Abstract
The new species, Pseudocirrhipathes mapia, is herein described from specimens coming from the coral reefs of the Bunaken Marine Park (North Sulawesi, Indonesia). The species is characterized by an unbranched thin corallum up to 1 m high (maximum basal thickness 4 mm), with large polyps arranged irregularly on one side of the stem, and by tentacles that are not completely contractile. The skeleton shows an array of spine morphologies along the stem, although most are typically verticillated in the apical section and highly tuberculated in the central portion. The study of its cnidome revealed the presence of an extremely long basitrich isorhiza. The morphological analysis has been coupled with a molecular study of the rDNA ITS sequences confirming the existence of the new genus Pseudocirrhipathes and its inclusion, together with the genus Allopathes, in a separate clade related to the Antipathidae.
Introduction
Antipatharians (Cnidaria: Anthozoa), commonly known as black corals, are colonial hexacorallians with a worldwide distribution. The order comprises approximately 235 species displaying 6‐tentacle polyps and a wide range of corallum morphologies, characterized by a rigid, horny, spiny skeleton. The area of the Bunaken Marine Park (North Sulawesi, Indonesia) hosts the richest and most diversified shallow‐water antipatharian assemblage that has ever been described, being composed of about 50 species belonging to eight genera and three families (Tazioli et al. Citation2007). The Bunaken Marine Park is placed in the centre of the so‐called coral triangle, comprising South Philippines and Central Indonesia, and recognised as a centre of marine biodiversity (Roberts et al. Citation2002).
In this area some of the most characteristic elements of the coral reefs are whip, or single axis, black corals, sometimes reaching several metres in length and forming dense meadows on cliffs lashed by strong currents. Although these monopodial, unbranched antipatharians are very common, their taxonomy is not completely clarified. Two genera belonging to the family Antipathidae, Cirrhipathes (Blainville, 1857) and Stichopathes Brook, Citation1889, are currently recognized as valid. Their separation is traditionally based on the arrangement of polyps along the stem: in Cirrhipathes the polyps are present all around the axis, while in Stichopathes they form a unique line on one side (Brook Citation1889).
Moreover, another genus of the family Antipathidae, the genus Allopathes Opresko and Cairns, Citation1994, was recently described for species characterized by tufts of monopodial branches, almost identical to Stichopathes colonies, but arising from a common base.
In this study we apply an integrated approach by combining traditional morphological characters, in situ observations, ecology, and genetic taxonomy in the description of the new genus Pseudocirrhipathes, based on the new species P. mapia characterized by a very thin unbranched axis, a peculiar spine and polyp arrangement, and tentacles unable to completely contract.
Materials and methods
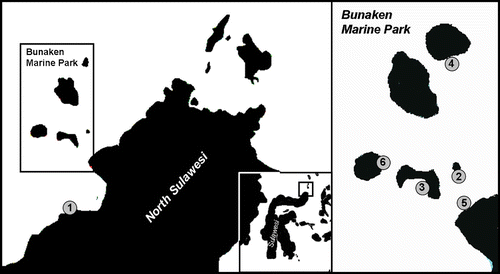
Twelve specimens belonging to the new species were collected between September 2005 and February 2007 by diving along the coast of Manado (Mapia and Wreck) and on some vertical reefs in the Bunaken Marine Park (Likuan 3 and Raymond's Point). Six other specimens were recorded, but not collected, from other sites in the Park (Siladen and Nain) (Figure ); two more specimens from Ambon Island (South Moluccas); and one from North Bali Island.
Figure 1 Map of localities and occurrences of Pseudocirrhipathes mapia in the Marine Park of Bunaken. 1, Mapia (type locality) (Coast of Manado); 2, Onong (Siladen Island); 3, Likuan 3 (Bunaken Island); 4, Nain (Nain Island); 5, Wreck of Manado (Coast of Manado); 6, Raymond's Point (Manado Tua Island).
The site known as Mapia is a gentle slope made up of black volcanic coarse sand and sparse rocks characterized by scattered coral gardens. Here specimens of the new species are quite common and reach their maximum size. They are usually found on rocky slopes with their basal plates sometimes covered by sand. Other locations include vertical coral reefs or plateaus that are often lashed by strong multi‐directional currents. Collections were made using shears to cut either entire colonies or portions of them. Each colony was photographed using digital underwater photography in order to record in situ aspects, especially colour, morphology and arrangement of the polyps along the stem, and presence of epibionts.
A portion of each sample was preserved in 4% formaldehyde while the remaining pieces were dried. The cnidome of each specimen (considered in different portions – mouth, tentacles and interpolypar coenenchyme) was studied using an optical microscope, while the histological study of mesenteries was conducted after cold‐curing resin inclusion (Technovit 8100), cutting with a microtome, and staining with Toluidine Blue.
Two specimens, one collected from the Mapia station and another from the Siladen coral reef, were preserved in 95% EtOH and used for the genetic analysis.
Type material of the new species has been deposited in the Natural History Museum of Genova, Italy (MSNG).
Phylogenetic analysis
We have sequenced the nuclear rDNA internal transcribed spacer sequences (ITS) of two specimens of our material and of Allopathes desbonni (Duchassing and Michelotti, 1864) (USNM 88327), Aphanipathes pedata (Gray, 1857) (USNM 74819), Aphanipathes cf. sarothamnoides Brook, Citation1889 (USNM 1007094) and Phanopathes rigida (Pourtalès, 1880) (USNM 88335), coming from the Smithsonian National Museum of Natural History of Washington. This comparison was decided on the basis of the results of the morphological analysis, which revealed for our specimens a typical verticillated spine pattern, similar to that of A. desbonni, and an overall similarity to the tuberculated spines of the other three species.
The procedures used for DNA extraction, amplification, and sequencing are described in Lapian et al. (Citation2007). The sequences obtained (GenBank accession numbers: FM882166–FM882171) were compared with those of the specimens analysed by Lapian et al. (Citation2007): Antipathes elegans (Brook, Citation1889), Antipathes sp. 1, Antipathes sp. 2, Antipathes sp. 3; Stichopathes sp., Cirrhipathes cf. anguina (Cirrhipathes sp. in Lapian et al. Citation2007), Cirrhipathes spiralis Brook, Citation1889, Myriopathes myriophylla (Pallas, 1766), Cupressopathes abies (Linnaeus, 1758), Antipathella subpinnata (Ellis and Solander, 1786) and Rhipidipathes reticulata (Esper, 1795).
The sequences (partial 18S rDNA, full‐length ITS1, 5.8S, ITS2 and partial 28S rDNA) were aligned using ClustalW (Thompson et al. Citation1994). To ensure reliability of data, different alignments were obtained using default settings within ClustalW, except for gap penalty, gap extension and gap distance, which were attempted using values ranging from 2 to 25, 2.5 to 7.5 and 2 to 6, respectively; all parameter combinations resulted in identical results.
The trees were constructed using maximum parsimony (MP) and maximum likelihood (ML) methods using the beta version of PAUP 4.8 (Swofford Citation1998). The MP tree was constructed following a heuristic search with TBR (tree bisection–reconnection) branch swapping and using random stepwise additions with 100 replications, and 1000 bootstrap replicates. Only minimal trees were retained. For the heuristic ML analysis, the optimum substitution model was determined using Model Test 3.06 (Posada & Crandall Citation1998). Once the appropriate model was determined (TrNef+G) (Tamura & Nei Citation1993), ML analysis was performed using all parameter values provided by Model Test (Gamma distribution shape parameter = 0.2623; substitution model: Rate matrix A–C = 1.0000; A–G = 2.7625; A–T = 1.0000; C–G = 1.0000; C–T = 4.0721; G–T = 1.0000; equal base frequencies). The search was carried out with TBR branch swapping, and bootstrap support for the ML tree was determined using 1000 replications. The stony coral Porites lutea Edwards and Haime, 1860 was used as an outgroup.
Results
Genus Pseudocirrhipathes Bo and Bavestrello n. gen.
Diagnosis
Monopodial corallum, not ramified nor pinnulated, straight or slightly coiled in the distal portion. The stem of the colony is thin, flexible and characterized by a very large hollow central canal, especially in the apical portion. Spines triangular or sub‐triangular, mainly perpendicular to the axis, with numerous rounded tubercles at least in a portion of the stem; arranged in verticils at the apex of the colony. Polyps large, 1–4 mm in transverse diameter, with long sagittal tentacles (up to 7 mm), not completely contractile, with 10 mesenteries; arranged in irregular rows along the stem, and leaving free most of the abpolypar side. Typical large isorhizae are present.
Remarks
Pseudocirrhipathes is the third genus of black coral, together with Cirrhipathes and Stichopathes, characterized by a monopodial, unbranched and unpinnulated corallum. It differs from Stichopathes by having irregularly arranged polyps and from Cirrhipathes by having tuberculated verticillated spines and, as observed in situ, tentacles unable to completely contract (Figure ). The genus Allopathes is always distinguishable due to the shape of the colonies, which are composed of several elongated stems arising from a short trunk‐like base or primary stem (Opresko Citation2003).
Etymology
The generic name is derived from the words “pseudo” (false) and “cirrhipathes” in reference to the apparent similarity of this genus to Cirrhipathes. [Note: The original genus name Cirrhipathes was later cited as Cirripathes; however, this is considered an incorrect emendation (see Opresko & Cairns Citation1994).]
Pseudocirrhipathes mapia Bo and Bavestrello n. spec.
Cirripathes? sp. Thomson and Simpson, Citation1905
Cirripathes rumphii van Pesch, Citation1910 (pro parte)
Eucirripathes rumphii van Pesch, Citation1914 (pro parte)
(Figures – )
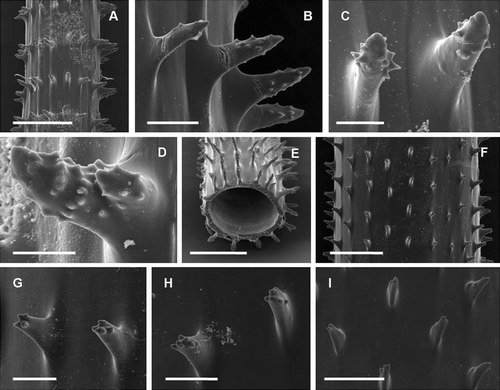
Figure 3 Spines of Pseudocirrhipathes mapia. A, spiral arrangement of spines along the basal portion of the stem; B, close up view of the basal region; C,D, slightly tuberculated triangular spines in the basal region; E, close up view of the central region, where spines are arranged in regular longitudinal rows; F, tuberculated sub‐triangular spines in the intermediate region; G,H, circular arrangement of tubercules on the surface of spines situated in the central portions of the colony; I,J, different arrangement of tubercules on the proximal side of spines, either covering the entire surface or absent. The tip of the spines is always free of tubercules; K, close‐up view of the apical region, where the spines are arranged into well‐separated verticils. Ridges were produced by the collapse of the skeleton due to dehydration; L, smooth triangular spines in the apical verticils; M,N, variation in diameter of the central canal in the central and apical portion of the colony, respectively. Scale bars: A, 1 mm. B, E, 0.5 mm. K, M, N, 0.2 mm. C, D, F–J, L, 0.1 mm.
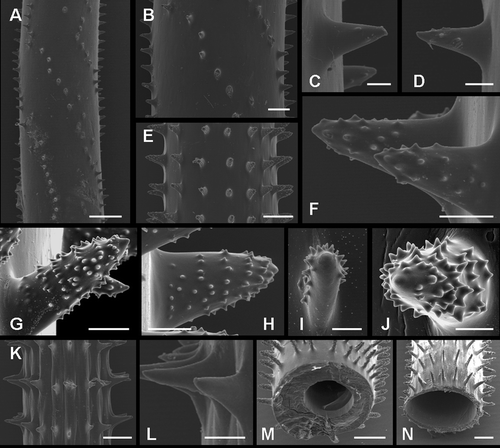
Figure 4 Underwater photographs of Pseudocirrhipathes mapia. A, macro‐photograph of P. mapia showing an expanded polyp surrounded by resting zooids that do not completely contract their tentacles; B, abpolypar side of a P. mapia colony showing the polyp‐free side of the axis; C, polypar side of a P. mapia colony; D, macro‐photograph of P. mapia showing expanded polyps. Sagittal tentacles are inserted at a lower level; E, two colonies of P. mapia; F, expanded polyp of P. mapia showing the double margin mouth; G, open mouth of an egesting polyp; H, a large discharged basitrich isorhiza, showing the spiny stylet and the incompletely extruded filament; I, patch distribution of nematocysts on the ectodermal surface of the tentacle; J, colony of P. mapia hiding a mimicking Pontonides unciger; K, extremely long sweeper tentacles of a white P. mapia, touching a dead Acropora. Images A, D, F, G, J are courtesy of Massimo Boyer. Scale bars: H, 25 µm. G, I, 1 mm. A, D, F, J, 2 mm. B, C, 5 mm. K, 20 mm. E, 20 cm.
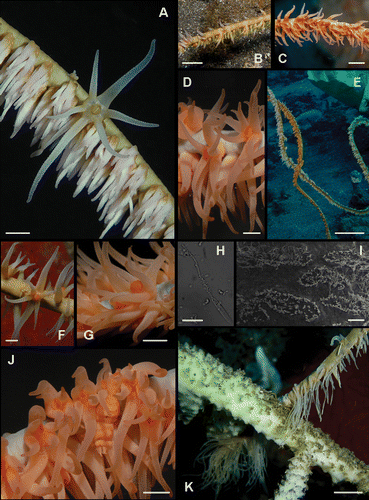
Figure 5 Spines of Cirripathes rumphii. Coel. 02559b A, arrangement of spines in the apical portion of the colony; B, close up view of a verticil of spines; C,D, highly tuberculated spines; E, diameter of the central canal. Coel. 02559a F, arrangement of spines in the apical portion of the colony; G,H, close‐up views of the apically tuberculated spines; I, frontal view of the laterally compressed spines. Scale bars: C, D, 50 µm. B, G, H, 100 µm. I, 200 µm. A, E, F, 500 µm.
Type material
Holotype: MSNG 54483, Indonesia, Marine Park of Bunaken, Mapia House Reef, 32 m depth, collected on 19.11.2006 using SCUBA, entire colony (1 m in height) with basal plate, 95% EtOH; Paratype: MSNG 54484, Indonesia, Marine Park of Bunaken, Mapia House Reef, 17 m depth, collected on 19.06.2006 using SCUBA, entire colony (70 cm tall) with anchorage, dried specimen. Specimens collected by M. Bo and G. Bavestrello.
Other material examined
Mapia House Reef: ANT4 (28.09.05, 15–30 m, apical portion of a 50 cm tall colony, 4% formaldehyde), ANT5 (28.09.05, 15–30 m, intermediate portion of a 50 cm tall colony, 70% EtOH, GenBank Accession Number: FM882167), BUK3 (19.06.2006, 33.3 m, intermediate portion of a 1 m tall colony, 95% EtOH), BUK4 (19.06.2006, 33.3 m, intermediate portion of a 1 m tall colony, 95% EtOH), PM1 (19.11.06, 32 m, apical and intermediate portions of a 1 m tall colony, 95% EtOH); Likuan 1: CMLIK (22.11.06, 35 m, apical and intermediate portions of a 30 cm tall colony, 95% EtOH, GenBank Accession Number: FM882168); Raymond's Point: MAS04 (01.03.2007, 45 m, apical and intermediate portions of a 70 cm tall colony, 95% EtOH); MAS05 (01.03.2007, 45 m, apical portion of a 1 m tall colony, 95% EtOH). Coel. 02559b (08.02.1900, 113 m, apical and intermediate portions, 70% EtOH, collected with dredge on a stony bottom, Stn. 305 Flores, Solor Strait, Mid Channel off Kampong Menanga).
Diagnosis
Monopodial straight corallum not ramified nor pinnulated, up to 1 m tall (maximum basal thickness 4 mm), in the bigger colonies slightly bent at apex in one or more loose spiral coils. Stem subdivided into three regions on the basis of the different arrangement and morphology of spines. In the basal region the triangular spines are almost smooth and organized in spiral rows. In the intermediate region the sub‐triangular spines are highly tuberculated and arranged in parallel longitudinal rows, giving rise to close verticils. The apical zone is characterized by widely spaced verticils of smooth triangular spines. Polyps large, up to 4 mm in transverse diameter, with 4.5 mm long sagittal tentacles not completely contractile, white to salmon pink with a greenish coenenchyme. Polyps distributed in irregular rows on one side of the stem. Cnidome composed of spirocysts (19×3 µm) and two types of basitrich isorhizae, a normal (25×3 µm) and a peculiar large type (35×5 µm).
Description
The colonies are made of a long (in the holotype 1 m tall and 2.5 mm thick near the base), thin, flexible stem, partially coiled in the distal end. In smaller colonies (less than 50 cm and 1.5–2 mm thick near the base) the stem is generally straight. The stem is anchored to rock with a flat basal plate, covered by tiny conical spines, and often incorporating sessile organisms like cirripeds (barnacles). The basal plate is covered by living tissue without zooids.
SEM analysis reveals three distinct regions of the stem, characterized by a different arrangement and morphology of the spines. The basal region, a few cm long above the basal plate, is characterized by triangular spines spirally arranged around the stem (Figure ). They are 0.2–0.3 mm tall, spaced 0.2–0.4 mm within each row, and have few rounded tubercules on their distal end (Figure ). In the intermediate region, sub‐triangular spines 0.2–0.3 mm tall are organized in distinct and regular longitudinal rows, distanced 0.3–0.6 mm from each other (Figure ). These spines are characterized by prominent rounded tubercles spread almost along the entire length of the spine (figure 3F–), both irregularly or, in many cases, with a peculiar circular arrangement (Figure ). The tuberculation can be distributed on the entire surface of the spine or just in its superior side, leaving the inferior side completely or partially smooth (Figure ). In the distal region of the colony, primarily smooth triangular spines, 0.1–0.2 mm tall, and spaced 0.3–0.6 mm from each other, are organized in perfectly regular rows giving rise to separated verticils (Figure ). In this region the skeleton is so thin that after dehydration the walls collapse giving rise to evident ridges between spines (Figure ). The maximum basal diameter recorded of the corallum (1 m high) is 4 mm and the minimum apical diameter is 0.2 mm. Transverse sections of the stem depict a significant decrease in thickness of the corallum wall due to an enlargement of the central canal from the base (up to 0.32 mm) to the apex (up to 0.88 mm) (Figure ).
The number of rows of spines visible in frontal view is variable along the stem going from the basal portion (4) to the apex (9). An increase in width, measured at the base of the spine, is also evident, from the base (0.05 mm) to the apex (0.1 mm). Spines are primarily orientated perpendicularly to the axis, but at times may be slightly curved upwards or downwards. Spine density is on average 2.9 spines/mm (measured in a single row). The spines have the same morphology and size on all sides of the axis. Bifid spines, or more rarely trifid, have been recorded in the central portion of colonies.
Generally the colonies have salmon pink polyps with a greenish coenenchyme, but specimens with orange or white polyps were also recorded (figure 4A–). Polyps show an irregular arrangement on the polypar side and are almost completely absent on the abpolypar section (Figure ). Underwater observations indicate that the basal region of the colony is characterized by irregularly distributed radial polyps, while in the distal portion polyps are more crowded, sagittally elongated, and reach their maximum size. They are large, with a transverse diameter ranging from 1 to 4 mm. The interpolypar distance, measured from, but not including, the distal lateral tentacles of one polyp to the proximal lateral tentacles of an adjoining one (Opresko Citation1972), is on average 1.1 mm. Their average density is about 4.7 zooids/cm. Tentacles are conical with a swollen base and a pointed tip when fully expanded, while the sagittal tentacles are very long (1.5–6.5 mm) and are inserted at a slightly lower level relative to the lateral tentacles. The tentacles have partial contractile ability and when they are not completely expanded, hang in the water as soft cones (Figure ). The mouth is elevated on an oral cone 0.5 mm tall and, as shown in the pictures, displays a well‐defined double margin, darker on the ectodermal side (Figure ).
The cnidome of Pseudocirrhipathes mapia is composed of spirocysts and basitrich isorhizas of two types:
-
spirocysts (19×3 µm) are abundant in all the examined portions of the polyps, but are densely packed in batteries within the tentacular epidermis;
-
basitrich isorhizas occur in two sizes, a smaller one (25×3 µm) and a very distinctive larger one (35×5 µm, with the spiny basal portion of the thread 24.8 µm long) (Figure ). The first type is present exclusively in the tentacles, while the second is present in all those portions of the polyp examined (tentacles, mouth and interpolypar coenenchyme), but is found primarily in the tentacles. SEM images of the tentacular surface, showing the discharged threads (maximum length recorded in the giant isorhiza, excluding the basal spiny portion, is 187 µm) revealed the typical patchy distribution of these nematocysts (Figure ).
Remarks
Living specimens of Pseudocirrhipathes can be distinguished from those belonging to the other monopodial genera Cirrhipathes and Stichopathes by the fact that polyps are not completely contractible (Figure ). The microscopical study of the polyps moreover indicates that typically P. mapia does not have mastigophores in its nematocyst set (unlike Cirrhipathes and Stichopathes, unpublished data), while it shows a particularly large, easily detectable type of basitrich isorhiza. Our analysis of Allopathes desbonni cnidome revealed that this species shares with P. mapia the abundant batteries of large isorhizae (41.8 µm×5.7 µm). Moreover, P. mapia shares with A. desbonni and A. robillardi (Bell Citation1891) the verticillated organization of the spines and a very large central canal in the apical skeleton (Opresko & Cairns Citation1994). The new species is, however, easily distinguishable from those of genus Allopathes by the single stem shape of the colony, the arrangement of the zooids (irregular in P. mapia, monoserial in the Allopathes), the large size of the polyps (up to 4 mm in transverse diameter in P mapia, 1–1.2 mm in A. desbonni, not available for A. robillardi), and several details concerning the spines. The spines of P. mapia are highly tuberculated only in the middle portion of the stem and are organized in verticils only at the apex of the colony.
In 1909 Cooper described two new species, Stichopathes regularis from Ceylon and Stichopathes bournei, both characterized by a peculiar verticillated arrangement of spines (Cooper Citation1909: figures and , pp. 306–308). For S. regularis a monoserial arrangement of polyps is described, while for S. bournei no data about the polyps are available. Moreover, in both species the spines are unequal in size on opposite sides of the axis, whereas in P. mapia they are equal in size on all sides of the stem; hence, we do not consider these two species synonyms of P. mapia.
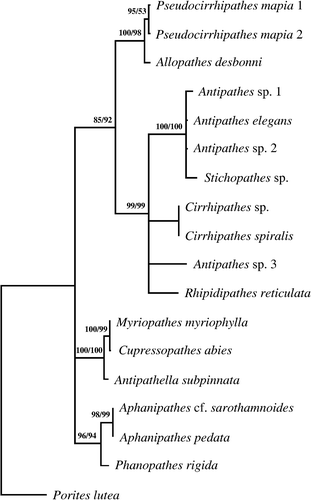
Figure 6 Phylogenetic tree. Consensus tree obtained by maximum parsimony (length 673; CI 0.792; RI 0.793) and maximum likelihood for 16 antipatharian species based on alignment (1.030 positions long and containing 206 parsimony informative sites) of internal transcribed spacer rDNA sequences. Numbers along the branches represent bootstrap estimates: MP value (left) and ML value (right).
Thomson and Simpson (Citation1905) recorded an unidentified Cirrhipathes collected at 84 m depth at Ceylon. This specimen was later included by Summers (Citation1910) in a new species described as Cirrhipathes indica from Portuguese East Africa. Thomson and Simpson's specimen is a long, unbranched colony, coiled at the apex. The polyps are not described, but the authors clearly indicate that the papillose spines are arranged differently in three regions along the stem. The base shows 20 rows of irregularly distributed spines while the apex is characterized by 9 rows of 0.1 mm high verticillated ones (Summers Citation1910: figure 8a–c, pp. 95, 107). Summers' C. indica is a long, simple stem wound in a large apical spiral, with minute papillose spines (size not reported) arranged, at the base, in 24–30 irregular rows (plate V, figure 9). In this species polyps are distributed all around the stem, as in a true Cirrhipathes. Even if Summers (Citation1910) synonymized the Cirrhipathes sp. of Thomson and Simpson with her C. indica, the latter species is so poorly described that it cannot even be determined if the spines occur in verticils. We believe Cirrhipathes sp. to be different from C. indica and to be much more similar to P. mapia, even if the absence of a basal spiral arrangement of spines, the lack of polyps and the loss of the type material do not allow a certain identification of this species.
In 1910 van Pesch described from several localities of the Indonesian Archipelago the new species Cirripathes rumphii (transferred in 1914 to Eucirripathes), also including the specimen of Thomson and Simpson. The author did not define a holotype, but described its species on the base of six specimens. We have studied two of these specimens (Coel. 02559a and b) from the Zoological Museum of Amsterdam. Of the four remaining specimens, three (Coel. 01761; 01762; 01755) are lost while the fourth is not present in the register of the Siboga collection at of the Amsterdam Museum.
The two specimens examined by us belong to different species detectable on the base of their spine morphology (figure 5A–), as visible also from the original figures of van Pesch (Citation1914: figure 245, p. 170; figure 249, p. 171). Coel. 02559b is characterized by almost smooth, triangular spines arranged in verticils at the apex and highly tuberculated spines in a proximal portion of the fragment (figure 5A–). This specimen shows large isorhizae 35×5 µm. The characteristics of this specimen completely agree with those of our P. mapia. Coel. 02559a shows triangular, laterally compressed spines with few rounded tubercles only at their tips, and arranged in parallel longitudinal rows never forming distinct verticils (figure 5E–). The polyps of this specimen do not possess the large isorhizae. The descriptions of van Pesch concerning the other four specimens suggest that one of those coming from St. 299 and characterized by entirely rough, knobby spines, can be included in P. mapia while the others probably belong to a third species. Under these circumstances it is impossible to refer to a unique species the taxon Cirripathes rumphii that must be therefore considered as a nomen nudum.
Etymology
The specific name refers to the Indonesian term “mapia” meaning “beautiful”. Mapia is also the name of the dive site where it was collected for the first time and where the population shows its highest density.
Ecology
In the Bunaken area the depth range of this species varies between 15 and 50 m. The specimens of van Pesch were collected at 34 and 113 m depth, while Thompson and Simpson recorded their specimen at 84 m depth.
In the Bunaken Marine Park colonies are quite abundant on sandy bottoms with low current, where they show more apical coils and reach a considerable height (usually 1 m). On vertical reefs lashed by strong currents, colonies tend to live in deeper water (under 30 m), and are usually smaller (less than 70 cm tall).
Two colonies, one collected in September 2005 and another in November 2006, were fertile female colonies, and from histological sections, it was possible to estimate an average of 78 eggs per polyp, with a range of diameters between 0.08 and 0.14 mm.
The shrimp Pontonides unciger, perfectly mimicking the colour patterns of the polyps, is a common epibiont of this species (Figure ).
In a specimen of P. mapia, the development of exceptionally long sweeper tentacles (five times their normal length) has been observed in a portion of the colony in contact with an Acropora coral (Figure ), while in another case sweeper tentacles were produced against a polychaete tube that had settled at the base of the black coral. The existence of sweeper tentacles in black corals has been until now reported only for colonies of Antipathella fiordensis defending against a soft coral (Goldberg et al. Citation1990).
Distribution
Indonesian Archipelago (Bunaken Marine Park, Ambon, Bali, Timor, Flores), Ceylon.
Phylogenetic analysis
The sequences of partial 18S rDNA, full‐length ITS1, 5.8S, ITS2 and partial 28S rDNA were studied to explore the phylogenetic relationships between our specimens, the specimen of Allopathes desbonni and 11 species previously analysed by Lapian et al. Citation2007. Moreover, we have analysed three specimens belonging to the family Aphanipathidae (Aphanipathes pedata, A. sarothamnoides, and Phanopathes rigida).
The phylogenetic tree clearly indicates three main clades (Figure ). The first include two sub‐clades: one comprises our specimens of P. mapia close to Allopathes desbonni while the second comprises all the species belonging to Antipathes, Stichopathes, Cirrhipathes and Rhipidipathes.
The second main clade includes the genera belonging to the family Myriopathidae (Cupressopathes, Myriopathes and Antipathella), while the third groups the two species belonging to the genus Aphanipathes together with Phanopathes rigida.
General remarks
The phylogenetic analysis clearly indicates that Pseudocirrhipathes is close to the genus Allopathes. The decision to place our material in a separate genus is mainly based on morphological characters including the general aspect of the colony, polyp size, shape and arrangement of spines. However, it is intriguing to propose the hypothesis that the Allopathes characteristic growth form could have evolved from the aggregation of several simple colonies. Yet, in accordance to what was previously stated by Opresko and Cairns (Citation1994), both the simple and the aggregated growth forms represent distinct generic characters, while the occurrence of spines in verticils can be considered a specific character. In support of this statement are several species with verticils that are not related to Pseudocirrhipathes, like the Stichopathes and Cirrhipathes species described above or the branched Aphanipathes verticillata Brook, Citation1889. Also, within a typical verticillated genus there are some exceptions, Allopathes denhartogi Opresko, Citation2003 possesses in fact a quincuxial spine pattern. Unfortunately, a molecular analysis of A. denhartogi was not possible.
At super‐generic level Allopathes and Pseudocirrhipathes must be probably included in a new family, related to Antipathidae. This idea is also supported by the phylogenetic analysis and the common presence in both A. desbonni and P. mapia of an unusually large isorhiza, but, up to now, data about this family are much too scarce for a formal description.
Our analysis also indicates a possible re‐arrangement of the family Aphanipathidae Opresko, 2004. The three tested species belonging to the Aphanipathes and Phanopathes belong to a separate clade (representing the subfamily Aphanipathinae), but the genus Rhipidipathes, placed by Opresko (2004) in the sub‐family Acanthopathinae on the basis of its anisomorphic spines, does not appears to be related to the former and should probably be included in the Antipathidae family. Anyway, all these conclusions must be considered provisional until more specimens and additional taxa are analysed.
Pseudocirrhipathes is the third black coral genus characterized by an unbranched, unpinnulated corallum. The presence of this kind of colony in the two sub‐clades of the “Antipathes” group suggests that an unbranched ancestor could be basal in this group. This idea was previously proposed by Roule (Citation1905), who regarded the unbranched genera as primitive and branched colonies as phylogenetically younger.
Acknowledgements
We would like to thank Dr Dennis Michael Opresko (Oak Ridge National Laboratory, Tennessee) and Dr Tina Molodtsova (Shirshov Institute of Oceanology) for the taxonomic consultancy. Dr Stephen Cairns and Dr Rob van Soest for the samples exchange, respectively, with the Smithsonian National Museum of Natural History and the Zoological Museum of Amsterdam. We would also like to thank Dr Cabrinovic of the British Museum, Dr Massimo Boyer (www.edge‐of‐reef.com) for the underwater photographs of the specimens, Dr Cristina Gioia Di Camillo for the drawings and the two referees for their important contribution to this manuscript.
References
- Bell , J. 1891 . Contributions to our knowledge of the Antipatharian corals. . Transactions of the Zoological Society of London , 13 : 87 – 92 .
- Brook , G. 1889 . Report on the Antipatharia collected by H. M. S. Challenger during the years 1873–1876. . Reports of the Scientific Results of the Voyage of H. M. S. Challenger , 32 : 1 – 222 .
- Cooper , C. F. 1909 . Antipatharia. Reports of the Percy Sladen Trust Expedition to the Indian Ocean. . Transactions of the Linnean Society London , 12 : 301 – 323 .
- Goldberg , W. M. , Grange , K. R. , Taylor , G. T. and Zuniga , A. L. 1990 . The structure of sweeper tentacles in the black coral Antipathes fiordensis. . Biological Bulletin , 179 : 96 – 104 .
- Lapian , H. F. N. , Barucca , M. , Bavestrello , G. , Biscotti , M. A. , Bo , M. , Canapa , A. , Tazioli , S. and Olmo , E. 2007 . A systematic study of some Black Corals species (Antipatharia, Hexacorallia) based on rDNA internal transcribed spacers sequences. . Marine Biology , 151 : 785 – 792 .
- Opresko , D. M. 1972 . Redescriptions and re‐evaluations of the Antipatharians described by L.F. de Pourtalès. . Bulletin of Marine Science , 22 : 950 – 1017 .
- Opresko , D. M. 2003 . A new species of Allopathes (Cnidaria: Antipatharia) from the eastern Atlantic. . Zoologische Verhandelingen, Leiden , 345 : 275 – 280 .
- Opresko , D. M. and Cairns , S. D. 1994 . Description of the new genus Allopathes (Cnidaria: Antipatharia) and its type species Cirripathes desbonni. . Proceedings of the Biological Society of Washington , 107 : 185 – 192 .
- Posada , D. and Crandall , K. A. 1998 . Modeltest: Testing the model of DNA substitution. . Bioinformatics , 14 : 817 – 818 .
- Roberts , C. M. , McClean , C. J. , Veron , J. E. N. , Hawkins , J. P. , Allen , G. R. , McAllister , D. E. , Mittermeier , C. G. , Schueler , F. W. , Spalding , M. Wells , F. 2002 . Marine biodiversity hotspots and conservation priorities for tropical reefs. . Science , 295 : 1280 – 1284 .
- Roule , L. 1905 . Description des Antipathaires et Cerianthaires recueillis par S.A.S. le Prince de Monaco dans l'Atlantique nord (1886–1902). . Résultats des Campagnes Scientifiques Accomplies sur son Yacht par Albert I Prince Souverain de Monaco , 30 : 1 – 96 .
- Summers , S. L. M. 1910 . Antipatharia from the Indian Ocean. . Journal of the Royal. Microscopical Society , 8 : 273 – 281 .
- Swofford , D. L. 1998 . PAUP*. Phylogenetic analysis using parsimony (* and other methods) , Sunderland, MA : Sinauer Associates . Version 4
- Tamura , K. and Nei , M. 1993 . Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. . Molecular Biology and Evolution , 10 : 512 – 526 .
- Tazioli , S. , Bo , M. , Boyer , M. , Rotinsulu , H. and Bavestrello , G. 2007 . Ecology of some common antipatharians from the Marine Park of Bunaken (North Sulawesi, Indonesia). . Zoological Studies , 46 : 227 – 241 .
- Thompson , J. D. , Higgins , D. G. and Gibson , T. J. 1994 . CLUSTALW: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position‐specific gap penalties and weight matrix choice. . Nucleic Acids Research , 22 : 4673 – 4680 .
- Thomson , J. A. and Simpson , J. J. 1905 . Report on the Antipatharia collected by Prof. Herdman at Ceylon, 1902. . Royal Society Report on the Pearl Oyster Fisheries , 4 : 93 – 106 .
- van Pesch , A. J. 1910 . Bijdragen tot de Kennis van het Genus Cirripathes , 96 Leiden : EJ Brill .
- van Pesch , A. J. 1914 . The Antipatharia of the Siboga Expedition. . Siboga‐Expedition Monographies , 17 : 1 – 258 .