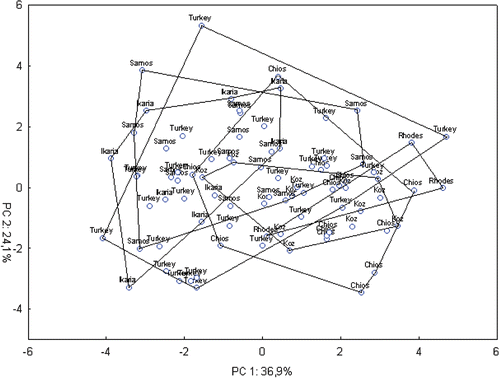
Figure 6 Bidimensional diagram of relationships between 28 specimens ofCicada cretensis in a principal component analysis based on a correlation matrix between 13 acoustic characters (standardized data).
Concerning C. mordoganensis (Figure ), the situation resembles that of C. orni. The first five extracted components accounted for 95.4% of the total variation and again more than half (61%) of the variation in the study was explained by the first two components. Interestingly, specimens from Ikaria and Samos (islands where only C. mordoganensis occur) tend to appear mostly on the left side of the diagram, while those from Rhodes, Chios and Koz tend be on the right side. Nevertheless, they tend to mix with populations from the Turkish mainland.
In respect of C. cretensis (Figure ), the PCA resulted in the extraction of four components accounting for 88.6% of the total variation. The single specimen from Karpathos and those from Kithira clustered with the typical C. cretensis from Crete, in spite of tending to be on the upper part of the scatterplot of the two first components (Figure ). In fact, they showed some acoustic differentiation due mostly to spectral characters in the first component and temporal characters in the second component. Moreover, Mann–Whitney tests between Kithira and Crete populations gave statistically significant differences (P<0.05) in the echeme duration and the related variables number of echemes(s) and echeme period. In short, the echeme duration tended to be shorter in the specimens from these two small islands when compared with Crete (Table ).
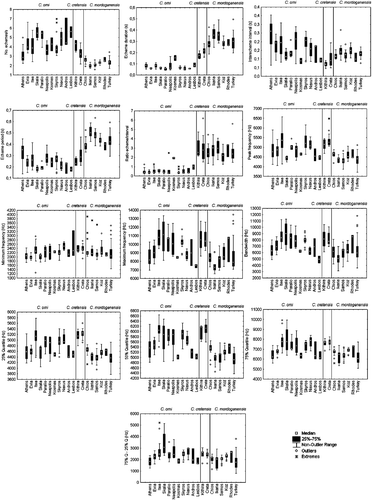
Similarly, when comparing the populations of C. orni with those of C. mordoganensis, some deviated from others for some variables (Figure ). On the other hand, Kruskal–Wallis tests (P<0.05) gave significant differences for all variables except peak frequency, quartile 25% and minimum frequency for C. orni. Equally, for C. mordoganensis most temporal and frequency variables showed differences among populations with the exception of the ratio echeme/inter‐echeme interval, bandwith, quartile 25% and quartile 50%. However, there were no consistent differences between groups of populations or variables.
Regarding these three species, coefficients of variation within and between individuals are low and with values usually below 20% (Table ). When considering the variation of song parameters from different species, it is clear that frequency characters were less variable than the temporal ones. The character presenting the highest coefficient of variation for all levels and species was the ratio echeme/inter‐echeme interval. In contrast, the temporal character echeme duration and echeme period had much lower CVs at all levels and for all species. Inter‐echeme interval was particularly variable, especially in C. cretensis. By contrast, it was less variable for C. mordoganensis.
Table IV. Coefficients of variation for each acoustic variable analysed within specimens (CVind) within populations (CVpop) (average±standard deviation) and within species (CVsp) for the three species investigated.
In general, and for all the three species investigated, the mean intraindividual CVs were lower than intrapopulational CVs (Table ). This allowed the use of a mean value of each variable for each specimen. On the other hand, CVpop values were more similar to CVsp for most of the variables than were CVind.
Discussion
The persistence of a deep sea barrier between the western and eastern Aegean islands explains the present distribution of C. orni and C. mordoganensis. Cicada orni occurs in the west and central Aegean side, while C. mordoganensis appears along most of the Anatolian coast, on the other side and not just in Samos and Ikaria as referred to by Drosopoulos et al. (Citation2006). Other studies based also in insects and other animal groups also revealed this faunal discontinuity between the Western islands on one side and the Eastern ones on the other, in accordance with the paleogeography of the Aegean islands (e.g. Kuhnelt Citation1986; Fattorini Citation2002; Kasapidis et al. Citation2005).
Despite the fact that the Aegean Sea has acted as a prevailing geographic barrier, there has been some contact between the C. orni populations through the mainland across the north of Greece and Turkey. In fact, C. orni occurs in the north of Turkey (Assos), and in the island of Lesbos. Additionally, molecular studies have shown that populations of this island share a 12s RNA haplotype with those from Skyros island on the other side of the Aegean Sea (Pinto‐Juma, personal communication). This similarity is particularly interesting since these two islands are very far apart.
Concerning song structure variation, the acoustic analyses carried out did not completely separate any population and overlap between specimens of different populations was consistently observed. Such homogeneity of calling songs in the Aegean area was already noted in a study performed by Pinto‐Juma et al. (Citation2005) for C. orni populations and is now confirmed when we included further populations from new Greek islands. The same applies to the populations of C. mordoganensis, which also constitute a very homogeneous group.
In respect to Crete, this island exhibits a strong degree of isolation as shown for other insect species and other animal groups, which, like C. cretensis, are also absent in the Peloponnesos and in most of the other Aegean islands (e.g. Kuhnelt Citation1986; Beerli et al. Citation1994; Kasapidis et al. Citation2005). Moreover, populations from Kithira and Karpathos in spite of being very similar to C. cretensis show some small differences to it, namely in the echeme duration. Such differentiation also occurs in other insect groups such as carabids and tenebrionid beetles, whose distribution is restricted to Kithira and others to Karpathos (e.g. Kuhnelt Citation1986).
According to the paleogeographic history of the Aegean area (cf. Figure ), which gives the origin of Crete as the Miocene, it would be expected that the Cretan species might correspond to the first divergent lineage of the insular Aegean Cicada species. This expectation is in agreement with the results achieved by Pinto‐Juma (personal communication), which suggest a basal position of C. cretensis in relation to C. orni and C. mordoganensis and from which they would have been derived. Moreover, C. cretensis is a species with a calling song of the same pattern as the calling songs of both C. orni and C. mordoganensis. In fact, comparing both C. orni and C. mordoganensis to the Cretan species, great similarities can be found with C. orni in the spectral domain and with C. mordoganensis in the temporal domain (cf. Figure ).
Therefore, C. cretensis might have differentiated very probably by isolation with the geographic separation of Crete from the Aegean landmass in the north and during the middle and late Miocene (5 Myr) (Welter‐Schultes Citation2000). On the other hand, the Karpathos populations might have diverged from the remaining Cicada populations about 3 Myr ago. Similarly, Kithira has been an isolated island, separated from Crete and the Peloponnesos during most of the Pliocene (Kasapidis et al. Citation2005). However, it should be taken into account that the present differences found among the specimens from Kithira and Karpathos might be an effect of the small sample investigated.
The hypothesis that the distribution patterns of recent taxa on the Aegean islands reflect paleogeographical pattern was statistically tested by Hausdorf and Henning (Citation2005). Their results show that there is a strong concordance between the age of the islands and the degree of divergence of the species. As such, the composition of the island faunas was found to be strongly influenced by recent geography as well as by Pliocene and even also by Miocene events. On the other hand, climatic parameters were generally hardly found to have influenced the faunal composition. The same appears with other cicadas, namely from the west Pacific island arc, where their distribution and phylogeny clearly reflect the geotectonic history of the area (De Boer Citation1995; De Boer & Duffels Citation1996).
Considering the cicadas here analysed, temporal song characters were found to be more variable than spectral ones, as expected since latter characteristics are very constrained by physical properties of the sound‐producing organ.
In addition, values of within and between individual variation are low and in general below 20%. Therefore, the song characters analysed could be assigned as ‘static traits’ (Gerhardt Citation1991) and since they vary little, they may be used for species recognition within and between individuals.
Moreover, echeme was the most constant variable along the range of variation for C. orni, corroborating results already achieved by Pinto‐Juma et al. (Citation2005). The same happens for C. mordaganensis and C. cretensis. This is another result in agreement with the suggestion that echeme duration is an important parameter following Paterson's (Citation1985) concept of a specific mate recognition system.
Conclusions
The present study gives evidence that the geographic distribution of the genus Cicada can, in general, be correlated with the major paleogeographical separations during the tectonic evolution of the eastern Mediterranean. Moreover, results from Pinto‐Juma (personal communication) suggest that colonization events of the Greek islands occurred prior to the expansion events observed in continental Europe, for which the Pleistocene climate changes are the most likely explanation.
Therefore, despite the fact that there may have been posterior colonizations and since species are mostly allopatric, results support mainly a vicariant pattern of differentiation for Cicada, rather than a dispersal one. In fact, poor dispersal ability is another factor favouring endemism in this group, suggesting that source areas can still be traced from the present day distribution patterns. Cicadas are not, in general, good fliers, have limited ecological tolerance and the adult life is short (Boulard Citation1965; Boulard & Mondon Citation1995; Quartau Citation1995; Sueur & Puissant Citation2001) which are reasons for their apparently poor dispersal ability (the entire life‐cycle may take about 3–4 years, but winged adults only live 2–4 weeks).
It is suggested that the common ancestor that gave origin to the cicada species present in the eastern Mediterranean area appeared prior to the Pliocene, contrary to what was suggested by Quartau and Simões (Citation2006). Indeed, it is very probable given the existence of several fossil records of the genus Cicada in Europe dating from the Tertiary (Riou Citation1995). Therefore, the ancestor might have appeared during the Miocene by the time of disconnection of the Aegean plate.
Cicada evolutionary history may have been subsequently determined by the glacial‐interglacial alternating periods of the Pliocene and Pleistocene which are then considered the periods of Cicada continental expansion events and of regional differentiation.
The present suggestions must be seen as tentative and care should be taken since the evolutionary processes in the Aegean area are quite complex and seem not to follow a stable and uniform pattern (Giokas Citation2000; Chatzimanolis et al. Citation2003). Moreover, further material should also be incorporated in future studies to clarify this matter, namely from Cyprus and such studies should be conducted on both the acoustic and molecular levels.
Acknowledgements
For help in the field, thanks are due to Irfan Kandemir (Department of Biology, Zonguldak Karaelmas University, Turkey) and Sakis Drosopoulos and Penalope Tsakalou (Department of Agricultural Biology and Biotechnology, Agricultural University of Athens, Greece). Thanks are also extended to the referees for their helpful contributions in improving the manuscript.
References
- Augustinos , A. A. , Mamuris , Z. , Stratikopoulos , E. E. , Amelio , S. D. , Zacharopoulo , A. and Mathiopoulos , K. D. 2005 . Microsatellite analysis of olive fly populations in the Mediterranean indicates a westward expansion of the species. . Genetica , 125 : 231 – 241 .
- Beerli , P. , Hotz , H. , Tunner , H. , Heppich , S. and Uzzell , T. 1994 . Two new water frog species from the Aegean islands Crete and Karpathos. (Amphibia, Salientia, Ranidae). . Notulae Naturae , 470 : 1 – 9 .
- Boulard , M. 1965 . Notes sur la biologie larvaire des cigales (Hom. Cicadidae). . Annales de la Société entomologique de France , 1 : 503 – 521 .
- Boulard , M. and Mondon , B. 1995 . Vies Et Mémoires De Cigales–Provence Languedoc Méditerranée , Barbentane : Équinoxe .
- Chatzimanolis , S. , Trichas , A. , Giokas , S. and Mylonas , M. 2003 . Phylogenetic analysis and biogeography of Aegean taxa of the genus Dendarus (Coleoptera: Tnebrionidae). . Insect Systematics and Evolution , 34 : 295 – 312 .
- Çiplak , B. 2004a . Systematics, phylogeny and biogeography of Anterastes (Orthoptera, Tettigoniidae, Tettigoniinae): Evolution within a refugium. . Zoologica Scripta , 33 : 19 – 44 .
- Çiplak , B. 2004b . Biogeography of Anatolia: The marker group Orthoptera. . Memorie Societa Entomologica Italiana , 82 : 357 – 372 .
- De Boer , A. J. 1995 . Islands and cicadas adrift in the west Pacific. Biogeographic patterns related to plate tectonics. . Tijdschrift voor Entomologie , 138 : 169 – 244 .
- De Boer , A. J. and Duffels , J. P. 1996 . Historical biogeography of the cicadas of Wallacea, New Guinea and the West Pacific: A geotectonic explanation. . Paleogeography, Paleoclimatology, Paleoecology , 124 : 153 – 177 .
- Drosopoulos , S. , Eliopoulos , E. and Tsakalou , P. 2006 . “ Is migration responsible for the peculiar geographical distribution and speciation based on acoustic divergence of two cicadas in the Aegean archipelago? ” . In Insect sounds and communication. Physiology, behaviour, ecology and evolution , Edited by: Drosopoulos , S and Claridge , M. F . 219 – 226 . Boca Raton : Taylor & Francis .
- Dytham , C. 2003 . Choosing and using statistics, a biologist's guide , Oxford : Blackwell Science . 2nd ed
- Fattorini , S. 2002 . Biogeography of the tenebrionid beetle (Coleoptera, Tenebrionidae) on the Aegean Islands. . Journal of Biogeography , 29 : 49 – 67 .
- Garfunkel , Z. 2004 . Origin of the eastern Mediterranean basin: A reevaluation. . Tectonophysics , 391 : 11 – 34 .
- Gerhardt , H. C. 1991 . Female mate choice in treefrogs: Static and dynamic acoustic criteria. . Animal Behaviour , 42 : 615 – 635 .
- Giokas , S. 2000 . Congruence and conflict in Albinaria (Gastropoda, Clausiliidae). A review of morphological and molecular phylogenetic approaches. . Belgium Journal of Zoology , 130 : 93 – 100 .
- Hausdorf , B. and Henning , C. 2005 . The influence of recent geography, paleogeography and climate on the composition of the fauna of the central Aegean Islands. . Biological Journal of the Linnean Society , 84 : 785 – 795 .
- Hewitt , G. M. 1999 . Post‐glacial re‐colonization of European biota. . Biological Journal of the Linnean Society , 68 : 87 – 112 .
- Kasapidis , P. , Magoulas , A. , Mylonas , M. and Zouros , E. 2005 . The phylogeography of the gecko Cyrtopodion kotschyi (Reptilia: Gekkonidae) in the Aegean archipelago. . Molecular Phylogenetics and Evolution , 35 : 612 – 623 .
- Kuhnelt , W. 1986 . Contribution to the knowledge of historical biogeography of the Balkan peninsula, especially of Greece. . Biologia Gallo‐Hellenica , 12 : 71 – 84 .
- Oosterbroek , P. 1994 . Biodiversity of the Mediterranean region. . Systematics and Conservation Evaluation , 50 : 289 – 307 .
- Oosterbroek , P. and Arntzen , J. W. 1992 . Area‐cladograms of Circum‐Mediterranean taxa in relation to Mediterranean paleogeography. . Journal of Biogeography , 19 : 3 – 20 .
- Paterson , H. E. H. 1985 . “ The recognition concept of species. ” . In Species and speciation Edited by: Vrba , E. S . 21 – 29 . Transvaal Museum Monograph no 4. Pretoria
- Patterson , J. , Massei , G. and Genov , P. 1997 . The density of cicadas Cicada orni in mediterranean coastal habitats. . Italian Journal of Zoology , 64 : 141 – 146 .
- Pinto‐Juma , G. , Simões , P. C. , Seabra , S. and Quartau , J. A. 2005 . Calling song structure and geographic variation in Cicada orni Linnaeus (Hemiptera: Cicadidae). . Zoological Studies , 44 : 81 – 94 .
- Puissant , S. and Sueur , J. 2001 . Contribuition à l'étude des cigales de Corse (Hemiptera, Cicadidae). . Bulletin de la Societé Entomologique de France , 106 : 429 – 436 .
- Quartau , J. A. 1995 . Cigarras esses insectos quase desconhecidos. . Correio da Natureza , 38 : 19 – 33 .
- Quartau , J. A. and Simões , P. C. 2005 . Cicada cretensis sp. n. (Hemiptera, Cicadidae) from southern Greece. . Biologia , 60 : 489 – 494 .
- Quartau , J. A. and Simões , P. C. 2006 . “ Acoustic evolutionary divergence in cicadas: The species of Cicada L. in southern Europe. ” . In Insect sounds and communication. Physiology, behaviour, ecology and evolution , Edited by: Drosopoulos , S and Claridge , M. F . 227 – 237 . Boca Raton : Taylor & Francis .
- Riou , B. 1995 . Lyristes renei n. sp., cigale fossile du Miocène ardéchois (Homoptera Cicadidae). . EPHE Biologie et Evolution des Insectes , 7–8 : 73 – 76 .
- Simões , P. C. , Boulard , M. and Quartau , J. A. 2006 . On the taxonomic status of Cicada orni Linnaeus (Hemiptera, Cicadidae) from Lesbos island in Greece. . Zootaxa , 1105 : 17 – 25 .
- Simões , P. C. , Boulard , M. M. , Rebelo , M. T. , Drosopoulos , S. , Claridge , M. F. , Morgan , J. C. , Quartau and J. A. 2000 . Differences in the male calling songs of two sibling species of Cicada (Hemiptera: Cicadoidea) in Greece. . European Journal of Entomology , 97 : 437 – 440 .
- Sokal , R. R. and Rohlf , F. J. 1981 . Biometry. The principles and practice of statistics in biological research , New York : H. Freeman and Company .
- Sueur , J. and Puissant , S. 2001 . Une vie de cigale. . Le Courrier de la Nature , 191 : 18 – 23 .
- Sueur , J. , Puissant , S. , Simões , P. C. , Seabra , S. , Boulard , M. and Quartau , J. A. 2004 . Cicadas from Portugal: Revised list of species with eco‐ethological data (Hemiptera: Cicadidae). . Insect Systematics and Evolution , 35 : 177 – 187 .
- Taberlet , P. , Fumagalli , L. and Wust‐Saucy , A. G. 1998 . Comparative phylogeography and postglacial colonization routes in Europe. . Molecular Ecology , 7 : 453 – 464 .
- Welter‐Schultes , F. W. 2000 . The paleogeography of late Neogene cental Crete inferred from the sedimentary record combined with Albernaria land snail biogeography. . Paleogeography, Paleoclimatology, Paleoecology , 157 : 27 – 44 .