Abstract
The aim of this study was to evaluate the efficacy of sperm storage in the seminal receptacle in females of Armadillidium granulatum after the first mating and in the absence of further mating. Females of A. granulatum were bred in the laboratory, isolated from males, until they were sexually mature, and then they were allowed to mate. They were then isolated again from the males and observed to evaluate their reproductive behaviour until their natural death. Some females were sacrificed at various time intervals to carry out ultrastructural observations of the seminal receptacle and of the spermatozoa inside. All the females had the first brood at about 20 days after mating; a little less than 50% of the females had a second brood during the first reproductive season; only 10% of the females had another brood during the second reproductive season. The average number of juveniles produced by each female was about 25 at the first brood, but fall with the successive broods; the sex ratio of juveniles was about 1 male : 4 females. The ultrastructural observations showed some variations in the seminal receptacle wall over time with respect to reproductive activity while there was no appreciable modification as regards the spermatozoa present in the organ, the number of which progressively decreased during the period of reproductive activity.
Introduction
The females of numerous species of terrestrial Isopods are able to store the spermatozoa received during mating up to their death, thus assuring repeated fertilisation even in the absence of further mating (Schöbl Citation1880; La Vallette Saint‐George Citation1883; Vandel Citation1925, Citation1937, Citation1941; Howard Citation1942; Arcangeli Citation1948; Lueken Citation1963). According to Vandel (Citation1941), the survival of the spermatozoa in the female genital apparatus in the oniscideans is related to the degree of their morphological evolution: in the most ‘primitive’ forms, such as the Trichoniscidae, it does not exceed 3–4 months; in others, such as Chaetophiloscia elongata, it is about one year; while in the most advanced forms, for example Armadillidium vulgare, it can reach 17 months.
In many species of oniscideans, the need to have an effective long‐term sperm storage inside the female genital apparatus is closely related to the more or less marked reduction in the number of males, which means, in some cases, a real phenomenon of spanandry: in fact, while in some species, such as Trichoniscus pygmaeus and Armadillidium nasatum, the sex ratio is generally 1 : 1 (Vandel Citation1925), in others, such as Philoscia muscorum, the male/female ratio is 1 : 11 and in Trichoniscus pusillus it is 1 : 200 (Frankel et al. Citation1981). Moreover, in some species such as Platyarthrus aiasensis, the sex ratio varies in relation to the geographical area of the species studied, from 1 : 1 to a marked spanandry or even a total absence of males thus making parthenogenesis necessary (Caruso Citation1968).
Another reason that could influence the sperm storage inside the oniscideans' female genital apparatus is certainly the diversity of the physiology of the reproductive process and, more precisely, of the variable relationships between mating, vitellogenesis and ovideposition found at the specific level (Bessé Citation1976).
The contribution offered by research into the understanding of the morphological, histological, ultrastructural and functional aspects of the female genital apparatus of the oniscideans and of those relative to the sperm storage has been, however, very poor, and only recently have we been able for the first time to show the ultrastructural organisation of the seminal receptacle and how spermatozoa are preserved inside it in Porcellio laevis (Longo et al. Citation1998). This research did not, however, investigate the long‐term storage of the spermatozoa within the seminal receptacle and the efficacy of their use, even at a relatively long‐time after mating. With the intent to clarify these aspects, at least in part, in this paper we carried out an experimental investigation on Armadillidium granulatum Brandt.
These investigations were aimed at evaluating the long‐term conservation capacity of the spermatozoa inside the seminal receptacle and their efficacy in guaranteeing, during the reproductive life of the female, the fertilisation of the eggs after a single mating at the beginning of sexual maturity. The investigations also had the aim of acquiring some preliminary data on the reproductive biology of A. granulatum, in that for this species the knowledge of this aspect is extremely poor, as it is also for numerous other species of oniscideans.
Materials and methods
The investigation was carried out on a population of A. granulatum collected in Porto Palo (Syracuse, Sicily) and transferred to the laboratory under natural conditions of photoperiod and temperature. Some sexually mature females were dissected and the genital apparatus was processed for morphological and ultrastructural observations.
Some mated females were held under observation until the mancas hatching and immediately after isolated with their young in plastic boxes with gypsum bases covered with chestnut leaf litter kept constantly moist; the young were then observed over time up to the appearance of sexual characters that, in the oniscideans, become exteriorly evident at stage V, after the fourth cycle of moulting (Juchault Citation1967). Successively, young females were isolated from the males and bred in a single group until they reached sexual maturity, to obtain a consistent number of sexually mature virgin females. A number of these females were then placed in the presence of males and watched closely until mating took place.
After mating, the females were isolated and placed individually in breeding boxes and observed up to the birth of the young. Some of these females were sacrificed after the first brood for histological and ultrastructural observations; others were progressively sacrificed either after the second brood or in the following year, after a further brood or, in the absence of this, at the end of the reproductive season. None of the females under observation survived beyond the second reproductive season.
Morphological observations
For the evaluation of the general characteristics of the female genital apparatus the animals were killed by decapitation and dissected in Ringer's saline solution modified for land Isopods according to Legrand (Bessé Citation1976). The female genital apparatus, after removal, was observed and photographed with the aid of a stereo‐microscope Wild M400, both fresh and after a brief fixation in 2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.3; some specimens were in toto stained with 0.1% toluidine blue in 0.1 M phosphate buffer, pH 7.3 or with Sudan black IV B (saturated solution in isopropilic alcohol).
Light and electron microscopy
For the ultrastructural study some females were killed by decapitation and immediately immersed in Ringer's saline solution for land Isopods and then the female genital apparatus was removed.
Fixation was carried out in 2.5% glutaraldehyde in 0.1 M Na‐cacodilate buffer, pH 7.3 for 4 h at room temperature; after repeated washing in the same buffer the specimens were post fixed in 1% OsO4, in the same buffer, for 1 h at room temperature.
The samples were dehydrated in ethanol followed by propylene oxide and embedded in Embed 812 (EMS).
For light microscopy, semi‐thin sections cut on an Ultracut Leica ultramicrotome with diamond blades were stained with 0.5% toluidine blue in 0.1 M phosphate buffer, pH 7.3 or with the polychromatic staining method of Sato and Shamoto (Citation1973).
For transmission electron microscopy, ultra‐thin sections, collected on Cu/Rh grids of 200 or 300 mesh, were stained with uranyl acetate and lead citrate (Reynolds Citation1963) and examined in a Philips CM 10 electron microscope, at 60 or 70 kV.
Results
As in the other oniscidean species previously studied (Bessé Citation1976; Longo et al. Citation1998), the female genital apparatus of A. granulatum consists of two separate ovaries located dorso‐laterally to the intestine. In the sexually mature females the ovaries, which have the appearance of two small, dorso‐ventrally flattened sacs (Figure ), extend from the 2nd to the 7th segment of the pereion. At the level of the 5th segment there is a short oviduct that opens into the gonopore situated at the base of the corresponding pereiopod. Each ovary is connected to the lateral wall of the body by suspension ligaments.
Figure 1 A, Genital apparatus of mated female: ovary (ov) containing growing oocytes; od, oviduct; sr, seminal receptacle. B, Genital apparatus of mated female stained in toto with toluidine blue; g, germigen; od, oviduct; oo, oocytes; sr, seminal receptacle filled with spermatozoa (sp).
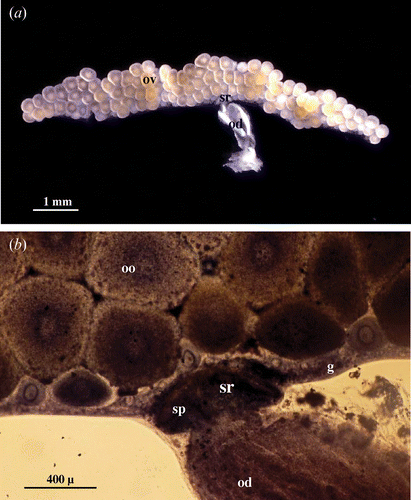
The germigen, where the primary oogonia and follicular cells are contained, is found along the lateral margin of each ovary. In the median region of the germigen the lumen of the ovary is occupied by the developing oocytes, whose diameter reach, at maturity, about 500–600 µm (Figure ).
The germigen is interrupted in correspondence to the site of insertion of the oviduct into the ovary where the seminal receptacle is present; this looks like a small cup protruding from the ovary wall. The dimensions of the seminal receptacle are subject to significant variations: small and not very prominent in the virgin females, increasing notably in size in the mated females where its morpho‐functional organisations change. In mated females, it is easy to see the presence in the lumen of the seminal receptacle of the numerous spermatozoa deposited, which form a dense skein (Figure ).
Experimental study of the sperm storage in the course of the reproductive process in captivity
In the females born in captivity during the summer and bred in isolation from males until their sexual maturity, the start of reproductive activity began only in the year after birth and the reproductive period was between June and October.
Fifty females were placed singularly in the presence of some males until mating took place; for all the females this was during the last days of June and the first days of July. The females had their parturial moult at about the middle of July and gave birth at about 18–20 days from the moult. The mean number of young from the first brood was 24.5±4.5 (Table ). After hatching, the young remained with their mothers until the fourth moult and after the determination of their sex were removed. The sex ratio at birth was 1 males : 5.3 females. Only 24 of the females produced a second brood during the year; the second parturial moult occurred at the end of August and the hatching of young at mid September, at about 18 days from the moult. The mean number of young was 19.4±7.4 and the male/female ratio was 1:3.4 (Table ).
Table I. Some data on reproductive behaviour of the females under observation.
About one‐third of the females under observation had a natural death or, more frequently, died through attack by parasites such as nematodes and mites during the winter. Of the females that survived, only four had a parturial moult in August of the following year and a third brood in the first 10 days of September. The mean number of young was 20.0±1.0 and the male/female ratio was 1 : 4 (Table ).
None of the females survived the following winter.
Ultrastructural investigations
The ultrastructural observations were carried out both on the seminal receptacles of sexually mature females coming from a population of A. granulatum collected in the wild and on the receptacles of females bred in captivity, after one mating, and killed after giving brood 1, 2 or 3 times during the successive reproductive seasons.
(a) Ultrastructural organisation of the seminal receptacle in sexually mature, mated females
In sexually mature females the seminal receptacle appears as a bulb‐shaped dilatation of the ovarian wall, in correspondence to the insertion of the oviduct and, if mating has taken place, inside it there is a considerable number of spermatozoa (Figure ).
The seminal receptacle wall is made up of a simple epithelium surrounded by a thick basal lamina. The epithelial cells have an irregular aspect and their nucleus, which is roundish or slightly oval in shape, is generally located in the basal portion (Figure ).
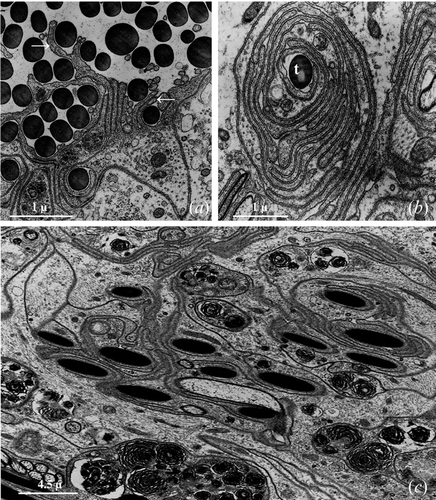
Figure 2 Seminal receptacle of mated female.A, The wall of the seminal receptacle is constituted by an epithelium monolayered laying on a thin basal lamina (b). B, In the ventral and ventro‐lateral regions of seminal receptacle the epithelial cells are much shorter and appear strongly interdigitated. The basal lamina (b) forms small protrusions (arrow head) that enter into cavities present at the base of the epithelium; n, nucleus; st, sperm tail. C, Numerous small plaques of electron dense material (arrowhead) are present on the cytoplasmatic side of the basal plasmalemma. D, Near to the transition zone between the seminal receptacle and the ovary the basal lamina (b) becomes progressively thicker up to 6 µm or more.
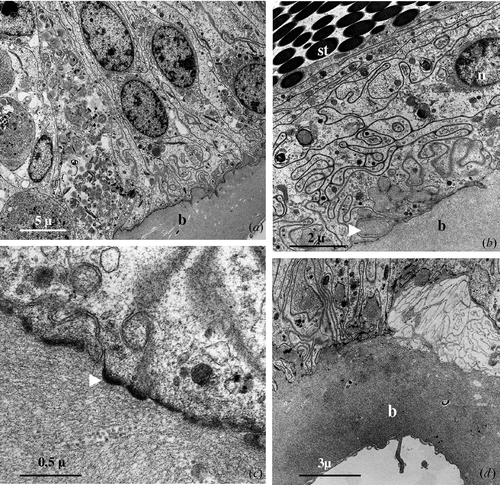
In the ventral and ventrolateral region of the seminal receptacle, the epithelium has a reduced height and the cells have a relatively ample base and a thin and oblong supra‐nuclear portion that extends obliquely towards the lumen of the organ and forms numerous interdigitations with the same portion of the adjacent cells (Figure ).
The basal lamina, from 3 to 4 µm thick, has a relatively homogeneous appearance and is made up of finely fibrillar material. The basal surface of the epithelial cells appears to be incredibly sinuous and in the region of contact between the plasmatic membrane and the basal lamina, on the cytoplasmatic side, numerous small plaques of electron dense material are present (Figure ). The basal lamina frequently enters into numerous, small extracellular cavities present at the base of the epithelial cells forming a continuous series of small protrusions that probably have the role of making the anchorage of the epithelial cells to the basal lamina more solid (Figure ).
Outside the basal lamina, particularly in proximity to the oviduct, there are thin, isolated muscle fibres that go obliquely towards the oviduct and small chromatophores, pigment cells rich in electron dense granules.
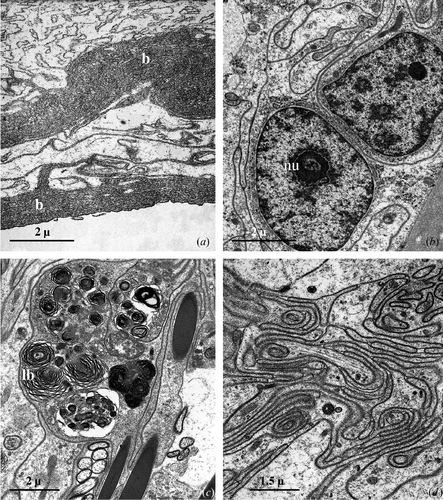
In correspondence to the transition zone between the seminal receptacle and the ovary the basal lamina profoundly modifies its appearance (Figure ): it becomes, in fact, progressively thicker up to 6 µm or more, and in correspondence to the ovary takes on a multilayered appearance (Figure ).
Figure 3 Seminal receptacle of mated female.A, In correspondence of the ovary the basal lamina (b) appears tick and multilayered. B, The nucleus of the epithelial cells is roundish, with abundant heterochromatin patches lying against the nuclear envelope and a large central nucleolus (nu). C, Numerous lamellar bodies (lb) grouped in the cytoplasm of an epithelial cell. D, Apical region of the epithelial cells forming a rich system of interdigitations.
Independently from the considered region, the epithelial cells have a simple organisation.
The nucleus, which is always relatively large, shows areas of condensed chromatin prevalently localised at its periphery and contains one or sometimes two nucleoli (Figure ).
The cytoplasm is characterised by a variable density: in some cells it appears rarefied and electron‐transparent, while in other cells it is clearly denser: this latter aspect is observed, above all, in the supranuclear portion near the lumen of the organ.
The epithelial cells, independently of their various cytoplasmatic density, generally appear rather poor in organelles with the exception of mitochondria, small and polymorph, often grouped together in considerable numbers in restricted areas in the sub‐nuclear portion or near the basal lamina. Still in the subnuclear portion large vesicles are often seen with a flocculent and electron transparent content.
The rough endoplasmic reticulum is present in the form of small, flattened cisternae localised in small areas near the nucleus; the Golgian structures are sporadic and made up of an exiguous number of small piled up sacs. The smooth endoplasmatic reticulum is almost absent.
The most peculiar aspect, characterising the cytoplasm of the epithelial cells, is certainly the presence of numerous lamellar bodies of variable dimension that fill, in some cases, almost all the cellular body (Figure ).
The cell junctions, rather rare in the basal region of the cells, become more numerous near the lumen, where the cell membrane of the adjacent cells assumes an extremely sinuous course (Figure ); they have extensive septate (Figure ) and adherens junctions. The apical portion of the cells appear very rich in microtubules disposed generally along the longitudinal axis of the cell (Figure ).
Figure 4 A, Apical region of the epithelial cells: extensive septate junctions are present between the plasma membranes while numerous microtubules are scattered in the cytoplasm. B, Lumen of the seminal receptacle filled with bundles of spermatozoa; t, sperm tail. C, Irregular protrusions (arrows) of the cell surface extend toward the lumen of the seminal receptacle which thus appears divided into numerous cavities filled with spermatozoa (sp). D, Numerous spermatozoa present in the lumen show the heads (h) detached from the tails (t).
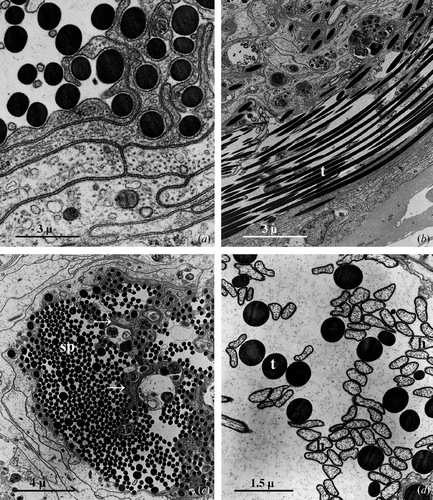
In the virgin females the lumen is very narrow; in the mated females it is, instead, much dilated and contains bundles of spermatozoa (Figure ) twisted together to constitute a skein that often takes the shape of an 8.
In the seminal receptacle of mated females, the apex of the epithelial cells forms numerous and more or less irregular protrusions that extend towards the centre of the lumen which is thus generally divided into a series of inter‐connecting lodges filled with spermatozoa (Figure ).
Some of the spermatozoa present in the lumen appear perfectly intact, while in others the head (the portion of the spermatozoa containing the nucleus and acrosoma) is detached from the long immobile tail (Figure ).
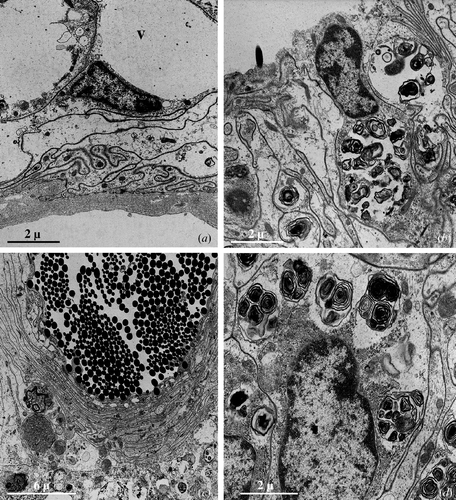
When the spermatozoa come into contact with the surface of the epithelial cells or with their protrusions, the cell membrane forms one or more pseudopodial‐like expansions (Figure ) that are first arranged around the spermatozoa and then wrapped around to form a sort of myelinic covering (Figure ).
Figure 5 Seminal receptacle of mated female.A, Some spermatozoa present in the lumen are captured by pseudopodial‐like extensions (arrows) produced by the epithelial cell surface. B, Frequently, the pseudopodial‐like extensions form, around the sperm tail (t), a membranous envelopment resembling a myelinic sheath. C, Thin cellular cavities housing heads and tails of captured spermatozoa.
This ‘capturing’ process principally involves the sperm tails while the head is only sporadically captured; between the epithelial cell membrane and that of the sperm tail there is always a discrete space (Figure ) while when it is the head of the spermatozoa that is captured, the two cell membranes are always in close contact.
Many of the spermatozoa thus captured are progressively internalised into thin tubular cavities that are formed within the epithelial cells (Figure ). These cavities are often deep almost reaching the basal lamina.
(b) Ultrastructural organisation of the seminal receptacle in the females after the first brood
In these females, while the general organisation of the seminal receptacle is very similar to that found in the control females, significant differences have been observed regarding the ultrastructural features of the epithelial cells.
The most considerable difference concerns the aspect of the epithelial cells whose cytoplasm appears highly vacuolated (Figure ) and considerably richer in lamellar bodies that are localised prevalently in the apical region of the cell, up to the lumen (Figure ); some of the vacuoles have a weakly flocculent content while other vacuoles are characterised by a content of amorphous material of various density and, above all, by complex membranous structures. In the epithelial cells with a particularly vacuolated cytoplasm, the nucleus appears to often be localised in an apical position (Figure ).
Figure 6 Seminal receptacle of female after the first brood.A,B, Large vacuoles (v) containing electron transparent material (A) or lamellar bodies (B) are present in the cytoplasm of the epithelial cells; in some cells, the nucleus is localised very near the cell surface. C, Numerous spermatozoa are still present in the lumen of the seminal receptacle. Seminal receptacle of female after the second brood. D, In the epithelial cells, the extension of the areas vacuolised and containing lamellar bodies is ulteriorly increased.
In its dorsal region the seminal receptacle has a wide regular lumen while ventrally it appears notably irregular; inside the lumen there are still numerous spermatozoa (Figure ), some of which have had their heads detached from the tail. A lot of sperm tails and heads are still present, morphologically unmodified, inside the tubular cavities of the epithelial cells.
(c) Ultrastructural organisation of the seminal receptacle in the females after the second brood
After the second brood, the seminal receptacle does not show marks of further ultrastructural modifications in comparison with those observed in primiparae females.
The epithelial cells show, at the most, a slight increment in the number of lamellar bodies (Figure ) and a higher degree of cytoplasmatic vacuolisation.
The spermatozoa inside the lumen are still numerous and those captured by the epithelial cells still seem well preserved.
(d) Ultrastructural organisation of the seminal receptacle in the females after the third brood
Only a few females were able to produce a third brood without having to mate a second time; this last brood took place in the course of the second breeding season (females three years old).
In their seminal receptacle the lumen width is very much reduced and few spermatozoa were still present inside. The spermatozoa captured by the epithelial cells are, on the contrary, always numerous and apparently unmodified.
The number of lamellar bodies in the epithelial cells is even higher as is the degree of cytoplasmatic vacuolisation.
Discussion
In A. granulatum the general morphology and histological and ultrastructural organisation of the seminal receptacle are generally similar to those that we observed in Porcellio laevis (Longo et al. Citation1998). Also in this species, in fact, this specialised region of the female genital apparatus appears as a goblet shaped protrusion of the ovarian wall at the insertion of the oviduct.
The histological structure of seminal receptacle wall is the same as that of P. laevis (Longo et al. Citation1998) and, in general, of all the specialised regions for the conservation of spermatozoa in many vertebrates and invertebrates (Selmi Citation1992). It appears to be made up of a simple epithelium resting on a thin basal lamina that in turn is lined by thin, isolated muscle fibres.
The basal lamina has a structure and a thickness almost homogeneous for all its course and, as was already observed by Longo et al. (Citation1998) in P. laevis, it is clearly different from that lining the ovary where the basal lamina becomes much thicker, more sinuous and bilayered.
The epithelium is made up of narrow, sinuous cells; their basal region forms a rich series of invaginations that probably have the role of making the anchorage with the basal lamina stronger. The lateral surface of the epithelial cells has a more regular course in its median part where the membranes of the adjacent cells are close but do not form extensive junctions. In the apical third the cells become markedly sinuous and form numerous interdigitations in correspondence to which septate and adherens junctions are present. This disposition of cell junctions has, probably, the aim of allowing reciprocal movements of the basal part of the epithelial cells so as to allow the notable dilatation of the seminal receptacle when it receives the spermatozoa after mating.
The ‘light’ and ‘dark’ cells already described in the seminal receptacle of P. laevis (Longo et al. Citation1998) have also been found in A. granulatum; this feature is to be attributed only to a different density of the cytoplasm because at the ultrastructural level there are no significant differences in the organisation of the two types of cells.
The ultrastructural organisation of the epithelial cells always appears rather simple and the cell organelles, above all those having biosynthetic and secretory activities, are scarce in all the various phases of the examined activity: scattered ribosomes, small cisternae of the rough reticulum localised almost exclusively near the nucleus, rare Golgi complexes are present while the smooth reticulum is almost nonexistent. The mitochondria are, on the contrary, more abundant though small in size, polymorph and generally concentrated in the basal region of the cells; a considerable variety of cytoplasmatic vesicles are present.
In many epithelial cells a large number of these vesicles contain isolated membranous structures, sometimes multiple and tightly packed together so that they have the typical shape of dense lamellar bodies. These lamellar bodies are principally found in the apical cell region, close to the plasmalemma: often, there is a conspicuous number of these lamellar bodies scattered between the spermatozoa in the lumen of the seminal receptacle.
Selmi (Citation1992), in a review on the conservation of spermatozoa, pointed out how the epithelium of the regions specialised in this role is characterised, both in vertebrates and in invertebrates, by a notable activity of secretion aimed at creating a particular ionic environment in the lumen of the organ and giving a valid trophic support to the spermatozoa that stay there for long periods. The chemical characteristics of the secretion produced by the epithelial cells in the species investigated are variable; in some species of molluscs, insects, amphibians, reptiles, birds and chiropteran, the secretion products consist of glycogen or other polysaccharides; in other species of molluscs, insects and amphibians they are made up of proteins and/or glycoproteins and, in still other species of insects, birds and chiropteran, by lipoproteins and phospholipids.
In P. laevis (Longo et al. Citation1998) the secretion is fundamentally lipidic; in this species, the cells of the seminal receptacle after mating undergo a notable development of the smooth endoplasmatic reticulum and in the lumen of this organ there are numerous lipidic globules of various dimensions, as was shown by the strong positivity to Sudan Black B.
In A. granulatum the ultrastructural characteristics of the epithelial cells do not indicate a marked secretory activity; it is therefore possible that in this species the formation and release into the lumen of numerous lamellar bodies represents the way in which a trophic support is given to the spermatozoa present there.
In those cases in which the secretion is made up of lipoproteins or phospholipids it has been hypothesised that, besides its trophic importance, it has a role as the source of material necessary for the modifications that take place in the plasmalemma of the spermatozoa during their stay in the female genital tracts (Daly & Golding Citation1977; Giusti & Selmi Citation1985; Selmi & Giusti Citation1986). This does not seem to be the case in A. granulatum, since the spermatozoa do not appear to undergo a notable structural modification of any type while they are in the seminal receptacle.
The spermatozoa present inside the seminal receptacle have, in A. granulatum, the same behavioural pattern as in P. laevis; the majority of the spermatozoa are free in the lumen while only some are ‘captured’ by the cells of the wall. The capture of the spermatozoa by the epithelial cells takes place, as in P. laevis, thanks to the formation of pseudopodia that extend from the cell apex after contact with the surface of the spermatozoa and then wrap the spermatozoa many times pulling it into the tubular cavities that are formed within the epithelial cells.
The majority of the spermatozoa present in the lumen undergo the traumatic detachment of the head from the tail; as was affirmed by Cotelli et al. (Citation1976) in their work on spermatozoa of land Isopods, the structure that joins the head to the tail is very delicate and the tail can become easily detached from the head during the manipulation of the spermatozoa that follows their preparation for microscopic observation. Even if nothing is known about the way that gametes interact in these animals we can suppose that the single spermatic heads, even if they are separated from their tails, maintain their fertilising capacity.
We have tried to give an answer to the question of how long the fertilising capacity is maintained in the seminal receptacle and how the spermatozoa are modified during storage. Even though in 1941 Vandel affirmed that in Armadillidium vulgare the spermatozoa present in the seminal receptacle conserved their fertilising capacity for up to 17 months after mating, no experimental or ultrastructural information has ever been given with respect to this aspect.
This research, besides trying to clarify, at least in part, these aspects has allowed the acquisition of some significant data on the reproductive biology of the species under investigation for which, up to now, have not been available in the literature. It should be remembered that the present data come from investigations carried out under artificial conditions of breeding and, considering that the reproductive process in land Isopods is extremely sensitive to any variation in environmental parameters (Warburg Citation1993), we cannot attribute absolute values. It is necessary to compare and support them with adequate verifications conducted directly in the natural environmental conditions of this species.
Under artificial breeding conditions the females of A. granulatum, after a single mating following sexual maturity and bred in isolation for all of their natural life, gave a brood at least once during the first reproductive season. About 40% of the females examined also gave a second brood, shortly after the first, during the same reproductive season, while only about 12% gave a third brood during the second reproductive season, demonstrating the possibility of using the spermatozoa stored in the seminal receptacle at about 15 months after mating.
Our observations concerning the sex of the new born show how also in A. granulatum, as in many other species of Oniscidea, there is a numeric relationship between sexes clearly biased towards the females that varied according to the different broods, between 1 : 3 and 1 : 5. Some of the females gave birth to only females, thus confirming, also for A. granulatum, the existence of the monogeny phenomenon already found in other species of the genus Armadillidium (Juchault & Legrand Citation1989).
The ultrastructural observations on the seminal receptacle of some females killed after the first, second or third brood showed some interesting aspects relative to the general aspect of this structure and, above all, to the way in which the conservation of the spermatozoa is carried out.
In the females killed immediately after the first brood, or a few months thereafter, the wall of the seminal receptacle showed ultrastructural characteristics generally similar to those of the control females; the only noteworthy aspects concern an increase in the number of lamellar bodies present that tended to be found in an apical position in association with a parallel increase in cytoplasmatic vacuolisation, particularly evident in the peripherical region of the epithelial cells. The spermatozoa present in the lumen were still numerous while those that had been captured and internalised by the cells of the wall were in a good state of conservation.
The changes found in the seminal receptacle of the females sacrificed after the second brood seemed to be minimal; the spermatozoa present in the lumen of the receptacle or captured by the epithelial cells were still numerous and in a good state of conservation.
In the females sacrificed after the third brood, and thus at a time relatively distant from mating—about 15 months—the epithelial cells seemed to undergo a further degree of involution, in part possibly linked to the ageing of the animal, which appeared as a greater degree of cytoplasmatic fluidification and consequent vacuolisation and with an increase in the lamellar bodies; in the lumen of the organ the spermatozoa were present in a much reduced number as were those localised in the wall of the organ; however, their appearance was still normal.
Aspects relative to the activity of capturing and internalisation of the spermatozoa that are very similar to those found in P. laevis (Longo et al. Citation1998) and in A. granulatum were described for the spermatheca of some species of Anellida Oligochaeta (Richards & Fleming Citation1982); this activity, described by the authors as a type of spermiophagy would be aimed, as the authors say, at the removal of old spermatozoa; similar spermiophagic activity of the seminal receptacle in an Annellida Polychaeta, Pisione remota (Westheide Citation1988), was, on the other hand, interpreted by the author as providing an additional trophic supply for the growing oocytes.
Longo et al. (unpublished data), during preliminary ultrastructural investigations on the female genital apparatus of some species of Oniscidae—Halophiloscia couchi, H. hirsuta and Stenophiloscia zosterae—found clear signs of spermiophagic activity of groups of epithelial cells located in the region of transition between the seminal receptacle and the ovaries; spermiophagy seems to mainly involve the spermatozoa tails, captured by the cells of the walls of the organ, which show clear signs of a progressive demolition and re‐absorbtion.
As the sperm tail of the land Isopods is essentially of a proteic nature, it seems logical and consequential to attribute to this activity only a trophic finality that is a further example of a way of transferring from the male to the female, by means of mating, of a large quantity of nutritive substances supporting its reproductive activity, as found in many insect species (Leopold et al. Citation1971; Terranova et al. Citation1972; Leopold, Citation1976).
Such a hypothesis does not seem to be supported by the findings at the ultrastructural level relative to the females of this experimental study; in all the cases examined, it was always possible to see an almost perfect state of conservation both of the spermatozoa present in the lumen and of those captured by the wall of the organ without ever finding aspects of spermiophagy phenomena, and thus the use of a trophic potential represented by the sperm tails.
In his paper published in 1902, Nichols stated: ‘The function of the extraordinarily long fibers, if the spermatozoa remain motionless, is to me a matter of great perplexity’; the perplexity as regards the meaning of the long motionless tails of the spermatozoa of the Isopods remains the same after almost a century!
Further research must be carried out and some more years must pass before a solution is found to this enigma.
References
- Arcangeli , A. 1948 . Appunti sulla riproduzione degli Isopodi terrestri (Crostacei). Bollettino del Museo di Zoologia. . Università di Torino , 1 : 2 – 11 .
- Bessé , G. 1976 . Contribution a l'étude expérimentale de la physiologie sexuelle femelle chez les crustacés Isopodes terrestres 296 p Thèse Doctorat d'Etat, Université de Poitiers, CNRS France, n° AO 13 017
- Caruso , D. 1968 . Partenogenesi e spanandria in Platyarthrus aiasensis Legrand (Crustacea Isopoda). . Bollettino dell'Accademia Gioenia di Scienze Naturali di Catania Serie IV , 9 : 451 – 457 .
- Cotelli , F. , Ferraguti , M. , Lanzavecchia , G. and Donin , C. L. L. 1976 . The spermatozoon of Peracarida. 1. The spermatozoon of terrestrial isopods. . Journal of Ultrastructure Research , 55 : 378 – 390 .
- Daly , J. M. and Golding , D. W. 1977 . A description of the spermatheca of Spirorbis spirorbis (L.) (Polychaeta: Serpulidae) and evidence for a novel mode of sperm transmission. . Journal of the Marine Biological Association of United Kingdom , 57 : 219 – 227 .
- Frankel , B. , Sutton , S. L. and Fussey , G. D. 1981 . The sex ratios of Trichoniscus pusillus Brandt (Crustacea: Oniscoidea). . Journal of Natural History , 15 : 301 – 307 .
- Giusti , F. and Selmi , M. G. 1985 . The seminal receptacle and sperm storage in Cochlostoma montanum (Issel) (Gastropoda: Prosobranchia). . Journal of Morphology , 184 : 121 – 133 .
- Howard , H. W. 1942 . The genetics of Armadillidium vulgare Latreille. II. Studies on the inheritance and monogeny and amphogeny. . Genetics , 44 : 143 – 159 .
- Juchault , P. 1967 . Contribution a l'étude de la différenciation sexuelle mâle chez les crustacés isopodes. . Annals of Biology , 6 : 191 – 212 .
- Juchault , P. and Legrand , J. J. 1989 . Sex determination and monogeny in terrestrial isopods Armadillidium vulgare (Latreille, 1804) and Armadillidium nasatum Budde‐Lund, 1885. . Monitore Zoologico Italiano (NS) , 4 : 359 – 375 .
- von La Vallette Saint‐George , A. 1883 . De Isopodibus commentatio anatomica Bonn
- Leopold , R. A. 1976 . The role of male accessory glands in insect reproduction. . Annual Review of Entomology , 21 : 199 – 221 .
- Leopold , R. A. , Terranova , A. L. , Thorson , B. J. and Degrugillier , M. E. 1971 . The biosynthesis of male housefly accessory secretion and its fate in the mated female. . Journal of Insect Physiology , 17 : 987 – 1003 .
- Longo , G. , Musmeci , R. , Privitera , R. and Sottile , L. 1998 . Ultrastructural organisation of seminal receptacle and sperm storage in Porcellio laevis Latreille (Crustacea: Isopoda Oniscidea). . Tissue & Cell , 30 : 464 – 474 .
- Lueken , W. 1963 . Zur Spermienspeicherung bei Armadillidien (Isopoda Terestria). . Crustaceana , 5 : 27 – 34 .
- Nichols , M. L. 1902 . The spermatogenesis of Oniscus asellus Linnaeus, with especial reference to the history of the chromatin. . Proceedings of the American Philosophical Society , 41 : 77 – 112 .
- Reynolds , E. 1963 . The use of lead citrate at high pH as an electron opaque stain in electron microscopy. . Journal of Cell Biology , 17 : 208 – 212 .
- Richards , K. S. and Fleming , T. P. 1982 . Spermatozoal phagocytosis by the spermathecae of Dendrobaena subrubicunda and other lumbricids (Oligochaeta, Annelida). . International Journal of Invertebrate Reproduction , 5 : 233 – 241 .
- Sato , T. and Shamoto , M. 1973 . A simple rapid polychrome stain for epoxy‐embedded tissue. . Stain Technology , 49 : 223 – 227 .
- Schöbl , J. 1880 . Ueber die Fortpflanzung Isopoder Crustaceen. Archiven Mikroskopie Anatomie . Entwick Mechanik , 17 : 125 – 140 .
- Selmi , M. G. 1992 . “ Sperm storage and capacitation. ” . In Sex origin and evolution. Unione Zoologica Italiana, Selected Symposia and Monographs Edited by: Dallai , R . Vol. 6 , 251 – 265 . Mucchi, Modena
- Selmi , M. G. and Giusti , F. 1986 . “ The reverse moving spermatozoon of Theodoxus fluvialis (L.). What happens in the seminal receptacle? ” . In Biology of reproduction and cell motility in plants and animals , Edited by: Cresti , M and Dallai , R . 205 – 210 . Siena : University of Siena .
- Terranova , A. C. , Leopold , R. A. , Degrugillier , M. E. and Johnson , J. R. 1972 . Electrophoresis of the male accessory secretion and its fate in the mated female. . Journal of Insect Physiology , 18 : 1573 – 1581 .
- Vandel , A. 1925 . Recherches sur la sexualité des Isopodes. I. Les conditions naturelles de la reproduction chez les Isopodes terrestres. . Bulletin Biologique de la France et de la Belgique , 59 : 317 – 371 .
- Vandel , A. 1937 . Recherches sur la sexualité des Isopodes. II. Les conditions de la fécondation chez Trichoniscus provisorius Racovitza. . Bulletin Biologique de la France et de la Belgique , 71 : 206 – 219 .
- Vandel , A. 1941 . Recherches sur la génétique et la sexualité des Isopodes terrestres. Sur la longévité des spermatozoïdes à l'intérieur de l'ovaire d'Armadillidium vulgare. . Bulletin Biologique de la France et de la Belgique , 75 : 364 – 368 .
- Warburg , M. R. 1993 . Evolutionary biology of lands isopods , Berlin : Springer‐Verlag .
- Westheide , W. 1988 . The ultrastructure of the spermatozoon in Pisione remota (Annelida: Polychaeta) and its transformation in the receptaculum seminis. . Journal of Submicroscopic Citology , 20 : 169 – 178 .