Abstract
This study has examined the presence and distribution of extrabulbar olfactory projections (nerve fibers that originate from cells in the olfactory epithelium, bypass the olfactory bulbs and project caudally into the brain) in larvae and adult stages of a semi‐aquatic frog, Rana esculenta. This species was chosen because previous work had suggested that the presence of an extrabulbar olfactory system (EBOS) may correlate with the detection of water‐borne odorants. The main result is that this extrabulbar system is present in both larval and adult specimens of this frog species. In stage 26, the earliest one used in our study, the EBOS is well‐developed and projects as far caudal as the rhombencephalon. During successive larval development and until the completion of metamorphosis, there occurs a progressive reduction in the caudal extent of the EBOS. This reduction is more dramatic during the metamorphic climax (stages 31–33). A reduction in the caudal extent of the extrabulbar projections in adult stages is similar to other species previously examined. We are inclined to believe that there is no correlation between a reduction in the caudal extent of the EBOS and transition from water to land.
Introduction
The notion that the olfactory bulbs are the only target of the axonal fibers originating from neurons located in the olfactory mucosa, as reported in many old and more recent international textbooks (see for instance Cajal Citation1911; Farbmann Citation1992), was challenged by Szabo and his collaborators in 1991. In an immunohistochemical study on the distribution of substance P in the brain of some bony fish species, Szabo's group (Citation1991) observed that although many of the immunostained fibers terminate on the dendritic tree of mitral cells, some of them bypass the olfactory bulbs without synapsing in this area. Originally, they called this fiber system ‘projection olfactive primaire extrabulbaire’ (extrabulbar olfactory projections = EBOP), later renamed as ‘extrabulbar olfactory system’ (EBOS; Schober et al. Citation1994). Since then, one of the most debated argument is whether the EBOS has to be considered a part of the nervus terminalis (NT) or not (see Pinelli et al. Citation2004; von Bartheld Citation2004). Apart from this controversy, here we will consider EBOS as a set of nerve fibers which originate from neurons located in the olfactory epithelium and bypass the olfactory bulbs to project into the brain more caudally.
The presence of a fiber system with these neuroanatomical features was confirmed in many vertebrate groups. In cyclostomes, an EBOS was described as NT (Meyer et al. Citation1987; von Bartheld et al. Citation1987; von Bartheld & Meyer Citation1988; Northcutt & Puzdrowski Citation1988). This fiber system was observed to project to the mesencephalon with targets also in the telencephalon and diencephalon. Among jawed fishes, in the only study available for elasmobranchs the absence of an EBOS was emphasized (Hofmann & Meyer Citation1995). In many teleostean species the EBOS has been described as being quite variable in extension, terminating in the telencephalon in some species (Demski & Northcutt Citation1983; Bazer et al. Citation1987; Riddle & Oakley Citation1992) and projecting as far caudal as the mesencephalon in some others (von Bartheld & Meyer Citation1986; Szabo et al. Citation1991; Hofmann & Meyer Citation1995). In amphibians, an EBOS was reported in some anurans (Hofmann & Meyer Citation1989a, Citation1989b, Citation1992; Pinelli et al. Citation2004) and gymnophiona (Schmidt & Wake Citation1990) with the caudal extent of the extrabulbar projections within the diencephalon. In urodeles, instead, an EBOS projecting as far caudal as the mesencephalon was reported in the axolotl and the rough‐skinned newt (Hofmann & Meyer Citation1989a); an EBOS extending beyond the diencephalon was described in ten species of salamanders (Schmidt et al. Citation1988). Among amniotes, except for a single description of some extrabulbar projections to the rat anterior olfactory nucleus (Monti‐Graziadei, Citation1992) there are no data on the presence of an EBOS in the adult. However, the transient occurrence during development of an EBOS was described in the duck (Meyer et al. Citation1987) and a well‐developed EBOS was also reported in rat embryos (Santacana et al. Citation1992).
Since an EBOS was consistently recorded in aquatic vertebrates, some authors assumed that such a system could be related to water‐borne odorants (Hofmann & Meyer Citation1992, Citation1995; Meyer & Rastogi Citation1998). This hypothesis was also supported by developmental studies performed in Xenopus laevis, with the EBOS more developed in tadpoles than in adults (Hofmann & Meyer Citation1989b; Pinelli et al. Citation2004). Moreover, Hofmann and Meyer (Citation1992) emphasized that in this species the nerve fibers of the EBOS derive from cells of a portion of the olfactory mucosa selectively related to water‐borne odorants.
Among anamniotes, the developmental analysis of an EBOS has so far been made in a perennially aquatic anuran, the African clawed frog, X. laevis (Pinelli et al. Citation2004). The earlier hypothesis that the EBOS regression (i.e. a progressive reduction in the caudal extent of its fiber projections during development) may be related to the evolutionary transition from water to land was strongly debated in this paper. To test this hypothesis, and to extend the knowledge on EBOS development in anuras, a semi‐aquatic anuran species, the edible frog Rana esculenta, was selected in the present study. This study on the developmental pattern of the EBOS, from an early larval stage to the adulthood, is also intended to extend our knowledge on EBOS development in the amphibian group and to test its evolutionary correlations.
Material and methods
Tadpoles and adults of R. esculenta were collected from a downstream affluent of Sele River in the Province of Salerno, Italy. The tadpole specimens were classified according to Witschi (Citation1956) for R. pipiens. At least four samples of each stage were used: stages 26 (hind limb buds), 28 (hind limbs grown with toes well‐developed), 30 (fore limbs emerged), 32–33 (metamorphic climax), newly metamorphosed froglets and adult males and females. All animals were anesthetized in a 0.1% solution of MS222 (tricaine methanesulfonate ethyl m‐aminobenzoate, Sigma Chemical Co.) before dissection. Adult frogs were transcardially perfused with freshly prepared 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer saline (PBS) pH 7.6. The brains, together with the noses, were rapidly dissected out under a binocular microscope and immersed in 4% PFA. After 4 h of fixation, crystals of DiI (1,1′‐dioctadecyl‐3,3,3′3′‐tetramethylindocarbocyanine perchlorate, Invitrogen, California, USA) were applied either into one of the olfactory chambers, or to the cut end of one of the olfactory nerves transected in proximity of the olfactory mucosa. Application sites were then sealed with a drop of agarose. In most cases the samples were left in PFA at 4°C for 4–5 weeks. This timing was selected because it was repeatedly observed in our laboratory that a much longer transport time (up to one year or more) does not reveal more extensive fiber projections within the larval or adult brain (see Figure ). After several washes in PBS the samples were embedded in 4% agarose and sectioned on a Vibratome (Leica VT1000 E). Horizontal sections, 80 µm thick, were collected and mounted on glass slides in PBS and examined in an epifluorescent photomicroscope (DMRB, Leica) equipped with appropriate filter sets and a digital Canon camera. Pictures were saved as TIFF files at 1200 dpi. The digital images were adjusted for brightness and contrast using Adobe Photoshop. Plate photomontage and lettering were made using CorelDraw.
All experimental procedures were in line with the European Community's Council Directive (86/609/EEC).
Results
The two technical approaches employed in this work, i.e. application of DiI to the olfactory chamber or to the cut end of the olfactory nerve, provided comparable results with regard to the EBOS neuroanatomy. Nevertheless, when the tracer was applied to the cut surface of the nerve, the results were comparatively more clear and readily interpreted. This is probably due to the fact that when the tracer is applied to the olfactory chamber, the olfactory epithelium is always slightly damaged and as a consequence in many samples the tracer was found to have diffused to the other olfactory chamber as well as in non‐neuronal structures.
The first larval stage in which it was possible to successfully employ our tracer technique was stage 26 when the hind limb buds begin to grow. In earlier stages of development, the olfactory structures were too small to allow a selective application of the tracer. Due to inherent methodological difficulties in discerning the synapses, we were unable to ascertain the precise innervation areas. As such, the terminal fields should be interpreted as putative innervation sites.
In all samples the entire olfactory epithelium and the olfactory/vomeronasal nerve complex containing EBOS fibers were heavily stained. At the same time, the glomerular layer of the main and accessory olfactory bulbs was also stained. Running caudally, compact fiber bundles and a number of scattered, closely lying, single fibers clearly bypassed the main olfactory bulbs. Running along a mediobasal route these stained fibers reached the ventromedial telencephalon. There are some major differences concerning different developmental stages such as the presence of some stained neurons in the premetamorphic tadpoles (stages 26, 28 and 30), variable frequency of EBOS fibers and their rostrocaudal extent of distribution within the brain and sometimes their putative target areas.
At stage 26 of development, the entire mass of the ipsilateral main olfactory bulb was stained; some stained fibers of the olfactory nerve innervated the contralateral glomeruli. Stained fibers bypassed the ipsilateral olfactory bulb rostroventrally. The stained fibers then emerged caudally, along the olfactory bulb's mediolateral extension, either as scattered single fibers or as 3 or 4 robust fiber bundles (Figures , ). All of the single fibers and fiber bundles converged medially and extended caudally along the mediobasal telencephalon up to the anterior commissure. Some fibers separated from the compact fiber bundles in the medioventral telencephalon and deviated laterally to terminate in and around the septal area. Although few, stained fibers were also observed in the more dorsal portions of the medial telencephalon. An interesting feature of the EBOS along its telencephalic course is that numerous single fibers anastomosed here. Thus augmenting the overall frequency of fibers that project posteriorly. Although less frequently than in the telencephalon, anastomosing stained fibers were also observed in other brain areas. Within the caudalmost telencephalon, furthermore, a majority of the stained fibers, single or those organized in bundles, decussated at the anterior commissure and projected to the contralateral side (Figures , ). Some of the fibers deviated laterally and reached the ipsilateral posterior pallium. Some of the stained fibers as far caudal as the anterior commissure spreading around the ipsilateral portion of the preoptic recess and randomly diffusing into the ipsilateral preoptic nucleus (Figure ). Further caudal to the anterior commissure, interestingly, all ipsilateral EBOS fibers continued as scattered single fibers, whereas on the contralateral side, single fibers as well as compact fiber bundle were observed. In fact, EBOS fibers were comparatively more abundant on the contralateral side than on the ipsilateral side (Figures , ). In the ventrolateral diencephalon, a few stained fibers left the main fiber bundle and projected to the infundibulum. The majority of fibers, nevertheless, crossed the thalamus and run caudally to project into the mesencephalon. From here some contralaterally located fibers were observed to re‐decussate at the level of the posterior commissure (Figure ), thus going back to the ipsilateral side and terminating in the immediate vicinity of the commissure. Further caudal to the posterior commissure, in the rhombencephalon, even less frequently, the stained EBOS fibers were scattered on the contralateral side. Besides the large number of stained fibers in this stage of development, some stained pericarya, usually round or slightly elongated, were also observed (Figure ). These were located ipsilaterally in the anterior basal telencephalon, just caudal to the ipsilateral main olfactory bulb and along the medial septal area. They were observed only in two or three sections in which the EBOS was also visible. Their presence (on an average 20 neurons per brain, distributed in a rostrocaudal fashion) was ascertained in tadpoles in stages 26, 28 and 30 of development.
Figure 1. Schematic line drawings illustrating the extension of the EBOS in horizontal ventral representative sections of the brain as obtained by anterograde DiI tracing technique.a, Stage 26−27; b, stage 28−29; c, 30−32; d, neometamorphosed and adult. Abbreviations: AC, anterior commissure; APOA, anterior preoptic area; INF, infundibulum; MES, mesencephalon; OBL, olfactory bulb; MS, medial septum; PC, posterior commissure; RHOM, rhombencephalon.
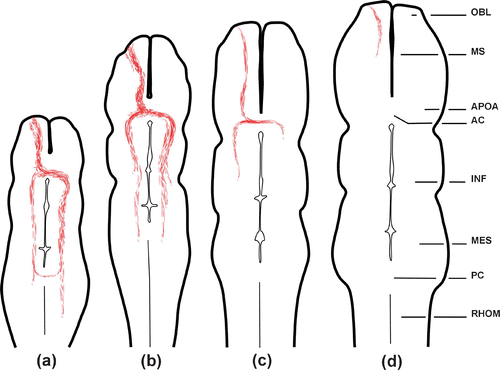
Figure 2. Horizontal sections of the brain of a stage 26 sample showing EBOS central projections after DiI application to the olfactory mucosa.A, Section at the forebrain level; many fibers (arrows) crossing the anterior commissure (AC) reach the contralateral side. OB, olfactory bulb; MS, medial septum; TEL, telencephalon. B, Section at mesencephalic level; note a fiber (arrowhead) crossing the posterior commissure (PC). C, Stained fiber bundle (asterisk) and single fibers (arrows) in the postbulbar basal telencephalon. D, Innervation (arrow) of the nucleus preopticus. POR, preoptic recess. E, Cell bodies in the anterior basal telencephalon, just behind the ipsilateral telencephalon ventricle after DiI application to the cut end of the olfactory nerve complex (stage 30). The vertical dotted line in A and B represents the midline. All images, except E, are oriented along the anteroposterior axis (a: anterior, p: posterior). Scale bars = 200 µm in A, B; 50 µm in C−E.
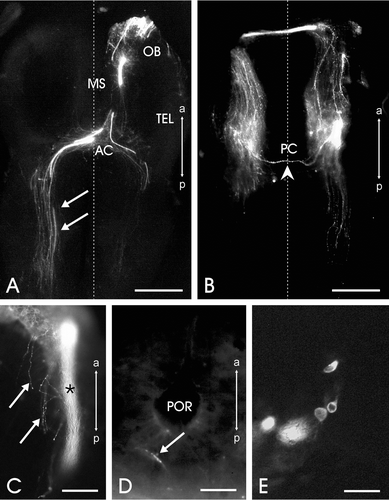
At stage 28, a comparatively less extensive fiber network was revealed after tracer application. In general terms, the distribution pattern of the stained fibers resembled to some extent that of the previous stage of development. The fiber bundles just behind the olfactory bulb appeared comparatively thicker than before, whereas the single fibers bypassing the olfactory bulbs were comparatively less frequent (Figures , ). Moreover, a few sets of fibers were observed to leave the olfactory bulb laterally; these fibers extended caudally in the immediate vicinity of the lateral wall of the telencephalon and terminated within a short distance. Tiny fiber bundles and single fibers were observed caudal to the anterior commissure (Figure ). These fibers invaded diffusely the thalamus and the hypothalamus. Invariably, a heavy innervation of the ipisilateral preoptic nucleus was observed, whereas the contralateral half of this nucleus contained only scanty fibers. Unlike the previous stage, the frequency of stained fibers and their extension in both halves of the brain, posterior to the anterior commissure, appeared equivalent and the fibers did not project beyond the mesencephalon (Figure ).
Figure 3. Horizontal sections of the brain of a stage 28 sample showing EBOS central projections after DiI application to the olfactory mucosa. A–C are enlargements of boxed areas in D. A, Section at the telencephalic level; heavy staining of the olfactory bulb (OB) and many stained EBOS fibers, single (arrowheads) and compact bundles (arrows). LV, lateral ventricle; MS, medial septum. B, Stained fibers converge anterior to the anterior commissure (AC). Many fibers reach the contralateral side and continue posteriorly as single fibers (arrowheads) or compact bundles (arrow). C, Fibers at the level of mesencephalon. D, Whole brain section. OM, olfactory mucosa. The vertical dotted line in A−D represents the midline. All images are oriented along the anteroposterior axis (a: anterior, p: posterior). Scale bars = 150 µm in A−C; 500 µm in D.
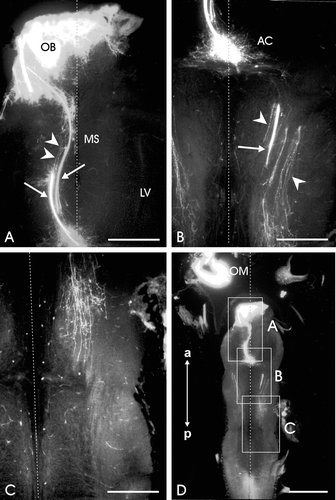
In stage 30, a general reduction in the rostrocaudal extent of distribution of the extrabulbar fiber system had occurred. Furthermore, this fiber system showed some remarkable differences with respect to the previous stages. Besides the substantial reduction in frequency than in earlier stages of development, the majority of stained fibres projected within the thalamic diencephalon. The bulk of the tracer‐stained fibers decussating at the anterior commissure was also greatly reduced and the ipsilateral fiber group appeared comparatively more conspicuous (Figures , ). No stained fibers were observed in any other brain area caudal to the diencephalon. During the metamorphic climax (stages 32 and 33) there were clear signs of a further progressive reduction in the caudal extent of the EBOS fibers.
Figure 4. Horizontal brain section of a sample at stage 30 showing EBOS after DiI application to the olfactory epithelium. The stained fibers are reduced if compared to the previous stages of development. AC, anterior commissure; LV, lateral ventricle; MS, medial septum. The vertical dotted line represents the midline. The image is oriented along the anteroposterior axis (a: anterior, p: posterior). Scale bar = 500 µm.
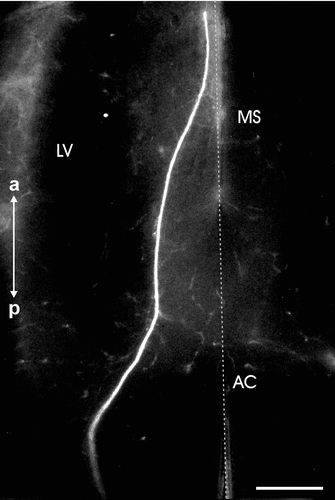
Figure 5. Horizontal section of the brain of an adult sample after DiI application to the cut end(in proximity of the olfactory mucosa) of the olfactory/vomeronasal nerve complex showing a solitary stained fiber (arrowhead) bypassing the olfactory bulbs and terminating in the medial septal area. The sample was kept for 13 months in PFA 4% at 4°C. Note that the bright staining of the main (OBL) and accessory (AOBL) olfactory bulbs is similar to that of samples in which the tracer transport time was 4–5 weeks. The vertical dotted line represents the midline. The image is oriented along the anteroposterior axis (a: anterior, p: posterior). Scale bar = 200 µm
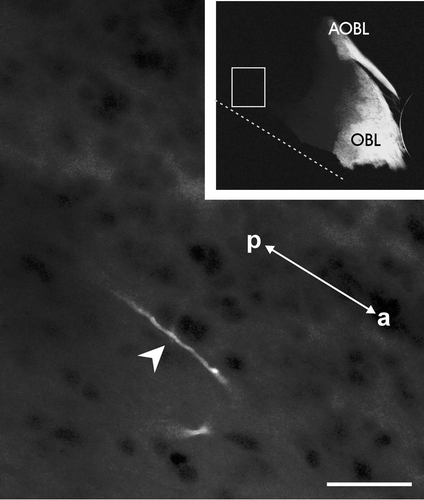
The reduction in the rostrocaudal extent of distribution of the EBOS fibers that had occurred during the metamorphic climax was seen immediately after metamorphosis, and this was essentially similar to that observed in the adults (Figure ). No fiber bundles were present in the telencephalon and the few solitary stained fibers bypassing the olfactory bulbs terminated in the medial septal area and were not observed in the vicinity of the anterior commissure (Figure ).
Discussion
Due to the inherent technical difficulties for tracer application in the early developmental stages, we cannot state when an EBOS first appears in R. esculenta. A similar problem was encountered in the study on X. laevis (Pinelli et al. Citation2004). However, considering the appearance and further development of the hind limb buds, in the latter species the technical approach was successful slightly earlier than in R. esculenta. According to the table of development (Nieuwkoop & Faber Citation1956), X. laevis tadpoles showed an EBOS which extended caudally to the diencephalon at stages 45–47, to the rhombencephalon at stages 50–56 and which was limited after metamorphosis to the diencephalon in its rostrocaudal extension (Pinelli et al. Citation2004). The earliest larval stage (stage 26) of R. esculenta in which the tracer technique worked successfully corresponds approximately to the stage 53 of X. laevis. Anyway, in both species, the maximal rostrocaudal extension of the EBOS during development included the rhombencephalon as the caudal most brain region. Similarly, in both species the EBOS appeared to undergo regressive changes during larval development, prior to the metamorphic climax, with the EBOS retracting to the level of the diencephalon. However, while in X. laevis this is almost the definitive configuration, in R. esculenta a further EBOS reduction takes place during the metamorphosis, resulting in the presence of very few fibres in the medial septal area as the caudal most EBOS extension. On the other hand, the newly metamorphosed froglets in both species show a distribution pattern of stained fibers which persists in the adulthood (Hofmann & Meyer Citation1989b, Citation1992; present data). An analogous decline in the caudal extent of EBOS fibers was observed during the development of rat (Santacana et al. Citation1992) and ducks (Meyer et al. Citation1987); in the latter, however, the EBOS disappears totally during later development.
In the adult clawed frog, the nasal cavities consist of three separate subsystems: the vomeronasal organ and the lateral and medial diverticula. A similar organization was observed in Pipa pipa and P. carvalhoi (Meyer et al. Citation1997). According to Hofmann and Meyer (Citation1992), the EBOS in X. laevis originates only from the lateral diverticulum of the olfactory organ, which appeared to be related to the perception of water‐borne odorants (Altner Citation1962; Foske Citation1934). These data make it plausible to assume that the presence of an EBOS in the adult green frog is functionally insignificant because of the absence of a water nose in this species. However, among anurans the adult Bufo marinus has an EBOS with a pattern identical to that of X. laevis (Hofmann & Meyer Citation1989b), but there is no evidence of a water nose in Bufo species. Furthermore, some authors, differ from Hofmann and Meyer (Citation1992), describing the origin of the EBOS from the air nose (Gaudin & Gascuel Citation2005). Besides anurans, this putative relationship of an EBOS with water‐borne odorants does not fit in other amphibian groups as well, in which an EBOS has been reported in species that usually do not smell underwater. For instance, Schmidt and Wake (Citation1990) studying the olfactory projections in some gymnophiona species, described an EBOS in Ichthyophis kohtaoensis and Typhlonectes natans extending as far caudal as the diencephalon. The first species is predominantly a subterranean species (Dünker et al. Citation2000), hence it smells air‐borne molecules; the second one is secondarily aquatic (Schmidt & Wake Citation1990). Further data on ten salamander species again showed that an EBOS develops without any significant differences in its neuroanatomical pattern between aquatic and terrestrial species (Schmidt et al. Citation1988).
Taken together, all these observations seem to describe a pattern in which the presence of an EBOS does not seem to be specifically correlated with the perception of water‐borne odorants as previously hypothesized (Hofmann & Meyer Citation1992, Citation1995; Meyer & Rastogi Citation1998); it may be connected with some primitive function that is less important or which disappeared during the evolution of amniotes. It is in line to emphasize that the regression of EBOS takes place in tadpoles prior to the metamorphosis, when they are supposed to be able to utilize their water nose. These seems to be an evolutionary tendency to a reduction in the caudal extent of an EBOS as noticed in the bony fish evolution, in which the more derived species show a less extensive EBOS (see Hofmann & Meyer Citation1995; Pinelli et al. Citation2004). Although caution is warranted, it can be speculated that the presence of a well‐developed EBOS during early development in mammals and birds (Meyer et al. Citation1987; Santacana et al. Citation1992) as well as in anurans (Hofmann & Meyer Citation1992; present data) may reflect some evolutionary event of recapitulation.
Another point that needs attention is the presence of stained neurons in the anterior telencephalon during the earliest larval stages studied. In amphibians, the NT is composed of neurons originating from the olfactory placode and migrating into the brain along the olfactory/vomeronasal nerve complex during development (see Schwanzel‐Fukuda & Pfaff Citation1989; Fiorentino et al. Citation2001). These neurons in the adults often project their fibers through the olfactory nerve to the olfactory mucosa. Therefore, it is possible that peripheral elements of such cells may incorporate and carry the tracer to the neurons located along the nerve. Indeed, fibers of NT, visualized by FMRFamide immunohistochemistry, have been described in the olfactory mucosa of R. esculenta until stage 28 (D'Aniello et al. Citation1996). However, it should be emphasized that FMRFamide immunoreactivity shows only a part of NT. In the more advanced stages of development, the NT appears less extended in the frog olfactory components (D'Aniello et al. Citation1995, Citation1996) and this may explain the total absence of tracer‐stained neurons during metamorphosis. Though it is plausible that such cells contribute to the mass of stained fibers observed by us, it is difficult to separate them from EBOS. However, it has never been shown that cells of the NT located within the brain project so extensively posteriorly. In urodeles, Hofmann and Meyer (Citation1989a) reported that some fibers projecting to the mesencephalon originated in the NT, but no cell bodies were described along this pathway. Hence, we may suggest that they described the EBOS.
Acknowledgments
This work was supported by the University of Naples Federico II and by PRIN 2003 (to RKR).
References
- Altner , H. 1962 . Untersuchungen über Leistungen und Bau der Nase des südafrikanischen Krallenfrosces Xenopus laevis (Daudin, 1803). . Zeitschrift fur Vergleichende Physiologie , 45 : 272 – 306 .
- Bazer , G. T. , Ebbesson , O. E. , Reynolds , J. B. and Bailey , R. P. 1987 . A cobalt‐lysine study of primary projections in king salmon fry (Oncorhynchus tshawytscha Walbaum). . Cell and Tissue Research , 248 : 499 – 503 .
- Cajal , R. S. 1911 . Histologie du systeme nerveux de l'homme et des vertebres , Madrid : C.S.I.C . Reprinted 1954
- D'Aniello , B. , Fiorentino , M. , Pinelli , C. , di Meglio , M. , Vallarino , M. and Rastogi , R. K. 1996 . Distribution of FMRFamide‐like immunoreactivity in the brain and pituitary of Rana esculenta during development. . Developmental Brain Research , 95 : 194 – 204 .
- D'Aniello , B. , Pinelli , C. , Di Fiore , M. M. , Iela , L. , King , J. A. and Rastogi , R. K. 1995 . Development and distribution of gonadotropin‐releasing hormone neuronal systems in the frog (Rana esculenta) brain: immunohistochemical analysis. . Developmental Brain Research , 89 : 281 – 288 .
- Demski , L. S. and Northcutt , R. G. 1983 . The terminal nerve: A new chemosensory system in vertebrates? . Science , 220 : 435 – 437 .
- Dünker , N. , Wake , M. H. and Olson , W. M. 2000 . Embryonic and larval development in the caecilian Ichthyophis kohtaoensis (Amphibia, Gymnophiona): A staging table. . Journal of Morphology , 243 : 3 – 34 .
- Farbmann , A. I. 1992 . Cell biology of olfaction , Cambridge : Cambridge University Press .
- Fiorentino , M. , Pinelli , C. , D'Aniello , B. , Iela , L. , di Meglio , M. and Rastogi , R. K. 2001 . Development and distribution of FMRFamide‐like immunoreactivity in the toad (Bufo bufo) brain. . Journal of Chemical Neuroanatomy , 21 : 201 – 13 .
- Foske , H. 1934 . Das Geruchsorgan von Xenopus laevis. . Zeitschrift fuer Anatomie und Entwicklungsgeschichte , 103 : 519 – 550 .
- Gaudin , A. and Gascuel , J. 2005 . 3D Atlas describing the ontogenic evolution of the primary olfactory projections in the olfactory bulb of Xenopus laevis. . Journal of Comparative Neurology , 489 : 403 – 424 .
- Hofmann , M. H. and Meyer , D. L. 1989a . Central projections of the nervus terminalis in four species of amphibians. . Brain Behaviour and Evolution , 34 : 301 – 307 .
- Hofmann , M. H. and Meyer , D. L. 1989b . The nervus terminalis in larval and adult Xenopus laevis. . Brain Research , 498 : 167 – 169 .
- Hofmann , M. H. and Meyer , D. L. 1992 . Peripheral origin of olfactory nerve fibers bypassing the olfactory bulb in Xenopus laevis. . Brain Research , 589 : 161 – 163 .
- Hofmann , M. H. and Meyer , D. L. 1995 . The extrabulbar olfactory pathway: Primary olfactory fibers bypassing the olfactory bulb in bony fishes? . Brain Behaviour and Evolution , 46 : 378 – 388 .
- Meyer , D. L. , von Bartheld , C. S. and Lindörfer , H. W. 1987 . Evidence for the existence of a terminal nerve in lampreys and birds. . Annals of the New York Academy of Sciences , 519 : 385 – 391 .
- Meyer , D. L. , Fackler , I. R. , Jadhao , A. G. , D'Aniello , B. and Kicliter , E. 1997 . Differential labelling of primary olfactory system subcomponents by SBA (lectin) and NADPH‐d histochemistry in the frog Pipa. . Brain Research , 762 : 275 – 280 .
- Meyer , D. L. and Rastogi , R. K. 1998 . “ Olfaction and Reproduction. ” . In Encyclopedia of reproduction , Edited by: Knobil , E and Neill , J. D . 445 – 456 . San Diego : Academic Press .
- Monti‐Graziadei , A. G. 1992 . Primary olfactory projections beyond the olfactory bulb in mammals. . Abstract Chemical Sense (Achems) , 79
- Nieuwkoop , P. D. and Faber , J. 1956 . Normal table of Xenopus laevis (Daudin) , Amsterdam : Elsevier .
- Northcutt , R. G. and Puzdrowski , R. L. 1988 . Projections of the olfactory bulb and nervus terminalis in the silver lamprey. . Brain Behavior and Evolution , 32 : 96 – 107 .
- Pinelli , C. , D'Aniello , B. , Polese , G. and Rastogi , R. K. 2004 . Extrabulbar olfactory system and nervus terminalis FMRFamide immunoreactive components in Xenopus laevis ontogenesis. . Journal of Chemical Neuoroanatomy , 28 : 37 – 46 .
- Riddle , D. R. and Oakley , B. 1992 . Immunocytochemical identification of primary olfactory afferents in rainbow trout. . Journal of Comparative Neurology , 324 : 575 – 589 .
- Santacana , M. , Heredia , M. and Valverde , F. 1992 . Transient pattern of exuberant projections of olfactory axons during development in the rat. . Developmental Brain Research , 70 : 213 – 222 .
- Schmidt , A. , Naujoks‐Manteuffel , C. and Roth , G. 1988 . Olfactory and vomeronasal projections and the pathway of the nervus terminalis in ten species of salamanders. . Cell and Tissue Research , 251 : 45 – 50 .
- Schmidt , A. and Wake , M. H. 1990 . Olfactory and vomeronasal systems of caecilians (Amphibia: Gymnophiona). . Journal of Morphology , 205 : 255 – 268 .
- Schober , A. , Meyer , D. L. and von Bartheld , C. S. 1994 . Central projections of the nervus terminalis and the nervus praeopticus in the lungfish brain revealed by nitric oxide synthase. . Journal of Comparative Neurology , 349 : 1 – 19 .
- Schwanzel‐Fukuda , M. and Pfaff , D. W. 1989 . Origin of luteinizing hormone‐releasing hormone neurons. . Nature , 338 : 161 – 164 .
- Szabo , T. S. , Blähser , S. , Denizot , J. P. and Veron‐Ravaille , M. 1991 . Projection olfactive primaire extrabulbaire chez certains poissons teleosteen. . Comptes Rendus de l'Academie des Sciences , 312 : 555 – 560 .
- von Bartheld , C. S. 2004 . The terminal nerve and its relation with extrabulbar ‘olfactory’ projections: Lesson from lampreys and lungfishes. . Microscopy Research and Technique , 65 : 13 – 24 .
- von Bartheld , C. S. and Meyer , D. L. 1986 . Tracing of single fibers of the nervus terminalis in the goldfish brain. . Cell and Tissue Research , 245 : 143 – 158 .
- von Bartheld , C. S. , Lindörfer , H. W. and Meyer , D. L. 1987 . The nervus terminalis also exists in cyclostomes and birds. . Cell and Tissue Research , 250 : 431 – 434 .
- von Bartheld , C. S. and Meyer , D. L. 1988 . Central projections of the nervus terminalis in lampreys, lungfishes, and bichirs. . Brain Behavior and Evolution , 32 : 151 – 159 .
- Witschi , E. 1956 . Development of Vertebrates , Philadelphia, PA : WB Saunders Co .