Abstract
The distribution of Antipathella subpinnata in Italian seas is herein given and discussed. A. subpinnata is a large, white, branched antipatharian with an Atlanto‐Mediterranean distribution. It is probably the most commonly observed black coral in this basin and it is possible to find large populations of this species at diving depths. Personal records of the occurrence of the species in the Strait of Messina, together with a wide census involving diving centres along all the Italian coasts, have been used to create a distribution data set. A description of the species, including in vivo measurements of the polyps, is given. Information concerning the habitat, the population density, the substrate, the epibionts and the environmental conditions of the sites where the species was found are also included. This study confirms the importance of A. subpinnata as a common component of the lower fringe of the circalittoral twilight environment, below 50 m depth, in localities where hard substrata are available.
Introduction
Black corals are among the most common azooxanthellate corals in tropical reefs, sometimes showing high diversities and considerable abundances (Tazioli et al. Citation2007). The group is also present in temperate and polar waters with fewer species but, in unique habitats, they may be found at very high abundances (Grange 1985, 1988; Grange & Singleton 1988). Data about ecology, distribution and population structure of black corals are rare mainly due to the paucity of field studies that have focused on this group.
Five species of black corals are known to occur in the Mediterranean Sea (Opresko & Försterra Citation2004): Antipathes dichotoma Pallas, 1766, Antipathes fragilis Gravier, Citation1918 (Family Antipathidae), Parantipathes larix (Esper, 1790) (Family Schizopathidae), Leiopathes glaberrima (Esper, 1792) (Family Leiopathidae), Antipathella subpinnata (Ellis and Solander, 1786) (Family Myriopathidae). A. subpinnata is probably the most widespread species according to the records in the literature. The aim of this paper is to describe the distribution of this species in Italian seas together with some morphological details of the colonies and polyps.
A. subpinnata represents the type species of the genus Antipathella Brook, Citation1889 (Family Myriopathidae, Opresko Citation2001). The inclusion of this species in the family has been recently confirmed by molecular analyses (Lapian et al. Citation2007). The genus, comprised of five species, is characterised by a branched corallum with simple elongated pseudo‐pinnules arranged irregularly in one to four rows (Gray Citation1857; Lacaze‐Duthiers Citation1865; Opresko Citation2001; Opresko & Baron‐Szabo Citation2001). Following the original description of A. subpinnata, various other authors found and redescribed the species. In many cases it was only matter of a new geographic record (Grasshoff Citation1985; Vafidis & Koukouras Citation1998) or a brief and un‐detailed description (Lamouroux Citation1821; Roule Citation1905; Gravier Citation1921); sometimes, however, some morphological information was given (Gray Citation1857; Brook Citation1889; von Koch Citation1889; Schultze Citation1896; Gravier Citation1918; Dantan Citation1920; Pax et al. Citation1987). The majority of these authors studied colonies or fragments collected from deep‐water using trawls and dredges. Lacaze‐Duthiers (Citation1865) thoroughly investigated the anatomy and morphology of the species maintaining colonies alive for several days in an aquarium. Brook (Citation1889) gave a detailed morphological description of the species and incorporated it in the new genus Antipathella, which at that time was included in the family Antipathidae. Dantan (Citation1920) considered not only the histological‐anatomical characteristics of A. subpinnata, but also some biological aspects, like reproduction, nutrition and defence.
In 2001, Opresko clarified the systematic position of the species by including the genus Antipathella in the new family Myriopathidae and, since the holotype of Ellis and Solander was lost, he designated a neotype collected in the Gulf of Naples, based on the concept of the species as presented by Brook (Citation1889) and later workers. From an historical point of view however it is possible that the first record of this species in the Mediterranean belongs to the Adriatic Sea. In fact, Pax and Müller (Citation1962) considered it likely that the Gorgonia dichotoma described by Linnaeus in 1758 for the Adriatic Sea was a specimen of A. subpinnata, a record nearly 30 years earlier than that of Ellis and Solander (1786) in the Gibraltar Strait. However, this record is now considered doubtful since it is possible that Linnaeus was describing a specimen of Antipathes dichotoma Pallas, 1766.
The two species can be distinguished by the morphology of spines, which are conical and smooth, 0.2 mm or more tall in A. dichotoma while 0.1–0.2 mm in A. subpinnata, where they can bifurcate on the stem, and for the polyp size (2.0–2.4 mm in transverse diameter in A. dichotoma and 1 mm in A. subpinnata) (Opresko Citation2001, Citation2003). Also the pattern of ramification is different: in the tall colonies of A. dichotoma, the long flexible branches are irregularly distributed on all sides of the stem and lower branches, occasionally uniserially arranged and inclined with an angle often close to 90° (Opresko Citation2003). At times branching is dichotomous and more branches arise at the same level (Rossi Citation1971). In A. subpinnata the elongated thin pseudo‐pinnules arise vertically with an angle of 30–70° and are arranged in 1–4 irregular rows around the stem giving rise to a more densely branched colony (Opresko Citation2001). The colonies are smaller and the apical branches show an almost feather‐like disposition (Rossi Citation1971). The depth range of A. dichotoma (60–250 m) overlaps that of A. subpinnata (Opresko & Försterra Citation2004).
Materials and methods
Specimen collection
Two specimens of A. subpinnata were collected by diving in the Messina Strait in October 2004 and November 2005 at a site on the Calabrian coast between Scilla and Favazzina. The bottom is composed of a geomorphologic system formed by six smaller shoals and a major one called “Secca dei Francesi” surrounded by depths ranging from 55 to 70 m. The eastern side of the explored shoal is directed towards the shore and gently levels off at 58 m depth on a sea bottom composed of detritic materials, whereas the western wall is directed towards the open sea and declines drastically and vertically to depths of 65–70 m onto a coarse sandy bottom. The habitat is exposed to strong currents in N–S and E–W directions.
Analysis of the material
The colonies were kept alive in natural seawater for laboratory analyses of the zooids and their cnidome. They were preserved dry for the study of pinnules and spines. Branches were preserved in 4% formaldehyde and alcohol 95°, and polyps were relaxed with MgCl2. The cnidome of the samples was examined by squeezing some polyps or different parts of polyps (mouth, tentacles and coenenchyme) on a slide and observing the cnidocysts with the optical microscope (100× oil immersion).
For the SEM analysis, fragments of branches were washed with distilled water, dehydrated in a graded ethanol series samples, and dried in a Critical Point Dryer. Finally, they were coated with gold–palladium in a Balzer Union evaporator and examined with a Philips EM 515 SEM.
Distribution data set
A data set of the distribution of A. subpinnata in Italian Seas was created using all available literature data, along with observations of professional divers, who were mainly diving instructors and professional photographers. We sent a questionnaire to many diving centres located along the Italian coasts, asking for information concerning the presence of black corals in their area of interest, the population density, the depth range and the habitat features. When possible the information was checked through the study of the images taken in situ by different authors.
Results
Description of the specimen
The largest specimen, that we are here describing, has a size of 78 cm in height, 39 cm in width and 33 cm in thickness (Figure ). Underwater the colour is transparent‐white (Figure ). Immediately after collection, even if the sample was kept in water, or after fixation, the polyps turned opaque white or light yellow and produced a considerable amount of mucous.
Figure 1 Antipathella subpinnata. A, dry colony. B, spines on an apical portion of the axis (2 mm in diameter). C, dendritic spines at the base of the axis. D,E, conical spines on a major branch (0.3 mm in diameter). F, spines on a primary pseudo‐pinnule (0.1 mm in diameter). G, arrangement of pseudo‐pinnules on a branch. H,I, arrangement of polyps on pinnules. L, living polyp. M, fixed polyps. N, p‐Mastigophore microbasic 29×6 µm (shaft 9.6 µm long). O, p‐Mastigophore microbasic 22×5 µm (shaft 8 µm long). P, SEM photo of tentacular spirocysts. Q,R, basitrich isorhizas 20×3 µm. S, basitrich isorhiza 9×2 µm. Scale Bar: N–S, 10 µm; E, 100 µm; C, D, F, L, 200 µm; H, I, M, 700 µm; B, 1 mm; G, 1 cm; A, 10 cm.
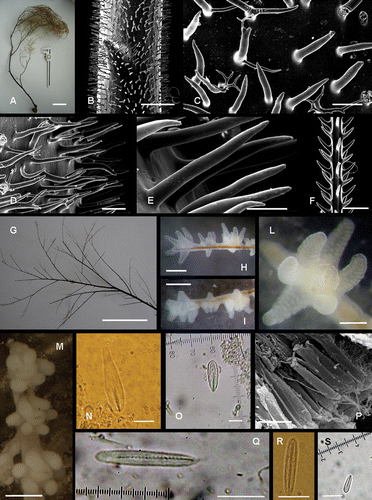
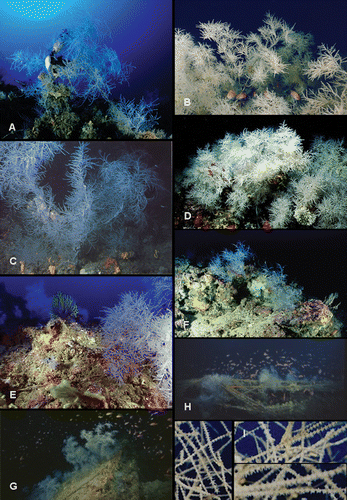
Figure 2 In situ photographs of Antipathella subpinnata. A, colony with shark's egg. Portofino. Photo Andrea Ghisotti. B, colonies offering refuge to some Anthias anthias. Messina Strait. Photo Gianni Neto. C, colonies photographed by ROV at 90 m depth in Portofino. Sponges Axinellae spp. present at the base of the coral. Courtesy of Prof. Riccardo Cattaneo‐Vietti. D, big colonies of white A. subpinnata. Messina Strait. Photo Francesco Turano. E, colony on the rocky substrate of Favazzina, Messina Strait (62 m depth). Colonies of Paramuricea clavata in the background. Photo Gianmichele Iaria. F, Capraia Island. Photo Andrea Ghisotti. G,H, colonies on the wreck “Ravenna”, Imperia (75–90 m depth). Photos Aldo Ferrucci, courtesy of Cristiano Aicardi. I, macro photos showing the expanded living polyps. “Scoglio della Formica”, S. Flavia. Photos Santo Tirnetta.
The colony is characterised by long, numerous and flexible ramifications. The major branches (up to the fifth order) converge basally in a single stem, 59 cm long, showing a basal diameter of 0.9 cm and a strong anchorage (approximately 11 cm2). Pseudo‐pinnules of various length (from 1 to 4 cm) are arranged irregularly in 1–4 rows around the ramification which bear them and are directed upwards with an angle of 30–45° (Figure ). Primary pseudo‐pinnules show a density of 2–3 per cm (in all rows) and can reach the second order of ramifications. Subpinnules are shorter than primary pinnules (from 0.5 to 1.5 cm) and show the same density per cm. No anastomosis between ramifications are detectable.
The SEM analysis revealed typical ‘Myriopathidae’ spines. On the stem, there are long, thin, acute, smooth, cylindrical spines (apical portion 0.2 mm in diameter; Figure ) which become dendritic at the base of the stem (Figure ), and are irregularly arranged. On the branches, the spines are mainly simple (Figures ). The spines are inclined generally upwards without differences in height between the polypar and abpolypar side. They reach up to 0.3 mm in height and 0.04 mm in width on the stem and major branches (range 0.2–0.3 mm), while decreasing to 0.2 mm on higher order branches. The number of rows is not countable on the stem, since the spines are extremely crowded, while there are 10–12 rows (from lateral view) on the major branches (6–7 spines per mm in one row, 0.16–0.21 mm apart). On the pseudo‐pinnules the spines show a slightly more triangular–subcylindrical shape (Figure ); they are 0.1–0.16 mm high on a pseudo‐pinnule 0.1 mm in diameter; are distally inclined and arranged in 5–6 rows (decreasing to 4–5 on the subpinnules) with a density of 5–6 spines per mm in each row (meaning that they are 0.18 mm apart, on average).
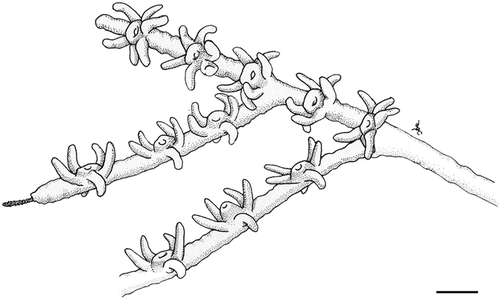
The polyps are monoserial (Figure ), with a slightly sagittally elongated outline, since the two lateral couples of tentacles are close to the oral cone and the sagittal tentacles are inserted at a lower level (Figures ). In vivo, polyps are at times so transparent that it is possible to observe the golden skeleton underneath (Figures ). Adult polyps have a transverse diameter of 0.7–0.9 mm and their tentacles in vivo are elongated (up to 0.7 mm long), cylindrical, with a rounded tip. The interpolypar distance is quite variable, ranging from 0.1 to 0.5 mm (up to 0.8–0.9 mm on certain pseudo‐pinnules), the density ranges from 8 to 10 polyps per cm and small polyps (0.5 mm in transverse diameter) are irregularly distributed between the adult ones (Figures ). There is no distinct polypar side in the colony, since the zooids are not arranged on the same side of all ramifications. The oral cone is elevated (0.16 mm on average) and the mouth opening is usually oval in shape and surrounded by a thick oral margin (Figures ).
Figure 3 Arrangement of polyps inA. subpinnata. Drawing by Dr Cristina Gioia di Camillo. Scale bar: 0.7 mm.
No sexually mature colonies were collected.
The cnidome of A. subpinnata is composed of microbasic p‐mastigophores, basitrich isorhizas and spirocysts.
-
Microbasic p‐mastigophores can be separated in two categories based of the size and the shape of the capsules. The first consists of drop‐like nematocysts, 29×6 µm (shaft 10 µm long), with a distinct V‐notch on the undischarged shaft (Figure ); the second consists of nematocysts 22×5 µm (shaft 8 µm long) characterized by a capsule of more uniform width (Figure ). Both categories of mastigophores are found in tentacles and coenenchyme, but a few of the first type are present also around the mouth.
-
Spirocysts (16−22×2 µm) are the most abundant cnidocysts in this species and are present in all the considered portions of the polyps, but are densely packed in batteries only in the tentacular epidermis (Figure ).
-
Basitrich isorhizas are recorded in two sizes, 20×3 µm (Figures ) and 9×2 µm (Figure ), respectively. The first isorhiza is present around the mouth, in the coenenchyme and tentacles, where it is more abundant. The second type is present in tentacles and mainly in the coenenchyme.
Remarks
Opresko (Citation2001) gave a detailed description of A. subpinnata based on a small, alcohol‐fixed colony. The analysis of our samples immediately after collection, has added new information about the morphology, the cnidome and the colour of living polyps (Figure ). In the past Lacaze‐Duthiers (Citation1865) described red‐grey polyps, Gravier (Citation1918) yellow‐white polyps, and von Koch (Citation1889) reported a colour toning from white‐grey to reddish after contraction, which was then lost after alcohol preservation.
The possibility of studying large colonies allowed us to check the morphological variability of the spines within the entire set of ramifications. Particularly, the SEM analysis of the basal part of the stem verified the presence of dendritic spines, which was previously only hypothesised. The data concerning the height of spines were in accordance with what was reported by Opresko (Citation2001) and most other authors, but not with Gili (Citation1987), who reported, probably erroneously, that the spines of his specimen were 0.6–1.7 mm high.
Moreover, our specimens, which were bigger than any ever reported in literature, had a more dense system of ramification made up of more numerous or thicker branches and pseudo‐pinnules. The study of the living polyps revealed a more sagittally elongated outline than that reported before and a different colour pattern than what was known for fixed samples. While the transverse diameter of zooids was smaller than the 0.1 mm reported by Opresko (Citation2001), the sagittal tentacles were slightly more elongated (0.7 mm rather than 0.6 mm) and more cylindrical when extended. Data concerning the oral cone, the mouth and the cnidome were for the first time included in the description.
Distribution
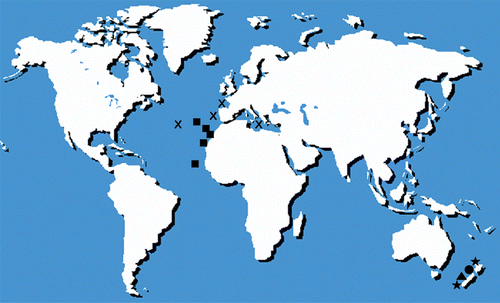
A map of the geographic distribution of A. subpinnata in the Mediterranean basin (Figure ) has been created summarising the data present in the literature (Table ) and those obtained from diving centres (Table ). For some historical records it has not been possible to verify the species determination, because it was lacking a systematical description, therefore some may be considered doubtful.
Figure 4 Distribution map ofAntipathella subpinnata in the Atlantic‐Mediterranean region. Legend of colours: Dark grey/red: historical record sites; light grey/green: current record sites. A, Near Gibraltar. B, Spanish coast. C, French coast. D, Ligurian Sea. Gulf of Naples, Tyrrhenian Sea: E, Bay of Naples; F, Capri Island; G, Nisida Island. H, Tunisian and Algerian coasts. Adriatic Sea: I, Albanian coasts of Otranto Strait, Croatian islands of L, Lastovo; M, Lissa and N, Lagosta. O, North Aegean Sea. Eastern Atlantic (not shown in the map): P, Josephine Seamount; Q, Great Meteor Seamount. R, West Coast of Portugal. S, West Coast of France: Biskaya Bay and Gascogne Gulf. T, Brest, Atlantic France, English Channel. Gulf of Genoa, Genoa, Ligurian Sea: a, Bordighera; b, Wreck ‘Ravenna’, Imperia; c, Capo Mele. Portofino Promontory, Genoa, Ligurian Sea: d, Secca dell'Isuela; e, Punta di Portofino. f, Secca fonda della Civitata, Capraia. G, Capo Comino, Sardinia. h, Ponza, Latina. i, Dorsale della Sciara del Fuoco, Stromboli, Sicily. l, Scoglio della Formica, S. Flavia, Palermo, Sicily. m, Secche di Favazzina, Bagnara, Strait of Messina, RC, Sicily. n, Pantelleria Island, Sicily. o, Gallipoli, Lecce.
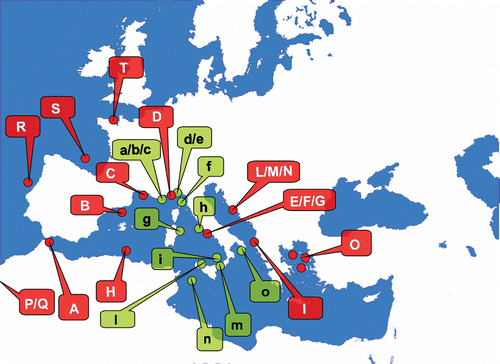
Table I. Summary table of the literature data concerning the distribution of A. subpinnata in the Mediterranean Sea.
Table II. Summary table of new data concerning the distribution of A. subpinnata in Italian Seas.
Eight records of A. subpinnata in the Mediterranean Sea have been reported in the literature since the species was first described from near Gibraltar by Ellis and Solander in 1786. The records available for the western basin are more numerous than for the eastern one, probably due to a different number of studies conducted in the two regions (Vafidis & Koukouras Citation1998). For the Western Mediterranean, the species has been reported from off the Spanish (Gili Citation1987), French (Riedl Citation1983; Rossi Citation1971), and North African coasts (Vafidis & Koukouras Citation1998); and from the Ligurian sea (Riedl Citation1983; Rossi Citation1971), Gulf of Naples (Lacaze‐Duthiers Citation1865; Brook Citation1889; von Koch Citation1889; Gravier Citation1918; Dantan Citation1920; Opresko Citation2001), and Adriatic sea (Heller Citation1868; Pax & Müller Citation1955). For the Eastern Mediterranean, the species has only been recorded from the Greek archipelago, situated in the North Aegean Sea (Vafidis & Koukouras Citation1998) (Figure ).
Outside the Mediterranean basin there are four records for the Eastern Atlantic Ocean: Great Meteor and Josephine Seamounts (Grasshoff Citation1985), west coast of Portugal (Nobre Citation1931; Grasshoff Citation1985), west coast of France (Roule Citation1896; Grasshoff Citation1985) and Brest, English Channel (Dantan Citation1920) (Figure ). For the non‐Mediterranean records the confusion may rise with the Atlantic species Antipathella wollastoni. The two species have a similar arborescent morphology, but in A. wollastoni the pinnules are frequently arranged in four rows with a total of 14–20 pinnules per cm (Opresko Citation2001).
Grasshoff (Citation1988) referred to Antipathes cf. subpinnata, a collection of several fragments and colonies up to 2 m tall, collected in a depth range between 80 and 220 m, around the islands of St. Paul and Amsterdam, in the southern Indian Ocean. The description of these specimens does not agree with the characteristics of the species and therefore we considered this attribution as doubtful.
Our investigation along the Italian coasts added twelve new records. The majority of these were from the Tyrrhenian and Ligurian Seas, in particular Bordighera, Imperia, Capo Mele, Portofino Promontory, Capraia Island, Capo Comino on the eastern coasts of Sardinia, and Ponza Island. Other new records were from Sicily and the surrounding islands (Stromboli Island, Palermo, Messina Strait, Pantelleria Island). The most eastern record of A. subpinnata in Italy is that for Gallipoli, situated on the Ionian Sea (Figure ).
Bathymetric range
The bathymetric distribution of the species, as reported by the divers, ranges from 54 m to 92 m (Table ). The shallower records are for Sardinia, the deepest for the wreck “Ravenna” in the Ligurian Sea. Specimens described in literature were collected mainly at depths greater than 100 m, sometimes over 200 m, and reaching a maximum of 500 m.
Substrate
Generally A. subpinnata was found on hard substrata (Table ). In the majority of the sites the species was found growing on rock, even if covered by fine muddy sediments (as for example in Stromboli island). A. subpinnata is also able to colonise artificial substrates as observed on the wreck “Ravenna” in the Ligurian Sea where numerous large colonies are anchored on the iron skeleton of the ship.
Colony size
Recorded colonies ranged in height from a minimum of 40 cm to a maximum of 1.2 m (Table ) without evident relation to the described environmental factors, like substrate (rock or artificial), population density (dense population or sparse colonies) and current (moderate or strong).
Concerning the literature data, only a few authors gave precise measures of the colony size: for example Gili (Citation1987) referred to some of the smallest colonies ever reported (20–35 cm high) and Vafidis and Koukouras (Citation1998) found colonies 27–56 cm high in the Eastern Mediterranean.
Population density
In some sites, A. subpinnata reaches high densities (Table ). The most dense and most extensive populations are those of Palermo with about 100 colonies covering 40 m2 and Portofino with 10 colonies for 100 m2. There are, however, sites in which the colonies are more spread out, as in the case of Capraia island, Capo Comino, Stromboli island, and Gallipoli.
Associated fauna
Our data confirm that A. subpinnata colonies are frequently mixed with gorgonias (Lütken Citation1872; Grasshoff Citation1985); in particular, we have recorded this black coral within dense meadows of Paramuricea clavata and Eunicella cavolinii together also with sponges (for example Axinellae spp.; Table and Figure ). These coral assemblages host a rich fish community (Figure ). The colonies generally do not harbour epibionts, with the exception of the bivalve Pteria hirundo (reported also by Vafidis & Koukouras Citation1998) or Scyliorhinus sp. eggs hanging from the ramifications of the coral (Figure ).
Discussion
Our study expands the known distribution of Antipathella subpinnata in the seas surrounding Italy and clearly indicates that this species is a common component of the lower fringe of the circalittoral twilight environment, below 50 m depth, where hard substrata are available. The large data set obtained in this study shows that in the Mediterranean Sea this black coral never lives at depths shallower than 50 m. It is very probable that the record of A. subpinnata from 10 m depth reported by Riedl (Citation1983) is erroneous, since there is no actual evidence of its presence at such shallow depths.
The bathymetric pattern highlighted in this study suggests that temperature is the main environmental factor involved in determining the bathymetric distribution of A. subpinnata. The data indicate that it is a stenothermal species not able to survive at water temperatures greater than 15°C. For this reason, A. subpinnata must be viewed as a characteristic species of a coral assemblage composed also of some species of gorgonians, mainly Paramuricea clavata and Eunicella cavolinii. Moreover, the optimum temperature range for this species agrees with that of the other species belonging to the genus Antipathella, for example A. aperta and A. fiordensis which live in the fiords of New Zealand.
In the Mediterranean Sea research on deep coral assemblages was until now limited to the white coral banks mainly composed of madrepores of the genus Lophelia Milne‐Edwards & Haime, 1849 and Madrepora Linnaeus, Citation1758 (Tursi et al. Citation2003), while the populations of flexible corals (black corals, gorgonians) are almost unknown. Moreover, these important assemblages are now endangered by trawling activity and the situation is particularly problematic in some areas where the coral assemblages host populations of important target species for commercial fisheries. This is, for example, the case of the middle slope horizon of the western Mediterranean, whose assemblage is characterised by meadows of the gorgonian Isidella elongata and by the shrimps Aristeus antennatus and Aristeomorpha foliacea. This assemblage, described at the beginning of the twentieth century (Issel Citation1930) has been heavily exploited and the gorgonian has now almost completely disappeared from the trawled bottoms of most of the Mediterranean areas (Sardà et al. Citation2004).
Coral assemblages characterised by A. subpinnata are found on hard substrata, the type of bottom that is not trawled by commercial fisherman; therefore, direct damage by trawling activities is minimal. On the other hand, in some places the boats, trawling very close to the base of the cliff, can produce a strong resuspension of the sediment that increases the water turbidity and may thereby damage the Antipathella assemblages. For all these reasons we strongly suggest the institution of Marine Protected Areas in places where hard substrata are present deeper than 50 m.
The genus Antipathella is comprised of five species living in temperate waters; three occur off New Zealand (A. aperta, A. strigosa, A. fiordensis), one in the northeastern Atlantic (A. wollastoni), and the third mainly in Mediterranean Sea (A. subpinnata) (Opresko Citation2001). The scarcity of taxonomic studies and the uncertainty of several old determinations about antipatharians, in general, have, until now, prevented biogeographic studies on black corals. Concerning the genus Antipathella, for example, the distinction between A. wollastoni and A. subpinnata has just been recently clarified (Opresko Citation2001), but some old identifications could be doubtful. Nevertheless, the world distribution of the entire genus strongly suggests the status of Thetyan relict (Figure ), a distribution pattern known for many genera, like the popular examples of the precious coral (Corallium rubrum, distributed with other species from the Atlantic to the Pacific Ocean passing through the Mediterranean basin) (Bayer Citation1964), and the sea grass Posidonia (with the only Mediterranean species Posidonia oceanica and a group of eight species around the Australian coast) (Kuo & McComb Citation1989).
Acknowledgements
We would like to thank Dr Dennis M. Opresko for the kind collaboration in the species determination and for his precious suggestions, and Dr Cristina G. Di Camillo for the drawing. We would also like to thank all the divers that patiently and kindly collaborated and helped with both precious information and photos: Andrea Ghisotti from Fins & Fans by Il Capodoglio s.a.s., Gianmichele Iaria from Oloturia Sub, Alfonso Santoro and Linda Scannavino from Blue Shark, Cristiano Aicardi and Aldo Ferrucci from Nautilus Technical Diving Centre, Luca Coltri from Pianeta Blu, Antonello D'Aietti from Green Divers, Andrea Donati from Ponza Diving Centre, D. C. Gallipoli Dromia Sub, Egidio Trainito, Gianni Neto, Francesco Turano, Santo Tirnetta and Prof. Riccardo Cattaneo‐Vietti (Università di Genova).
References
- Bayer , F. M. 1964 . The genus Corallium (Gorgonacea: Scleraxonia) in the Western North Atlantic Ocean. . Bulletin of Marine Science , 14 : 465 – 478 .
- Brook , G. 1889 . Report on the Antipatharia collected by H. M. S. Challenger during the years 1873–1876. . Reports of the Scientific Results of the Voyage of H. M. S. Challenger , 32 : 1 – 222 .
- Dantan , J. L. 1920 . “ Recherches sur les Antipathaires. ” . 245 [PhD Thesis]. Université de Paris, Paris Archives d'anatomie microscopique
- Ellis , J. and Solander , D. 1786 . The natural history of many curious and uncommon Zoophytes collected by the late John Ellis, systematically arranged and described by the late Daniel Solander , London : White and Son .
- Gili , J. M. 1987 . “ Estudio sistemático y faunístico des los cnidarios de la costa Catalana. ” . 565 [PhD Thesis]. Barcelona, Autonomous University of Barcelona
- Grange , K. R. 1986 . Distribution, standing crop, population structure, and growth rates of black coral in the southern fiords of New Zealand. . New Zealand Journal of Marine and Freshwater Research , 19 : 467 – 475 .
- Grange , K. R. 1988 . Redescription of Antipathes aperta, Totton (Coelenterata: Antipatharia), an Ecological Dominant in the southern fiords of New Zealand. . New Zealand Journal of Zoology , 15 : 55 – 61 .
- Grange , K. R. and Singleton , R. J. 1988 . Population structure of a black coral Antipathes aperta, in the southern fiords of New Zealand. . New Zealand Journal of Zoology , 15 : 481 – 489 .
- Grasshoff , M. 1985 . Die Gorgonaria und Antipatharia der Großen Meteor‐Bank und der Josephine‐Bank (Cnidaria: Anthozoa). . Senckenbergiana maritima , 17 : 65 – 67 .
- Grasshoff , M. 1988 . The geographical and bathymetric distribution of the Gorgonacea and Antipatharia (Cnidaria, Anthozoa) of St. Paul and Amsterdam Islands (Indian Ocean). . Mèsogée , 48 : 115 – 124 .
- Gravier , Ch. 1918 . Notes sur les Antipathaires de Golfe de Naples. . Pubblicazioni della Stazione Zoologica di Napoli , 2 : 223 – 240 .
- Gravier , Ch. 1921 . Antipathaires provenant des Campagnes des yachts Princess‐Alice et Hirondelle II. (1903–1913). . Résultats des Campagnes Scientifiques Accomplies sur son Yacht par Albert I Prince Souverain de Monaco , 59 : 1 – 20 .
- Gray , J. E. 1857 . Synopsis of the families and genera of axiferous zoophytes or barked corals. . Proceedings of the Zoological Society of London , 25 : 278 – 295 .
- Heller , C. 1868 . “ Die Zoophyten und Echinodermen des Adriatischen Meeres. Zoologische Botanische Verhandlungen. ” . 10 – 20 . Wien : Ueberreuter Carl .
- Issel , R. 1930 . La biologia del fondo a “scampi” nel Mar Ligure. Scopi e piano dell'indagine. . Bollettino dei Musei di Zoologia e Anatomia Comparata della R. Università di Genova , 10 : 1 – 3 .
- Kuo , J. and McComb , A. J. 1989 . “ A treatise on the biology of seagrasses with special reference to the Australian region. ” . In Biology of seagrasses. Aquatic plant studies 2 , Edited by: Larkum , A. W. D , McComb , A. J and Shepherd , S. A . 6 – 73 . Elsevier : Amsterdam .
- Lacaze‐Duthiers , H. 1865 . Deuxième mémoire sur les Antipathaires (Antipathes vrias). . Annales de Science Naturelles , 4 : 5 – 62 .
- Lamouroux , J. V. F. 1821 . Exposition Méthodique des Genres de l'Ordre des Polypiers : avec leur description et celle des principales espèces, figurées dans 84 planches, les 63 premières appartenant a l'Histoire naturelle des zoophytes d'Ellis et Solander , Paris : Veuve Agasse, Imprimeur‐Libraire .
- Lapian , H. F. N. , Barucca , M. , Bavestrello , G. , Biscotti , M. A. , Bo , M. , Canapa , A. , Tazioli , S. and Olmo , E. 2007 . A systematic study of some Black Corals species (Antipatharia, Hexacorallia) based on rDNA internal transcribed spacers sequences. . Marine Biology , 151 : 785 – 792 .
- Linnaeus , C. 1758 . Systema Naturae. Tomus I. Editio Decima Holmiae, Impensis Direct. Lurentii Salvii
- Lütken , C. F. 1872 . Antipathes arctica, a new species of black coral (Antipathidae) from the Polar Seas. . Annals and Magazine of Natural History , 10 : 77 – 83 .
- Nobre , A. 1931 . Contribuições para o estudo dos Coelenterados de Portugal , Porto : Instituto de Zoologia da Universidade do Porto .
- Opresko , D. M. 2001 . Revision of the Antipatharia (Cnidaria: Anthozoa). Part I. Establishment of a new family, Myriopathidae. . Zoologische Mededelingen, Leiden , 75 : 343 – 370 .
- Opresko , D. M. 2003 . Redescription of Antipathes dichotoma Pallas, 1766 (Cnidaria: Anthozoa: Antipatharia). . Zoologische Mededelingen, Leiden , 77 : 481 – 493 .
- Opresko , D. M. and Baron‐Szabo , R. C. 2001 . Re‐descriptions of the antipatharians corals described by E.J.C. Esper with selected English translations of the original German text. (Cnidaria, Anthozoa, Antipatharia). . Senckenbergiana biologica , 81 : 1 – 21 .
- Opresko , D. M. and Försterra , G. 2004 . “ Orden Antipatharia (corales negros o espinosos). ” . In El Mar Mediterraneo (Fauna, Flora, Ecologia), vol.2 , Edited by: Hofrichter , R . 506 – 509 . Barcelona : Omega .
- Pax , F. 1952 . Die Antipatharien, Zoantharien und Actinarien der “Havr” Expedition. . Reports Institut za Oceanografiju i Ribarstvo, Split , 6 : 1 – 24 .
- Pax , F. and Müller , I. 1955 . Gli Antozoi del Museo Civico di Storia Naturale di Trieste. Parte I. . Atti del Museo Civico di Storia Naturale di Trieste , 20 : 103 – 110 .
- Pax , F. and Müller , I. 1962 . Die Anthozoenfauna der Adria. . Fauna et Flora Adriatica , 3 : 1 – 343 .
- Pax , F. , van‐Praët , M. and Doumenc , D. 1987 . “ Ordre des Antipathaires. ” . In Traite de Zoologie. Anatomie, Systematique, Biologie. Vol. 3, Cnidaires Anthozoaires , Edited by: Doumenc , D . 189 – 210 . Paris : Masson .
- Riedl , R. 1983 . Fauna und Flora des Mittelmeeres. Ein systematischer Meeresfübrer für Biologen und Naturfreunde , Hamburg‐Berlin : Parey P Verlag .
- Rossi , L. 1971 . Guida a cnidari e ctenofori della fauna italiana. . Quaderni della Civica Stazione Idrobiologica di Milano , 2 : 1 – 101 .
- Roule , L. 1896 . Cœlentérés, Résultats scientifiques de la Campagne du CAUDAN dans le Golfe de Gascogne , Lyon : Annales de l'Université de Lyon .
- Roule , L. 1905 . Description des Antipathaires et Cerianthaires recueillis par S.A.S. le Prince de Monaco dans l'Atlantique nord (1886–1902). . Résultats des Campagnes Scientifiques Accomplies sur son Yacht par Albert I Prince Souverain de Monaco , 30 : 1 – 96 .
- Sardà , F. , Calafat , A. , Flexas , M. M. , Tselepides , A. , Canals , M. , Espino , M. and Tursi , A. 2004 . An introduction to Mediterranean deep sea biology. . Scientia Marina , 68 : 7 – 38 .
- Schultze , L. S. 1896 . Beitrag zur Systematik der Antipatharien. Abhandlungen der Senckenbergischen naturforschenden Gesellschaft. Antipathiden von Ternate nach den Sammlungen Prof. Kukenthal. . Zoologischer Anzeiger , 23 : 1 – 40 .
- Tazioli , S. , Bo , M. , Boyer , M. , Rotinsulu , H. and Bavestrello , G. 2007 . Ecology of some common antipatharians from the Marine Park of Bunaken (North Sulawesi, Indonesia). . Zoological Studies , 46 : 227 – 241 .
- Tursi , A. , Mastrototaro , F. , Matarrese , A. , Maiorano , P. and D'Onghia , G. 2003 . Biodiversity of the white coral reefs in the Ionian Sea (Central Mediterranean). . Chemistry and Ecology , 1 : 107 – 116 .
- Vafidis , D. and Koukouras , A. 1998 . Antipatharia, Ceriantharia and Zoantharia (Hexacorallia, Anthozoa) of the Aegean Sea with a check list of the Mediterranean and Black Sea Species. . Annales de l'Institut océanographique, Paris , 74 : 115 – 126 .
- von Koch , G. 1889 . Die Antipathiden des Golfes von Neapel. . Mitteilungen aus der Zoologischen Station zu Neapel , 9 : 187 – 204 .