Abstract
The aim of this study is to describe the taxonomical aspects, the biology and ecology of P. vaihiriae collected from Italy. This species was sampled from a pond of “Bosco di Policoro” (South of Italy), and a tropical fish farm (Central Italy). In the pond of Policoro the Plumatella vaihiriae colonies grew with those of P. fungosa and of a Victorellidae.
In the glass tank of the fish farm was found only P. vaihiriae, while in the sedimentation pond large colonies of P. casmiana and Plumatella sp were also present. Many mites of two species, which belong to Oribatidae and Halacaridae, were found only on the colonies collected in the glass tank, and in the same tubules seriously damaged statoblasts were present. During the maturation of the damaged immature statoblasts, the remaining material has been organized in an aberrant way. The two species are both predators. Although there are few findings, Plumatella vaihiriae has a widespread distribution. The finding of P. vaihiriae in Italy is of great importance, because it expands the distribution area of the species to include the European zone. This species has wide ecological valency for salinity, pH and transparency, but appears to be a stenothermic species. The presence of P. vaihiriae in the Italian habitats could be due to importations by migratory birds at Policoro and by tropical fish in the fish farm.
Introduction
Plumatella vaihiriae is present in Tahiti (Hastings Citation1929), Utah (Rogick & Brown Citation1942), Hawaii (Baily‐Brock & Hayward Citation1984) and Argentina (Cazzaniga Citation1988). It is now also reported from Thailand (Wood, personal communication).
Other authors (Wood & Marsh Citation1999) found P. vaihiriae in three wastewater plants in South Carolina, Wisconsin and Arizona. Its distribution seems to be extended throughout the United States (Wood Citation2001). Lacourt (Citation1968) reported this species for Australia, but it was not confirmed by Wood (Citation1998). P. vaihiriae is an uncommon but locally abundant species (Wood Citation2001).
This research is a part of a wider project aiming to extend the knowledge of the Italian freshwater bryozoan diversity. Here we describe the taxonomical aspects, the biology and ecology of P. vaihiriae collected from Italy.
Materials and methods
Sampling sites
The species was collected from two sites: a pond of “Bosco di Policoro” (South Italy) and a tropical fish farm (Central Italy).
Bosco di Policoro (Site 1): P. vaihiriae was sampled from this site in June 2005 and it was found inside one of the small perennial water ponds.
This site is close to the mouth of the River Sinni (Matera‐Basilicata). It is a Community Importance Site (SIC) and the natural environment consists of the sand littoral, ponds, marsh and the wood. The pond has a diameter of 30 m, is surrounded by dense phragmitetum that sinks roots into the bottom lime.
It is an important zone for migratory birds. The neritic zone is lacking and in the central part the depth is 2.5 m. On the bank the reeds are black from sulfur. The water is oxygenated at the surface, but near the bottom is anoxic. It has a conductivity of 3126 μS/cm due to the proximity of the sea and high evaporation.
The second site is a tropical fish farm close to Viterbo (Italy). The species was sampled in February and June 2006. P. vaihiriae was found in the glass collecting tank of the cycled water coming from the reproduction sector of the different Discus varieties and the chemical–physical parameters of water were: pH 5; conductivity 180 μS/cm; constant temperature 30°C. Furthermore, the species was also collected from the external sedimentation pond in the ground and the chemical–physical parameters were pH 9; conductivity 300 μS/cm; temperature 24°C. One part of the collected samples of bryozoans was immediately fixed with 90% ethyl alcohol; another part was transferred alive to the laboratory.
Analysis
From each colony, zoecial tubules, the richest in stato‐, floato‐ and sesso‐blasts, were isolated in order to ensure sufficient material for both light microscope and SEM. In this way the observed statoblasts surely belong to the same species. Some statoblasts, after treatment with a saturated solution of KOH for 12 h at 37°C, were observed with an Olympus CX 41 phase contrast microscope. The statoblasts were measured with image analysis Olympus DP soft system. The measures on the dorsal and ventral valves were: L = whole length; W = whole width; l = capsule length; w = capsule width; A = polar annulus width; a = lateral annulus width; F = fenestra length; f = fenestra width. Moreover, the ratios calculated are the following: L/W, l/w, F/f, A/a for both dorsal and ventral valves. Other statoblasts were treated with a saturate solution of KOH for 30 s at room temperature and washed in deionized water; some were cut in the middle with a micro‐dissection needle according to Wiebach (Citation1964) modified; then they were freeze‐dried in a freezer (Taticchi & Pieroni Citation2005), fixed to aluminum stubs and sputter‐coated with gold–palladium and viewed in a Philips XL 30 SEM.
All the observed material is stored in the private collection “A. Viganò” of M. I. Taticchi. The labels are: s359, s360, s361 for Policoro; s380 for the sedimentation pond; tm 52 for glass collecting tank.
Results
In the pond of Policoro the P. vaihiriae colonies were intertwined with those of P. fungosa and of a Victorellid bryozoan. In the fish farm while in the glass tank there was only P. vaihiriae, in the sedimentation pond large colonies of P. casmiana and Plumatella sp were also present. In the first site the colony is small; the tubules are packed with statoblasts and grow parallel to the substrate. Sometimes the coelomatic cavities are fused to form small common rooms from which the short tubules open upwards (this feature has never before been described in a plumatellid bryozoan). The tubule is transparent and has a circular section; septa and keel are lacking. In the glass tank of the second site, the tubules are very long and intertwined like a hank of rope; they are not fused as described by Baily‐Brock and Hayward (Citation1984) and Wood and Marsh (Citation1999), and are completely transparent and adhere to the glass wall loosely. On the contrary in the sedimentation pond the colony turns up to decomposing sticks; the tubules are not fused and the oldest are brownish. The mean tentacle number (11 counts for every site) is: 32.8 for Policoro; 43.0 for the glass tank; 44.1 in the pond of the fish farm. The large variation in the mean tentacle number is probably due to the different environmental conditions. The elliptic floatoblast has somewhat pointed poles. The dorsal valve is reticulated; the reticulum meshes wrap around both the annulus and the roundish fenestra, where they are smaller and have more evident crests (Figure ). The polar groove, also reticulated, is flattened and extended on the fenestra like a half‐moon (Figure ). Interstitial tubercles are completely lacking both on the annulus and fenestra. The annulus of the ventral valve is narrower than on the dorsal valve. The annulus was always inflated, while Wood and Marsh (Citation1999) reported that the floatoblast annulus was often not inflated upon release. A swelling of the fenestra makes a furrow between the annulus and fenestra clear (Figure ). In the central fenestra zone, the reticulum meshes are smaller and there is a protuberance like a button in the centre. The ventral valve is larger than the dorsal (Table ). At high magnification, we can see some nodules on the reticulum crests (Figure ). The suture has never been described before and consists of two thin cords surmounted by alternate tubercles where the reticulum meshes end. The parasutural zone is like a furrow (Figure ). In lateral view we can see that the floatoblast has a shape of little boat (Figure ) because the ventral valve is strongly convex, while the dorsal valve is nearly flat. In the median section (Figure ) we can see the numerous and small gas chamber pores irregularly disposed; they have a smooth rim with some tiny notches (Figures ).
Table I. Measures (µm) of the floatoblast of P. vaihiriae from Site 1 (Policoro).
Figure 1 a, P. vaihiriae: floatoblast dorsal valve; b, P. vaihiriae: polar groove; c, P. vaihiriae floatoblast: ventral valve; d, P. vaihiriae: the suture and the nodules on the reticulum meshes; e, P. vaihiriae: lateral view; f, P. vaihiriae: median section; g, P. vaihiriae: gas chamber pores; h, P. vaihiriae: tiny notches on the smooth rim of the pores; i, P. vaihiriae: sessoblast; l, P. vaihiriae: particular of the sessoblast.
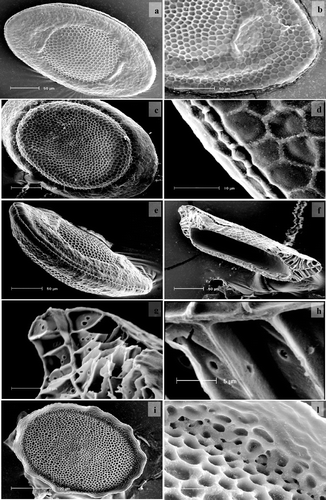
The floatoblast of the first site is smaller than the floatoblasts of other plumatellids (Table ). In the second site, the floatoblast is larger; its dimensions are in the range between those of P. repens and P. fungosa (Table ). The oval sessoblast is reticulated, as in Rogick and Brown (Citation1942) and Wood and Marsh (Citation1999) (Figure ). We can only add that the frontal valve reticulum consists of crests 3 µm high so that the valve has the appearance of a sieve (Figure ). Numerous nodules are present on the crests, and on the proximal part of the lamella, while on the distal part and on the ventral side the reticulum is incomplete (Figure ). The basal valve is reticulated as in P. repens. This kind of sessoblast is unmistakable and characteristic.
Table II. Measures (µm) of the floatoblast of P. vaihiriae from Site 2 (fish farm).
Many mites were found only on the colonies collected in the glass tank of the second site. In some decaying tubules there were also some open eggs. The mites belong to two species: Trimalaconothrus maniculatus Fain & Lambrechts, 1987 (Oribatidae) (Di Cicco et al. Citation2004) and Porohalacarus alpinus alpinus (Thor, 1910) (Halacaridae).
In the same tubules, aberrant statoblasts were present. We associate the mites activity to the presence of the deformed statoblasts. In these the dorsal valve was sometimes difficult to distinguish from the ventral. Some parts were lacking completely. The annulus completely covers the fenestra. During the maturation of the damaged immature statoblasts, the remaining material had been organized in an aberrant way (Figure ).
Up until now, only the Oribatidae Hydrozetes lacustris (Michael, 1882) had been found in freshwater bryozoans. According to Bushnell and Rao (Citation1979), Oribatidae are decomposers. According to Marcus (Citation1926) they are predators of Cristatella mucedo. Wiebach (Citation1959) is doubtful if they are lodgers or predators. According to the present results, both Trimalaconothrus maniculatus (Oribatidae) and Porohalacarus alpinus alpinus (Halacaridae) seem to behave as predators. However, more studies are required to affirm if both mite species are really predators.
Conclusive remarks
The dimensions of the floatoblast of the samples from Policoro and fish farm are smaller and larger, respectively, than those of the USA samples (Table ). According to Lacourt (Citation1968), the polypides from Australia have about 48 tentacles, while in our samples, also we found a large variation, the mean tentacle number is always lower.
Table III. Comparison between the mean values of the floatoblst from Sites 1 and 2 and those reported in the literature.
Considering the description of P. vaihiriae (Wood & Marsh Citation1999) from the USA, we can say that the colonies found in the natural Italian sites have no branches growing free of substrate as in Wood and Marsh's colonies. Moreover, we must note the complete lack of interstitial tubercles and the presence of minute nodules on the mesh crests of the dorsal reticulum of the floatoblast and sessoblast. The central button in the middle of the ventral fenestra is common to the Italian and Tahiti samples. The finding of P. vaihiriae in Italy is of great importance because it expands the distribution area of the species to include the European zone. Moreover, it testifies its ample ecological valency for salinity, pH and transparency. On the contrary P. vaihiriae appears to be a stenothermic species. The founding of two mite species adds new information on the biology of the bryozoan. The presence of P. vaihiriae in the Italian habitats could be due to importations by migratory birds at Policoro and by tropical fish in the fish farm.
Acknowledgments
This study was funded by Italian Ministero dell'Università e della Ricerca Scientifica (2003). We are indebted to Prof. Cicolani Bruno of University of L'Aquila (Italy) for the mite identification, to Dr. Gagliardi Flavio for allowing us to collect samples from the fish farm and to Mr. Saltalamacchia Piero of University of Camerino (Italy) for his help for collecting samples of P. vaihiriae from the pond close to Policoro.
References
- Baily‐Brock , J. and Hayward , P. 1984 . A freshwater bryozoan, Hyalinella vaihiriae Hastings (1929), from Hawaiian prawn ponds. . Pacific Science , 38 : 199 – 204 .
- Bushnell , J. H. and Rao , K. S. 1979 . “ Freshwater Bryozoa: Micro‐architecture of statoblasts and some Aufwuchs animal associations. Advances in bryozoology. ” . In Systematics Association Special Volume, 13 , Edited by: Larwood , G. P and Abbott , M. B . 75 – 91 . London : Academic Press .
- Cazzaniga , N. 1988 . Hyalinella vaihiriae (Ectoprocta‐Phylactolaemata) en la Provincia de San Juan (Argentina). . Revista de la Asociación de Ciencias Naturales del Litoral , 19 : 205 – 208 .
- Di Cicco , E. , Tarantino , C. , Magi , E. , Verni , F. , Gagliardi , F. and Rossi , G. 2004 . Role of Trmalaconothrus maniculatus in transmission of Aeromonas hydrophila to larval stages of Discus fish (Symphysodon aequifasciatus). . Parassitologia , 46 (Supplement 1) : 148
- Hastings , A. 1929 . Notes on some little‐known phylactolaematous polyzoa and description of a new species from Tahiti. . Annals and Magazine of Natural History , 10 : 300 – 310 .
- Lacourt , A. W. 1968 . A monograph of the freshwater Bryozoa‐Phylactolaemata. . Zoologische Verhandelingen , 93 : 1 – 159 .
- Marcus , E. 1926 . Beobachtungen und Versuche an lebenden Süßwasserbryozoen. . Zoologische Jahrbücher, Abteilung für Systematik, Ökologie und Geographie der Tiere , 52 : 279 – 350 .
- Rogick , M. D. and Brown , C. J. D. 1942 . Studies on freshwater Bryozoa. XII. A collection from various sources. . Annals of the New York Academy of Sciences , 43 : 123 – 144 .
- Taticchi , M. I. and Pieroni , G. 2005 . “ Freshwater Bryozoa of Italy. A survey of some species from the Italian bryozoan collection of A. Viganò with new records. ” . In Bryozan Studies 2004 , Edited by: Moyano , H. I , Cancino , J and Jackson , W . 317 – 327 . London : Taylor & Francis Group .
- Wiebach , V. F. 1959 . Kommensalen, Feinde und Parasiten der Süswasser – Moostierchen. . Mikrokosmos , 48 : 168 – 172 .
- Wiebach , V. F. 1964 . Untersuchunghen an süsswasser‐bryozoen aus zentralafrika. . Muséée Royal de l'Afrique Centrale‐Tervuren, Belgique Annales‐Serie in 8°‐Sciences Zoologiquies , 129 : 1 – 42 .
- Wood , T. S. 1998 . Reappraisal of Australian freshwater bryozoans with two new species of Plumatella (Ectoprocta: Phylactolaemata). . Invertebrate Taxonomy , 12 : 257 – 272 .
- Wood , T. S. 2001 . “ Bryozoans. ” . In Ecology and Classification of North American freshwater invertebrates , Edited by: Thorp , J and Corvich , A . 505 – 525 . New York : Academic Press . 2nd ed
- Wood , T. S. and Marsh , T. G. 1999 . Biofouling of wastewater treatment plants by the freshwater bryozoan, Plumatella vaihiriae (Hastings, 1929). . Water Research , 33 : 609 – 614 .
