Abstract
Bioacoustic signals are considered to be essential to the social communication of anuran amphibians. However, some anuran species lack a vocal sac or tympanum, or both. We hypothesise that the variation of anuran acoustic organs correlates to altitude. We reviewed published literature concerning anuran species which inhabit China, and tested the general relationship between anuran acoustic organs and altitude. The result showed that both the vocal sac and the tympanum had a significantly negative trend to altitude, and that the vocal sac also significantly correlated to the tympanum. The interaction by phylogenetic relationships could be excluded. We suggested that anuran acoustic organs decline with increasing altitude. With increasing altitude, lower population density, low temperature, hypoxia and food shortage could be responsible for such degeneration. Nontympanic pathways of sound reception and ultrasonic signal system may be utilised to compensate for anuran acoustic organ degeneration.
Introduction
Bioacoustic signals are essential to the social communication of anuran amphibians. Most anurans can be incredibly vocal, especially during breeding time, when the males advertise themselves using acoustic signals in an attempt to attract a mate. Inflation of a vocal sac characterises advertisement calling behaviour in nearly all male anurans, and the vocal sac can serve as a visual cue and is a salient part of the advertisement signal in at least some circumstances (Rosenthal et al. Citation2004). The sound receptor, tympanum or eardrum, are caused to vibrate by sound waves. The vibrations travel to the inner ear through the columella, which conducts the movements of the tympanum across the space of the tympanic cavity. However, some anuran species lack a vocal sac or tympanum, or both. Although such a lack usually does not cause any inconvenience for anuran vocalisation, it suggests that some factors must play a role on the variation.
Acoustic signals are considered playing a very important role on anuran activities. Males gain reproductive advantage by competing for advertisement privileges and by vocally suppressing neighbouring males (Tobias et al. Citation1999). There are many studies that demonstrate strong directional selection on various components of signals used to attract conspecific mates (Ryan & Keddy‐Hector Citation1992) and considerable variation in signals and receivers among populations of the same species (Claridge et al. Citation1988; Verrill & Arnold Citation1989; Ritchie Citation1991; Ryan & Wilczynski Citation1991; Ryan et al. Citation1992; Rowe Citation1999; Rosenthal et al. Citation2004). Mating success has been shown to be positively correlated to an individual's chorus tenure in some species (Murphy Citation1999). Combinations of acoustic and visual cues can also enhance spatial localisation (McDonald et al. Citation2000). The acoustic signals that most anurans produce are accompanied by inflation of a conspicuous vocal sac. The specificity of female response to vocal sacs, and the great diversity of form, colour and pattern in these structures (Duellman & Trueb Citation1986; Hödl & Amézquita Citation2001; Narins et al. Citation2003), suggests that these cues might play a role in conspecific mate recognition and mate preference.
Attracted by the fact that the communication systems of some arthropods show strong analogies with those of anurans, we tried to investigate the extension of such parallelisms and focused on the relation with altitude. Arthropods were found to be reduced and lacking wings with both increasing altitude and increasing latitude (Downes Citation1965; Mani Citation1968). The elytra and wings of the grasshoppers in the Tibetan Plateau had degenerated or were even found to be lacking (Yin Citation1984). As a grasshopper relies on the elytra and wings to make sound, the degeneration or lack of these lead to a default, which in turn resulted in a degeneration of their auditory organ, and it was positively correlated to altitude.
Being ectothermal animals, anurans are particularly sensitive to environmental factors. The reduction of arthropods' wings and auditory organ provides a cue for us to explore the variation of anuran acoustic organs. Along with the increasing altitude, environment changes rapidly. There is an underlying process of deterioration that is more rapid and severe the greater the altitude. Anurans had to adapt to this pressure by adjusting their behaviour and physiology, and this must be followed by changes in morphology. According to the literature (Hu et al. Citation1987; Yang Citation1991; Ye et al. Citation1993; Zhao and Yang Citation1997; Liu et al. Citation2000), there are more than 200 anuran species in China, which being distributed from sea level to 5100 m above sea level (a.s.l.) provide good material for our study. Our aim is to test whether anuran acoustic organs decline with increasing altitude. We tested whether the acoustic organs of the species within anuran orders correlate to altitude, and briefly discussed processes that may account for the results.
Materials and methods
We reviewed literature that concerned about 250 anuran species in China. To avoid confusion caused by different taxonomic systems, we dealt with anurans according to the publication that listed the most anuran species which belong to four suborders: Opisthocoela, Anomocoela, Procoela and Diplasiocoela (see Appendix I). Other publications were also referred in case of possible bias on species description.
Data analysis is usually reasonable for interpreting a result, so we transformed the characteristics described in the literature into data. We recorded the characteristics according to papers that clearly described the vocal sac and tympanum of each species. We collected information on 199 species out of 250 (79.6% of total) for which both vocal sac and altitude information were available, and 162 species (64.8% of total) for which both tympanum and altitude information were available. Each character was turned into a number: ‘2’ stands for external vocal sac, ‘1’ for internal vocal sac and ‘0’ for lack of or inconspicuous vocal sac. For tympanum, ‘2’ represented a tympanum larger than half the diameter of the eye, horizontal tympanum diameter less than half the horizontal eye diameter or with an indistinct tympanum was marked ‘1’, and no tympanum or a barely visible tympanum was marked ‘0’. The altitudes as indicated by the literature were adopted. When it appeared in a range, we used the average value. Those with no clear description of both vocal sac or tympanum and altitude at the same time were excluded for purposes of calculating the relationship between vocal sac and tympanum.
Because we hypothesised that the anuran acoustic organs vary along with altitude, we tested the general relationship between anuran acoustic organs and altitude by using the nonparametric correlation test. In this procedure, altitude was treated as the independent factor and the characteristics of anuran acoustic organs as dependent factors. Altitude range was divided into four categories (1⩽1000 m; 1000 m<2⩽2000 m; 2000 m<3⩽3000 m; 4<3000 m). We also tested whether tympanum was influenced by vocal sac using bivariate correlation analysis.
Anuran families in China have different altitudinal ranges (Figure ). The association between altitude and both vocal sac and tympanum might be an artefact and could be explained by phylogenetic relationships rather than by natural selection acting independently on all the species considered. Loglinear analysis is useful in order to classify subjects based on values of a set of predictor variables, so the data were dealt with according to such a procedure. Genus was also introduced as one of the variables at family level, and all the species were grouped into six categories according to their families.
Figure 1 Altitudinal range of anuran families in China. The six families are Discoglossidae(DIS), Pelobatidae (PEL), Bufonidae (BUF), Ranidae (RAN), Rhacophoridae (RHA) and Microhylidae (MIC).
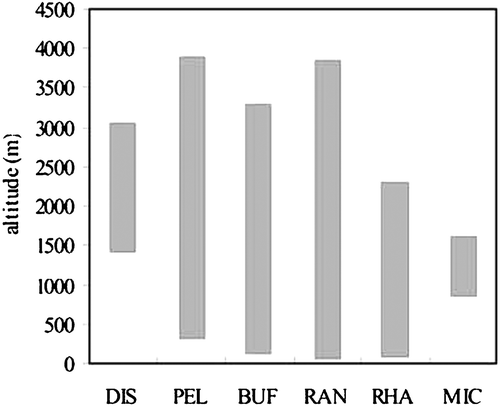
Since the responses had been aggregated, the cases were weighted by frequency first. Hierarchical loglinear analysis could remove some effects while evaluating the full model. The first model evaluated was the saturated model, which included all effects (altitude, family, vocal sac and tympanum) and thus perfectly fits the data. With our data, the quadruple and the triple interactions showed no significant effect (P values were larger than 0.577), so the quadruple and the triple interactions were removed. After a six‐step simple effect deletion, the final model was generated. Two remaining interaction terms were altitude–vocal sac and altitude–tympanum, and the interaction of phylogenetic relationships could be excluded. Then, two‐way interaction of altitude–vocal sac and altitude–tympanum were tested with General Loglinear Analysis.
Statistics were achieved by SPSS 12.0.
Results
The results of the nonparamatric test showed that anuran auditory organs showed a significant difference at different altitudes (Table ). As expected, the median test indicated that both vocal sac and tympanum showed a negative trend along with increasing altitude (Table ).
Table I. Kruskal–Wallis test for testing distribution of anuran auditory organs to different altitude.
Table II. Frequency of vocal sac and tympanum above and below the median.
Loglinear analysis also reached the conclusion that vocal sac and tympanum each had a significant association with altitude. The results showed a likelihood ratio chi‐square value of 18.80 (df = 16, P = 0.279), a Pearson's chi‐square value of 15.34 (df = 16, P = 0.500), so the model fitted the data adequately. It confirmed the hypothesis that anuran acoustic organs changed along with altitude.
In addition, bivariate correlation analysis showed that vocal sac and tympanum are positively correlated (r = 0.343, P<0.001, n = 188), and it could be inferred that the degeneration of the vocal organ led to the same trend in the auditory organ.
Discussion
Physical characteristics of the environment may influence the evolution of call characteristics related to morphology and physiology (Bosch & De la Riva Citation2004). Although some reports showed that differences in call structure could be more readily explained by differences among species in body size or by phylogenetic relationship than by differences in natural calling environment (Penna & Solis Citation1998; Kime et al. Citation2000), our results showed an association between acoustic organs and altitude instead of phylogenetic relationships.
High‐altitude populations are less resilient than low‐altitude populations (Morrison & Hero Citation2003). It was considered that, when calling in areas isolated from others, a male would tend to produce simple calls (Ryan & Rand Citation1999). Altitude, per se, had a negative effect upon species densities (Rogers Citation1976). Increasing with the altitude, lower‐density anurans will not make as much noise as those at low altitude; as a result, female detection of the male acoustic signal becomes somehow easier at this stage. Because individuals at high altitudes are not in such densities as those as at low altitudes, it is no longer necessary for them to keep either the complex call or diverse vocal sac.
Exhibiting the signal requires energy, whether the animal is displaying a morphological character in a courtship dance or producing a sound, pheromone, or electrical discharge (Ryan & Rand Citation1993). An increase in the signal to noise ratio increases the signal's conspicuousness, and complex, long calls appear to be more attractive to some females, but incur higher energy costs and oxygen consumption (Ryan & Rand Citation1993; Rosenthal et al. Citation2004). Taigen and Wells (Citation1985) indicated that calling can be metabolically very expensive, and during high levels of calling a large amount of oxygen can be consumed, with an increased metabolic rate of 10–20 times that of resting.
The fact is that there is less oxygen at higher altitudes. At 4000 m a.s.l., for example, the partial pressure of oxygen decreases by about 40% in comparison to pressure at sea level. In addition, it is common for anurans to suffer from food shortage. Those could not match the need for anuran calling. It is disadvantageous for anuran metabolic activity as well as calling. Marler and Ryan (Citation1996) stated that males given supplemental food were significantly more likely to call than males not given supplemental food. Anurans at high altitude must prevent continual calling and conspicuous vocal sac inflation in order to meet the limited food supply.
Temperature also decreases rapidly with increasing altitude, accompanied by low air pressure and lack of oxygen. Vocal activity becomes physiologically harder. Severe environments forced frogs and toads to distribute their energy more carefully. They had to adapt the pressure by adjusting their behaviour, physiology, and morphology.
Pough et al. (Citation1998) indicated that temperature affects several properties of the advertisement call of frogs and toads. The magnitude of a temperature effect, however, may differ between populations that live in different climates (Sullivan Citation1989; Navas Citation1996), and frogs from colder climates and higher elevations call at lower temperatures than frogs from warmer climates and lower elevations (Narins & Smith Citation1986; Schneider & Sinsch Citation1999). It could be inferred that frogs from colder climates had adjusted their physiological strategy to cope with a low‐temperature environment, although vocal activity is also physiologically harder at lower temperature, and high‐elevation tropical species use thermal niches to reduce exposure to the cold, as well as restricting maximum vocal activity to just after dusk, before temperatures become too low (Navas Citation1996; Murphy Citation1999). They will shorten the chorus period as much as possible. At the same time, temporal visual cues may be redundant with acoustic cues in localising and evaluating a call (Rowe Citation1999), so vocal sac degeneration or absence occurred.
It is often assumed that the evolution of an animal communication system involves co‐evolution of a signal and receiver (Ryan Citation1986; Ryan & Rand Citation1999). Within a taxon, signals and receivers are ‘congruent’ if the response and signal are either both present or both absent (Hill Citation1994). As a response to reduced vocal activity, tympanum degeneration followed.
The vocal sac and tympanum degeneration could be compensated by other means of sound transmission. Anuran amphibians can utilise nontympanic pathways of sound reception (body wall, head surface, and dorsal shoulder surface) (Hetherington Citation1992). So the reduction or lack of vocal sac and tympanum will not affect the communication of anurans inhabiting high‐altitude regions. It should be noted that males of the concave‐eared torrent frog (Amolops tormotus), whose tympana are recessed and invisible from the outside, living in or near noisy streams, reliably produce acoustic signals that contain prominent ultrasonic harmonics (Narins et al. Citation2004), and their unusual morphological features enable them to respond to ultrasonic signals (Feng et al. Citation2006). This is a sound evidence of frog species evolution resulting from natural selection.
We conclude that anuran acoustic organs declined with increasing altitude. Low population density, hypoxia, low temperature, and food shortage might be responsible for such a decline. Nontympanic pathways of sound reception or an ultrasonic signal system will efficiently maintain their communication.
Acknowledgements
We are particularly grateful to Massimo Delfino and an anonymous reviewer for their comments on the manuscript.
References
- Bosch , J. and De la Riva , I. 2004 . Are frog calls modulated by the environment? An analysis with anuran species from Bolivia. . Canadian Journal of Zoology , 82 : 880 – 888 .
- Claridge , M. F. , Hollander , J. D. and Morgan , J. C. 1988 . Variation in host–plant relations and courtship signals of weed‐associated populations of the brown planthopper, Nilaparvata lugens (Stal), from Australia and Asia: A test of the recognition species concept. . Biological Journal of the Linnean Society , 35 : 790 – 793 .
- Downes , J. A. 1965 . Adaptations of insects in the arctic. . Annual Review of Entomology , 10 : 257 – 274 .
- Duellman , W. E. and Trueb , L. 1986 . Biology of amphibians , Baltimore, Maryland : Johns Hopkins University Press .
- Feng , A. S. , Narins , P. M. , Xu , C. , Lin , W. , Yu , Z. , Qiu , Q. , Xu , Z. and Shen , J. 2006 . Ultrasonic communication in frogs. . Nature , 440 : 333 – 336 .
- Hetherington , T. E. 1992 . The effects of body size on functional properties of middle ear systems of anuran amphibians. . Brain Behavior and Evolution , 39 : 133 – 142 .
- Hill , G. E. 1994 . Geographic variation in male ornamentation and female mate preference in the house finch: A comparative test of models of sexual selection. . Behavioral Ecology , 5 : 20 – 30 .
- Hödl , W. and Amézquita , A. 2001 . “ Visual signaling in anuran amphibians. ” . In Anuran communication , Edited by: Ryan , M. J . 121 – 141 . Washington, D.C. : Smithsonian Institution Press .
- Hu , S. Q. , Fei , L. , Hu , Q. X. , Huang , Q. Y. , Huang , Y. Z. , Jiang , Y. M. , Tian , W. S. , Ye , C. Y. and Zhao , E. M. 1987 . Amphibia–Reptilia of Xizang , Beijing : Science Press .
- Kime , N. M. , Turner , W. R. and Ryan , M. J. 2000 . The transmission of advertisement calls in Central American frogs. . Behavioral Ecology , 11 : 71 – 83 .
- Liu , Y. M. , Xie , Y. H. and Ji , D. M. 2000 . General list of vertebrates in China , Shenyang : Liaoning University Press .
- Mani , M. S. 1968 . Ecology and biogeography of high altitude insects , The Hague, , The Netherlands : Dr. W. Junk .
- Marler , C. A. and Ryan , M. J. 1996 . Energetic constraints and steroid hormone correlates of male calling behaviour in the túngara frog. . Journal of Zoology , 240 : 397 – 409 .
- McDonald , J. J. , Teder‐Sälejärvi , W. A. and Hillyard , S. A. 2000 . Involuntary orienting to sound improves visual perception. . Nature , 407 : 906 – 908 .
- Morrison , C. and Hero , J. M. 2003 . Geographic variation in life‐history characteristics of amphibians: A review. . Journal of Animal Ecology , 72 : 270 – 279 .
- Murphy , C. G. 1999 . Nightly timing of chorusing by male barking treefrogs (Hyla gratiosa): The influence of female arrival and energy. . Copeia , 1999 : 333 – 347 .
- Narins , P. M. , Feng , A. S. , Lin , W. , Schnitzler , H. , Denzinger , A. , Suthers , R. A. and Xu , C. 2004 . Old world frog and bird vocalizations contain prominent ultrasonic harmonics. . The Journal of the Acoustical Society of America , 115 : 910 – 913 .
- Narins , P. M. , Hödl , W. and Grabul , D. S. 2003 . Bimodal signal requisite for agonistic behavior in a dart‐poison frog, Epipedobates femoralis. . Ecology , 100 : 577 – 580 .
- Narins , P. M. and Smith , S. L. 1986 . Clinal variation in anuran advertisement calls: Basis for acoustic isolation? . Behavioral Ecology and Sociobiology , 19 : 135 – 141 .
- Navas , C. A. 1996 . The effect of temperature on the vocal activity of tropical anurans: A comparison of high and low‐elevation species. . Journal of Herpetology , 30 : 488 – 497 .
- Penna , M. and Solis , R. 1998 . Frog call intensities and sound propagation in the South American temperate forest region. . Behavioral Ecology and Sociobiology , 42 : 371 – 381 .
- Pough , F. H. , Andrews , R. M. , Cadle , J. E. , Crump , M. L. , H.Savitzky , A. and Wells , K. D. 1998 . Herpetology , Upper Saddle River, NJ : Prentice‐Hall .
- Ritchie , M. G. 1991 . Female preference for ‘song races’ of Ephippiger ephippiger (Orthoptera: Tettigoniidae). . Animal Behaviour , 42 : 518 – 520 .
- Rogers , J. S. 1976 . Species density and taxonomic diversity of Texas amphibians and reptiles. . Systematic Zoology , 25 : 26 – 40 .
- Rosenthal , G. G. , Rand , A. S. and Ryan , M. J. 2004 . The vocal sac as a visual cue in anuran communication: An experimental analysis using video playback. . Animal Behaviour , 68 : 55 – 58 .
- Rowe , C. 1999 . Receiver psychology and the evolution of multicomponent signals. . Animal Behaviour , 58 : 921 – 931 .
- Ryan , M. J. 1986 . Factors influencing the evolution of acoustic communication: Biological constraints. . Brain Behavior and Evolution , 28 : 70 – 82 .
- Ryan , M. J. and Keddy‐Hector , A. 1992 . Directional patterns of female mate choice and the role of sensory biases. . American Naturalist , 139 : S4 – S35 .
- Ryan , M. J. , Perrill , S. A. and Wilczynski , W. 1992 . Auditory tuning and call frequency predict population‐based mating preferences in the cricket frog, Acris crepitans. . American Naturalist , 139 : 1370 – 1383 .
- Ryan , M. J. and Rand , A. S. 1993 . Sexual selection and signal evolution: The ghost of biases past. . Philosophical Transactions, Biological Sciences , 340 : 187 – 195 .
- Ryan , M. J. and Rand , A. S. 1999 . “ Phylogenetic inference and the evolution of communication in túngara frogs. ” . In The design of animal communication , Edited by: Hauser , M. D and Konishi , M . 535 – 557 . Cambridge, MA : The MIT Press .
- Ryan , M. J. and Wilczynski , W. 1991 . Evolution of intraspecific variation in the advertisement call of a cricket frog (Acris crepitans, Hylidae). . Biological Journal of The Linnean Society , 44 : 249 – 271 .
- Schneider , H. and Sinsch , U. 1999 . Taxonomic reassessment of Middle Eastern water frogs: Bioacoustic variation among populations considered as Rana ridibunda, R. bedriagae or R. levantina. . Journal of Zoological Systematics and Evolutionary Research , 37 : 57 – 65 .
- Sullivan , B. K. 1989 . Interpopulational variation in vocalizations of Bufo woodbousii. . Journal of Herpetololgy , 23 : 368 – 373 .
- Taigen , T. L. and Wells , K. D. 1985 . Energetics of vocalization by an anuran amphibian (Hyla versicolor). . Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology , 155 : 163 – 170 .
- Tobias , M. L. , Barnard , C. , O'Hagan , R. , Horng , S. H. , Rand , M. and Kelley , D. B. 1999 . “ Vocal communication between male Xenopus laevis. ” . In The design of animal communication , Edited by: Hauser , M. D and Konishi , M . 353 – 365 . Cambridge, MA : The MIT Press .
- Verrill , P. A. and Arnold , S. J. 1989 . Behavioral observations of sexual isolation among allopatric populations of the mountain dusky salamander, Desmognathus ochrophaeus. . Evolution , 43 : 745 – 755 .
- Yang , D. T. 1991 . The amphibian fauna of Yunnan , Beijing : China Forestry Publishing House .
- Ye , C. Y. , Fei , L. and Hu , S. Q. 1993 . Rare and economic amphibians. . Sichuan Publishing House of Science and Technology
- Yin , X. C. 1984 . The origin of apterous grasshoppers from Tibetan Plateau. . Acta Biologica Plateau Sinica , 2 : 57 – 65 .
- Zhao , E. M. and Yang , D. T. 1997 . Amphibians and reptiles of the Hengduan Mountains Region , Beijing : Science Press .