Abstract
The ecological correlates of dorsal colour pattern polymorphism were studied along the transition zone between two supposed subspecies ( = colour pattern types in this article) of the common wall lizard, Podarcis muralis, in a hilly area of Latium, Central Italy. In this area two supposed subspecies, i.e. P. m. brueggemanni and P. m. nigriventris, are known to occur. Lizards were studied along 500 m long transects within three different habitat types, i.e. wood, wall, and bushy pasture. A total of 279 adult lizards (154 males, 125 females) were examined. Three colour morphs were observed at each site, i.e. brueggemanni type (brown–green upper parts), nigriventris type (black–green upper parts), and a colour morph intermediate between the two. The distribution and abundance of brueggemanni and nigriventris colour morphs was clearly non‐random across habitat types: brueggemanni was abundant in walls and bushy pastures, and nigriventris in wood. To explain the observed pattern we tested the hypothesis of a differential predation exposure by the various colour morphs in different habitats by analysing the differences between colour morph frequencies of lizards with intact tail and with broken/regenerated tail in the various habitats of the study area. Our analysis would not support the differential predation‐risk hypothesis, because the frequency of individuals with broken tails was very similar in the three colour morphs among different habitats.
Introduction
Many studies have been devoted to investigate the correlates of colour pattern polymorphism in reptiles and to explain their ecological causes and consequences (see e.g. Camin & Ehrlich Citation1958; Andrén & Nilson Citation1981; Fernandez & Collins Citation1988; Malohtra & Thorpe Citation1991; Luiselli Citation1992; Capula & Luiselli Citation1994; Monney et al. Citation1995). The Mediterranean lacertid lizards seem to be particularly useful for this type of investigation because they are normally characterized by high inter‐ and intra‐population variability in colour pattern (e.g. Arnold & Ovenden Citation2002; Corti & Lo Cascio Citation2002). The evolutionary significance of the high colour pattern polymorphism of the Lacertidae has been unstudied for most taxa. However, there is evidence that colour pattern polymorphism is influenced by habitat constraints and complex interactions between environmental pressures and physiological traits in at least some lizard species (e.g. Martin & Forsman Citation1999; Lopez et al. Citation2004; Stuart‐Fox et al. Citation2006; Sacchi et al. Citation2007). In Podarcis muralis (Laurenti, 1768), it has been demonstrated that immune responsiveness is morph‐specific in males, suggesting that this physiological trait could play an important role in maintaining colour polymorphism in this species (Sacchi et al. Citation2007). In some cases at least it was pointed out that species which are characterized by a high degree of phenotypic plasticity in the pattern of the upper parts may have levels of genetic variability higher than those found in the morphologically low variable species (see e.g. Selander Citation1976; Capula Citation1994, Citation1996, Citation1997; Losos et al. Citation1997; Capula & Ceccarelli Citation2003). For instance, this is the case of the island populations of P. muralis occurring in the Tuscan Archipelago, which are characterized by high genetic and morphometric variability (Capula Citation1997). Moreover, it must be stressed that the results of mtDNA analysis (Caputo et al. Citation2008) indicate highly subdivided geographical and genetic structure of the P. muralis populations occurring in the Italian peninsula. In this paper we analysed a transition zone between the ranges of two colour pattern types of the common wall lizard, Podarcis muralis, in northern Latium (Central Italy) to study the ecological correlates of their colour pattern polymorphism. Podarcis muralis occurs in central Italy with at least two colour pattern types: one was described as typical for P. muralis brueggemanni (Bedriaga, 1879) and the other for P. muralis nigriventris Bonaparte, 1836. The former colour pattern type is widespread in Tuscany, Umbria, Marche, Romagna, Liguria, and in the northern part of Latium (Capula Citation2000; Corti & Lo Cascio Citation2002); the latter is present only in Latium, where it is widespread in the central and southern parts of the region (Capula Citation2000). The two colour pattern types are clearly different especially in the upper parts. The ‘brueggemanni’ type has a dark ground colour with a very variable pattern of brown blotches; the ‘nigriventris’ type is characterized by a black ground colour with green blotches, which are normally small and relatively sparse. It must be noted that the taxonomic status of the two subspecies is still under debate (see Capula Citation2000; Corti & Lo Cascio Citation2002), and that some authors (e.g. Gruschwitz & Böhme Citation1986) do not recognize brueggemanni as a valid subspecies.
Our goal was to answer to the following key questions: (1) Can colour pattern be informative enough to clearly discriminate among adult individuals and between the two supposed colour pattern types? (2) If yes, are there differences in the frequency of occurrence of the two colour morphs among habitat types? (3) If yes, what reasons can be invoked to explain a habitat‐dependent variation in the frequency of occurrence of the various morphs? That is, can predation risks explain an inter‐habitat differential frequency of occurrence of the various colour morphs? We tested the latter question by analysing the differences between colour morphs frequencies of lizards with intact tail and with broken/regenerated tail in the various habitats of the study area.
Materials and methods
Study area
The field study was carried out during July and August 2007. All lizards were captured in the vicinities of Oriolo Romano (450 m a.s.l., about 50 km north of Rome, Central Italy), a hilly village situated in the transition zone between the ranges of the colour pattern types P. m. brueggemanni and P. m. nigriventris. Three habitat types were surveyed during the present study: (a) shady, dense, and wet mixed oak forest dominated by Quercus spp. and Fagus sylvatica, with a canopy cover of approximately 60–80% (WOOD); (b) grassy territory interspersed by bushes (Rubus spp., Cytisus scoparius), with a canopy cover of approximately 20–40% (BUSHY PASTURE); (c) human‐made stony or cement walls delimiting gardens, roads, etc., very often in proximity of human settlements (WALL). In order to avoid problems in assigning lizard individuals to the correct habitat type, we did not survey sites with habitat features intermediate among the three selected habitats, or where these habitats do alternate at the local scale. Within each habitat type, we walked appropriate transects of 500 m each. The transect sites used for the three habitats were ⩽1 km each from another, in order to avoid that inter‐habitat colour pattern differences might be due to excessive geographic distance among sites rather than to micro‐geographic and ecological factors. A habitat unsuitable to lizards was always present between two transects, i.e. buildings without gardens and roads. Hence, all transects were certainly independent of each other in terms of lizard movements.
Protocol
Each 500 m transect was surveyed only once by walking very slowly in one direction, and recording all lizard sightings. Lizards were captured and identified to sex by their secondary sexual characters (enlarged femoral pores). In addition, the capture habitat was recorded. Pseudo‐replication of data was avoided by monitoring each transect only once and only in one direction, thus never replicating the eventuality of capturing the same lizard more than once.
Each captured lizard was assigned to one of the following colour morph categories, based on the colour pattern of the upper parts: (1) brueggemanni type (brown–green upper parts); (2) nigriventris type (black–green upper parts); (3) intermediate type (upper parts intermediate between brueggemanni and nigriventris). For this study we considered only adult individuals, i.e. individuals with snout–vent length ⩾5 cm, because juveniles of both brueggemanni and nigriventris have a similar colour pattern and this cannot be assigned to one of the three colour morph categories described above. The tail status (intact or broken/regenerated) was also recorded for each lizard captured at the study area.
Statistical analyses
The differences among habitats in the frequency of occurrence of colour morphs were analysed by a Monte Carlo randomization procedure for χ 2 test, with 10,000 permutations of the whole data matrix prior to apply the calculations. Then, the observed value was compared with the mean and the variance of the simulated matrices (Gotelli & Graves Citation1996). Non‐parametric tests were used in all cases because data variables were neither normally distributed nor were normalized with appropriate transformations. Non‐parametric Spearman's rank correlation coefficient was used to test for the correlation between percentages of lizards with intact tail and sample sizes, habitat types, and colour pattern types. Differences in frequency of lizards with broken/regenerated tails among colour morphs within each habitat type were tested by χ 2 test. All tests were two‐tailed and alpha was set at 5%. Statistical analyses were performed using STATISTICA 7.1 software. Monte Carlo analysis was performed after having generated the permutated matrices with Ecosym software 700 version.
Results
A total sample of 279 adult lizards, 154 males and 125 females (sex ratio 1.23:1, not different from equality at binomial test) were analysed. The distribution of observations of lizards by colour morph and by habitat type is given in Figure . Details of lizard distribution by sex and by habitat and the relative frequency of individuals with intact tail by sex, colour morph, and habitat type is shown in Table . The three colour morphs were always clearly recognizable at the study area. The distribution of the three colour morphs was non‐random across habitat types (Monte Carlo randomization procedure for χ 2 test: Observed index = 147.308; mean±variance of simulated indices = 8.110±15.975, P<0.00001), with brueggemanni accounting for the majority of P. muralis individuals in WALL (N = 80) and BUSHY PASTURE (N = 41), and nigriventris accounting for the majority of individuals in WOOD (N = 114; Figure ).
Figure 1 Distribution of observations of lizards by colour morph in the three habitat types. White bar: nigriventris type; black bar: brueggemanni type; grey bar: intermediate type.
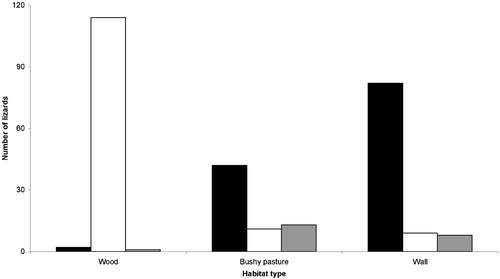
Table I. Colour morph categories, sex, habitat type, and frequency of occurrence of intact/regenerated tails in Podarcis muralis from the study area. Numbers indicate individuals examined for each column category. Numbers in parentheses indicate individuals with broken/regenerated tail.
Intermediate individuals accounted for a percentage of individuals less than brueggemanni and nigriventris, and were observed in all habitat types (Figure ).
There was no inter‐sexual difference in the frequency of occurrence of a given colour morph within each habitat type apart from WOOD (Table ). In this latter habitat the colour morph nigriventris was significantly more associated to males than to females (χ 2 = 4.246, df = 1, P<0.039).
Transect‐by‐transect (total N = 17), the percentage of individuals with intact tail (Table ) was not correlated with sample size (Spearman's r = −0.030, P = 0.908), sex (Spearman's r = 0.399, P = 0.113), habitat type (Spearman's r = −0.322, P = 0.207), and colour morph (Spearman's r = 0.076, P = 0.771). The frequencies of lizards with broken/regenerated tails did not differ significantly among colour morphs within each habitat type, either if sexes were pooled or entered separately in the analyses (in all cases, at least P>0.255 at χ 2 test).
Discussion
Within the studied geographic transition zone three colour morphs (brueggemanni, nigriventris, intemediate type) are always recognizable. The morphs brueggemanni and nigriventris are clearly linked to different habitat types, although sharing their habitat with individuals of the intermediate colour pattern. The morph brueggemanni is extremely rare in WOOD habitats, and very abundant in WALL and in BUSHY PASTURE habitats. On the other hand, the morph nigriventris is especially associated with wet, dense and shady wooded portions of territory (WOOD), where it represents over 95% of the common wall lizard population. These inter‐habitat differences between colour morphs are clearly unrelated to sex or sample size.
Differential habitat use among colour morphs of certain reptile species has been sometimes reported, and is generally attributed to predation avoidance by the least cryptic morph in the suboptimal habitat (e.g. Kjaergaard Citation1981). However, there is no univocal pattern in this regard, and some experimental studies have shown that different colour morphs may be nearly identical in terms of habitat choice, including even the choice of background colour (see e.g. Luiselli et al. Citation1994; Capula & Luiselli Citation1995). To explain the observed pattern in P. muralis, we hypothesized a differential predation exposure by the various colour morphs in different habitats: e.g. the nigriventris colour morph would be dominant over the brueggemanni one within wooded habitats because the former is more cryptic, that is less exposed to predators; the reverse might be true in wall and bushy pasture habitats. We tested this hypothesis comparing the frequency of occurrence of lizards with intact tail and with broken/regenerated tail. Since in reptiles frequency of tail damage and predation intensity are positively correlated (Capula et al. Citation1997; Luiselli et al. Citation2005; Placyk & Burghardt Citation2005), our assumption was that in inter‐morph comparisons for a given habitat type higher percentages of lizards with broken tails would indicate higher risk of predation for a given morph. However, our analysis would not support the differential predation‐risk hypothesis, because the frequency of individuals with broken tails was very similar in the three colour morphs among different habitats. The lack of significance in these analyses may, however, have been caused by the unbalanced, small sample sizes. Indeed, in suboptimal habitats for a certain morph, the number of individuals for that morph was much smaller than that of individuals for the other morph (e.g. in WOOD the sample size for brueggemanni was 2 versus 1 of intermediate morph and 114 of nigriventris), thus possibly biasing the statistical significance of comparisons. This obviously indicates that further data on several ecological aspects (e.g. thermal and reproductive ecology) are required to explain how the high degree of morphological variation observed in P. muralis is maintained by selection for different habitats in the absence of barriers to gene flow between adjacent populations.
References
- Andrén , C. and Nilson , G. 1981 . Reproductive success and risk of predation in normal and melanistic colour morphs of the adder, Vipera berus. . Biological Journal of the Linnean Society , 15 : 235 – 246 .
- Arnold , E. N. and Ovenden , D. 2002 . A field guide to the reptiles and amphibians of Britain and Europe , London : Harper Collins .
- Camin , J. and Ehrlich , P. 1958 . Natural selection in water snakes (Natrix sipedon L.) on islands in Lake Erie. . Evolution , 12 : 504 – 511 .
- Capula , M. 1994 . Population genetics of a colonizing lizard: Loss of genetic variability in introduced populations of Podarcis sicula. . Experientia , 50 : 691 – 696 .
- Capula , M. 1996 . Evolutionary genetics of the insular lacertid lizard Podarcis tiliguerta: Genetic structure and population heterogeneity in a geographically fragmented species. . Heredity , 77 : 518 – 529 .
- Capula , M. 1997 . High genetic variability in insular populations of the lacertid lizard, Podarcis muralis. . Biochemical Systematics and Ecology , 25 : 411 – 417 .
- Capula , M. 2000 . “ Podarcis muralis (Laurenti, 1768). ” . In Anfibi e Rettili del Lazio , Edited by: Bologna , M. A , Capula , M and Carpaneto , G. M . 84 – 85 . Roma : Fratelli Palombi Editori .
- Capula , M. and Ceccarelli , A. 2003 . Distribution of genetic variation and taxonomy of insular and mainland populations of the Italian wall lizard, Podarcis sicula. . Amphibia–Reptilia , 24 : 483 – 496 .
- Capula , M. and Luiselli , L. 1994 . Reproductive strategies in alpine adders, Vipera berus. The black females bear more often. . Acta Oecologica , 15 : 207 – 214 .
- Capula , M. and Luiselli , L. 1995 . Is there a different preference in the choice of background colour between melanistic and cryptically coloured morphs of the adder, Vipera berus? . Bollettino di Zoologia , 62 : 253 – 256 .
- Capula , M. , Luiselli , L. and Capanna , E. 1997 . The blue‐spotted morph of the slow worm, Anguis fragilis: Colour polymorphism and predation risks. . Italian Journal of Zoology , 64 : 147 – 153 .
- Caputo , V. , Giovannotti , M. and Olmo , E. 2008 . “ Refugia‐within‐refugia: The case of Podarcis muralis (Laurenti, 1768) in the Italian Peninsula. ” . Riassunti del 7° Congresso Nazionale della Societas Herpetologica Italica, 1–5 ottobre 2008, Oristano
- Corti , C. and Lo Cascio , P. 2002 . The lizards of Italy and adjacent areas , 165 Frankfurt‐am‐Main : Chimaira Verlag .
- Fernandez , P. J. Jr and Collins , J. P. 1988 . Effect of environment and ontogeny on color pattern variation in Arizona tiger salamanders (Ambystoma tigrinum nebulosum Hallowell). . Copeia , 1988 : 928 – 938 .
- Gotelli , N. J. and Graves , G. C. 1996 . Null models in ecology , Washington D.C. : Smithsonian Institution Press .
- Gruschwitz , M. and Böhme , W. 1986 . “ Podarcis muralis (Laurenti, 1768) – Mauereidechse. ” . In Handbuch der Reptilien und Amphibien Europas , Edited by: Böhme , W . 155 – 208 . Wiesbaden : Aula . 2/II, Echsen (Sauria) III (Lacertidae III: Podarcis)
- Kjaergaard , J. 1981 . Udbredelsen af sort hugorm I Danmark. . Flora og Fauna (Silkeborg) , 87 : 27 – 29 .
- Lopez , P. , Martin , J. and Cuadrado , M. 2004 . The role of lateral blue spots in intrasexual relationships between male Iberian rock‐lizards, Lacerta monticola. . Ethology , 110 : 543 – 561 .
- Losos , J. B. , Warheit , K. I. and Schoener , T. W. 1997 . Adaptive differentiation following experimental island colonization in Anolis lizards. . Nature , 387 : 70 – 73 .
- Luiselli , L. 1992 . Reproductive success in melanistic adders: A new hypothesis and some considerations on Andrén and Nilson's (1981) suggestions. . Oikos , 64 : 601 – 604 .
- Luiselli , L. , Angelici , F. M. , Di Vittorio , M. , Spinnato , A. and Politano , E. 2005 . Analysis of a herpetofaunal community from an altered marshy area in Sicily; with special remarks on habitat use (niche breadth and overlap), relative abundance of lizards and snakes, and the correlation between predator abundance and tail loss in lizards. . Contributions to Zoology , 74 : 41 – 49 .
- Luiselli , L. , Capula , M. , Rugiero , L. and Anibaldi , C. 1994 . Habitat choice by melanistic and cryptically coloured morphs of the adder, Vipera berus. . Bollettino di Zoologia , 61 : 213 – 216 .
- Malhotra , A. and Thorpe , R. S. 1991 . Experimental detection of rapid evolutionary response in natural lizard populations. . Nature , 353 : 347 – 348 .
- Martin , J. and Forsman , A. 1999 . Social costs and development of nuptial coloration in male Psammodromus algirus lizards: An experiment. . Behavioural Ecology , 10 : 396 – 400 .
- Monney , J. C. , Luiselli , L. and Capula , M. 1995 . Correlates of melanism in a population of adders (Vipera berus) from the Swiss Alps and comparison with other alpine populations. . Amphibia–Reptilia , 16 : 323 – 330 .
- Placyk , J. S Jr. and Burghardt , G. M. 2005 . Geographic variation in the frequency of scarring and tail stubs in eastern gartersnakes (Thamnophis s. sirtalis) from Michigan, USA. . Amphibia–Reptilia , 26 : 353 – 358 .
- Sacchi , R. , Rubolini , D. , Gentilli , A. , Pupin , F. , Razzetti , E. , Scali , S. , Galeotti , P. and Fasola , M. 2007 . Morph‐specific immunity in male Podarcis muralis. . Amphibia–Reptilia , 28 : 408 – 412 .
- Selander , R. K. 1976 . “ Genic variation in natural populations. ” . In Molecular evolution , Edited by: Ayala , F. J . 21 – 45 . Sunderland : Sinauer Associates .
- Stuart‐Fox , D. M. , Firth , D. and Whiting , M. J. 2006 . Multiple signals in chameleon contests: Designing and analysing animal contests as a tournament. . Animal Behaviour , 71 : 1263 – 1271 .