Abstract
Habitat‐based inventories provide critical reference data that are essential to track changes in amphibian communities and their habitats. We present the results of a pond inventory in a cultural landscape from central Romania. The presence/absence of amphibians was assessed through multiple‐year surveys during the breeding season and larval development. Ten amphibian species and a species complex were identified: Triturus cristatus, T. vulgaris, Bombina variegata, Bufo bufo, B. viridis, Rana dalmatina, R. temporaria, R. arvalis, Hyla arborea, Pelobates fuscus and the R. esculenta complex. The species richness is larger in the permanent ponds than in the temporary ones. Rana dalmatina, B. bufo and the R. esculenta complex are the most frequent in the permanent ponds, while Bombina variegata and R. temporaria were the most common in temporary ponds. The scarcity of B. viridis and R. arvalis is explained by the lack of available habitats. Our data allow a more complex analysis of the spatial and temporal determinants of amphibian habitat use in this cultural landscape, and provide a consistent baseline for future surveys and monitoring programmes.
Introduction
Pond‐breeding amphibians have complex life cycles and habitat requirements. The persistence of their populations is dependent on the presence and availability of diverse habitats such as aquatic habitats for reproduction, larval development and overwintering for some species, and various terrestrial habitats for feeding and wintering. Amphibians often migrate considerable distances between aquatic and terrestrial habitats (Sinsch Citation1990) and require safe corridors for their migration, especially if they must cross human‐made structures such as roads, that may cause severe mortality (reviewed in Puky Citation2006). Pond‐breeding amphibians are exposed to declines caused by habitat loss and landscape fragmentation, these factors being the dominant and most obvious cause of amphibian declines in Europe, although the causes may be multiple and interrelated (Stuart et al. Citation2004; Beebee & Griffiths Citation2005).
Romania still has many natural and seminatural ecosystems with a large biodiversity. Cultural landscapes in Romania are extremely important targets for conservation. Traditional land use practices created small‐scale perturbations/patchiness in the landscape, increasing landscape heterogeneity and ultimately leading to a high level of biodiversity (Palang et al. Citation2004). The Saxon cultural landscape from the middle section of the Târnava Mare Basin consists of a mosaic of species‐rich meadows and pastures and large deciduous forests of national and international value and importance (Mountford & Akeroyd Citation2005). Due to this recognition, around 85,374 ha of the area were recently designated as Natura 2000 Site of Community Interest (Ministerial Order 2007). Despite this, the expected land‐use changes, as a result of agricultural development caused by the implementation of the Common Agricultural Policy and infrastructural expansion, represent major potential threats and make the habitat‐based inventories even more important as baseline for detecting trends in future monitoring programmes. Previous studies on habitat availability and use by amphibians in this area focused on the characterization of the habitats (Hartel et al. Citation2006), on the relationship between the pond use of individual species and the pond‐ and landscape features (Hartel et al. Citation2005, 2007a, Citation2008), on the habitat use dynamic of temporary pond‐breeding communities (Hartel et al. Citation2005) and on long‐term studies of amphibian populations (Hartel Citation2004).
In this paper we will (1) compare pond occupancy of individual amphibian species and communities in the permanent and temporary ponds, and (2) provide distribution maps for these species and the habitats surveyed.
Materials and methods
Study area
The Târnava Mare Basin is located in southern Transylvania, central Romania (Figure ). Here we studied an area of approximately 4000 km2. The central section of the basin is dominated by hills ranging in elevation from 240 m to a maximum 600–800 m in the west to maximum 750–800 m in the east. The elevation range has an important effect on the climate, which is continental (Pop Citation2001). Mean annual temperatures decrease from the west to east, from 9°C to 6.5°C, mean annual rainfall ranges from 600 mm in the northwest to 700–800 mm in the east (Pop Citation2001).
The regulation of the river and its tributaries during the last 40 years resulted in the creation of new ponds along the river (Pop Citation2001), while large areas of the former floodplain were eliminated. The hydrological network is relatively dense (0.7–0.9 km/km2), and the average water flow of the Târnava Mare at the westernmost point is 15.1 m3/s (Pop Citation2001).
Currently there are two highways and one railway that cross the valley. The land use is mainly traditional (Pop Citation2001; Mountford & Akeroyd Citation2005). Intensive agriculture is practiced in the surroundings of the larger towns (Sighişoara, Mediaş, etc.) and along the Târnava Mare River. In these areas the valley is wide, and the formerly flooded areas are suitable for such activities, whereas in the rest of the area, mechanized agriculture is difficult, especially due to the geomorphology. The land use categories and their percentage in the studied area are shown in Table .
Table I. Percentage of habitat and land use types in the study area calculated on a 1:100.000 CORINE landcover map (European Environmental Agency 2006).
Surveys
We inventoried 96 permanent and 305 temporary ponds between 2000 and 2007. Some temporary ponds were situated at close distance to each other (i.e. tens of meters) indicating a patchy population structure (Petranka et al. Citation2004). We have considered the cluster of such temporary ponds as one breeding area and represented it as a single point on the map. Thus, at the end we had 138 sites with temporary ponds. We considered an amphibian species as present in a pond if one of the following life stages were identified: adults, eggs or larvae. Adult newts were studied using direct observation and dipnetting along the pond shoreline from February until the second part of May, whereas in the case of anurans direct visual observation, dipnetting and chorusing activity were used. Amphibian nomenclature does not follow the recent changes suggested by Frost et al. (Citation2006) since they were not included in national and international legislation yet. Only two species and a species complex are affected by these changes: Triturus vulgaris is Lissotriton vulgaris, Bufo viridis is Epidalea viridis and Rana esculenta complex is Pelophylax esculentus. Breeding adults of R. dalmatina, R. temporaria and B. bufo were searched intensively from the end of February (the first two species) and the end of March (Bufo bufo) until the second half of April. Breeding adults of B. variegata, Hyla arborea and R. esculenta complex were searched from the first part of May until the second part of June (H. arborea), and during the whole activity season (Rana esculenta complex, Bombina variegata). Chorusing activity was used for identification especially for H. arborea and R. esculenta complex. The larval period of the amphibians in this area lasts until the first part of August. In the Yellow Bellied Toad, larvae were found until the first part of September. To increase the probability of detecting reproductive adults in the water, we searched at least three to four times per season, including at least one night survey for each permanent pond. The temporary ponds were searched 1–2 times per breeding season. An additional 2–3 surveys were made to detect larvae until the first part of August. We verified that the sampling effort used was adequate to detect the amphibian species in this area by Pearson correlation and species accumulation curves.
In all, 23 permanent ponds were surveyed for 4–5 years between 2000 and 2007 (Hartel & Öllerer Citation2009), 42 other permanent ponds were also surveyed in 2004, an additional 20 permanent ponds in 2005, 7 permanent ponds in 2006 and 4 in 2007. In 2005, 12 ponds first located in 2004 were resurveyed, all ponds located in 2006 were resurveyed in 2007, whereas none of the ponds located in 2005 were resurveyed after this period. Thus, 51 permanent ponds were surveyed in one year, 19 in two, 2 in three, 3 in four and 21 in five years.
Most of the temporary ponds were surveyed in only one year because of the time constraints. Intensive monitoring was carried out in two clusters of temporary ponds. For these two sites, the details regarding the temporal and spatial pond use by amphibians were already presented elsewhere (Hartel et al. Citation2005, 2007b).
When presenting the use of temporary ponds, we grouped these ponds in three categories: ephemeral ponds with a short hydroperiod that usually dry within three weeks after formation (with up to 12 cm depth), medium hydroperiod ponds that usually dry up once or two times per year (with up to 25–30 cm depth), and long hydroperiod ponds that usually do not dry up throughout the year (with up to 40–50 cm depth). The altitude and the location of the ponds were established using handheld GPS accurate to 4–8 m. The distribution maps were made using the Manifold 7×GIS software, with land cover data obtained from the CORINE Land Cover 2000 1:100,000 version for Romania. The data on habitat occupancy throughout the years were summed and plotted on the maps.
Data analysis
Statistical analyses were carried out according to Faraway (Citation2005) using the R 2.5.0 software (R Development Core Team Citation2006). The “stats” package was used to estimate the coefficients in logistic regression with the altitude as predictor and the presence/absence of amphibians in ponds as binary response variable. Receiver Operating Characteristic (ROC) curves and area under the receiver operating characteristic curve (AUC) values were obtained using ROCR package (Sing et al. Citation2005). The ROC is a graphical technique used for visualizing and selecting classifiers based on their performance, e.g. the ability to correctly assign an observation based on the predictor variables. ROC curves are often reduced to a single index that facilitates comparisons of different tests, the area under the curve: AUC (Lasko et al. Citation2005). If AUC = 0.5, then the constructed binary classification model has no information about the response variables class. The model provides information about the response variables, and possesses a certain predictive power if AUC>0.5. If the observed AUC<0.5 then there is a structural error in the model. The relationship between the number of years at which the ponds were surveyed and the species richness in the permanent ponds was tested with Pearson correlation.
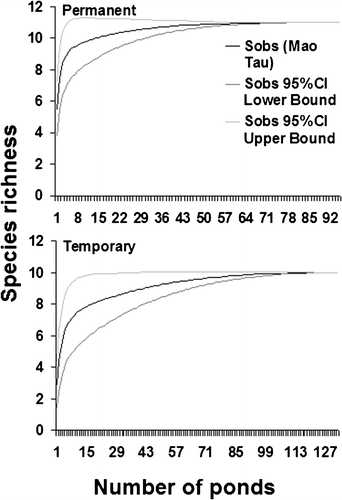
The species accumulation curves were computed using EstimateS 7.5 (Colwell Citation2005). Thompson and Withers (Citation2003) have shown that the shape of a species‐accumulation curve is influenced by both abundance and diversity. If rare species are present, or if there are few species with high abundance, accumulation curves have low shoulders and long trajectories to the asymptote. Conversely, areas with large numbers of abundant species have steep trajectories and reach asymptotes quickly. Species‐accumulation curves can also provide an index of the amount of sampling required to assess local and regional biodiversity. If the curve has not reached its asymptote, sampling probably has been inadequate.
Results
We identified 10 amphibian species and a species complex in the studied ponds: Triturus cristatus, T. vulgaris, Bombina variegata, Bufo bufo, B. viridis, Rana dalmatina, R. temporaria, R. arvalis, Hyla arborea, Pelobates fuscus and the R. esculenta complex. There are six species whose pond occupancy in permanent ponds exceeds 50% (Table ). Bombina variegata and R. temporaria are the most common in the temporary ponds, while Rana dalmatina, B. bufo and the R. esculenta complex are the most frequent in the permanent ponds (Table ). The lowest pond occupancy was found for B. viridis and R. arvalis (Table ). For this reason, these two species were not considered in the individual species analysis, only in the species richness comparisons.
Table II. The number of permanent and temporary ponds with the presence and absence of the amphibians in the middle section of the Târnava Mare basin.
There was no significant relationship between the sampling effort (i.e. the number of years each permanent ponds was surveyed) and the species richness in the ponds (r = 0.08, P = 0.42). The species accumulation curves have steep trajectories for both permanent and temporary ponds, and reach asymptotes quickly (Figure ). A complete inventory of species richness can be achieved with inventorying 58 permanent and 108 temporary ponds (Figure ).
The average species richness was larger in permanent ponds (5.46, SD = 2.31) than in the temporary ones (1.97, SD = 1.98) (t test, t = 14.42, df = 399, P<0.001). The ponds were situated at a mean altitude of 478 m (SD = 111.05, range 257–840). The permanent ponds were at a lower mean altitude (407 m, SD = 83.32) than the temporary ponds (535 m, SD = 97.99) (Mann–Whitney U test, Z = −8.15, P<0.001).
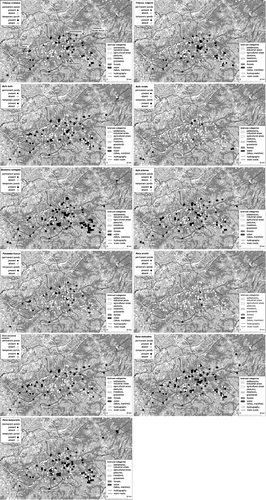
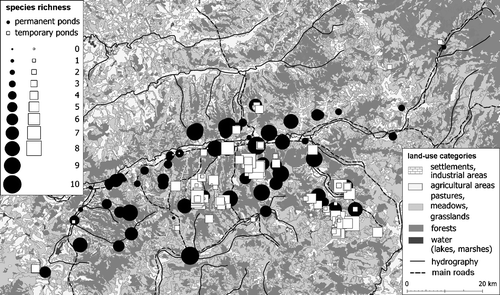
The distribution maps of these species in the two aquatic habitat types surveyed are presented in Figure . Figure shows the permanent and temporary ponds according to their species richness.
Figure 4 The distribution of the permanent and temporary ponds according to amphibian species richness.
There is a significant negative relationship between the pond occupancy of H. arborea, P. fuscus, R. dalmatina and R. esculenta complex and altitude (Table ). Bombina variegata and R. temporaria show a positive association with the altitude whereas there was no relationship in the case of T. cristatus, T. vulgaris and B. bufo (Table ).
Table III. The results of the logistic regression analysis of amphibian pond occupancy and altitude.
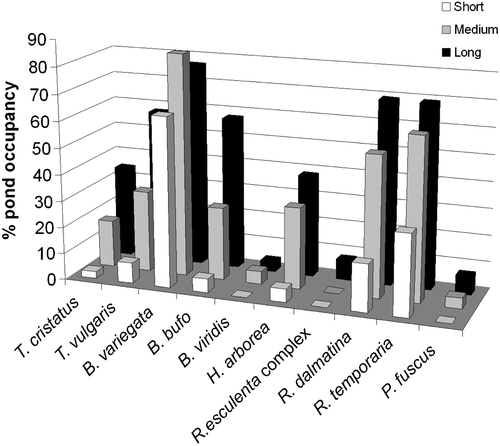
All species have larger temporary pond occupancy in the long duration ponds (Figure ). Bombina variegata and R. temporaria are the most frequent species in the short duration ponds (Figure ).
Discussion
The knowledge on the distribution of amphibians and their habitats is the first step for developing monitoring projects on this group (Cogălniceanu et al. Citation2006; Dodd et al. Citation2007). The present‐day approach commonly used to study the distribution of amphibians in Romania considers territorial administrative units as “distribution units” (see for example Ghira et al. Citation2002), or uses UTM quadrats. The use of such approaches in determining the status of amphibians is limited for a number of reasons. First, this method provides information about the presence of the different species in certain localities, but offers no information regarding the habitat types surveyed and their location (i.e. aquatic habitat types, terrestrial habitats, etc.). Second, they do not report data regarding the absence of species from the different surveyed sites. Recording absences besides presences in a certain habitat is of critical importance in documenting the spatial changes in the habitat use of amphibians when resurveys are done (Skelly et al. Citation2003). Third, the area of administrative units varies widely and does not allow for estimates of habitat availability and use. Therefore, providing data based only on locality name will not allow the assessment of the status of amphibians since it does not allow the resurvey of habitats due to the lack of information about them, making also impossible the setting up of a useful reference database for future comparisons and for the management plans that target amphibians and their habitats. Such reference data can be used to document long‐term changes in amphibian communities and to identify the contribution of different factors (e.g. habitat change, management practices, landscape composition and configuration, population fluctuations) to these modifications (Skelly et al. Citation2003; Crochet et al. Citation2004; Dodd et al. Citation2007). To our knowledge, pond habitat‐based inventories in Romania were made only in the Retezat National Park, Haţeg Geopark (Cogălniceanu et al. Citation2006), Ciuc basin (Demeter et al. Citation2006) and Târnava Mare basin (Hartel et al. Citation2006).
The species accumulation curves indicate that the sampling effort was adequate for the studied area as a whole, since the asymptote is reached quickly. The lack of relationship between the number of amphibian species and the number of survey years on each pond suggests that in real conditions the detection probability of the species are large, and they can be identified using the sampling methodology and intensity used by us.
Amphibian species that are documented to prefer permanent, vegetated ponds for reproduction, such as T. cristatus, B. bufo, R. dalmatina, H. arborea, R. esculenta complex (Vos & Stumpel Citation1995; Scribner et al. Citation2001; Nyström et al. Citation2002, Citation2007; Gustafson et al. Citation2006; Skei et al. Citation2006), have a large permanent pond occupancy (Figure ). The area studied by us allows proper conditions for these species and a large species richness since the permanent ponds are well represented and well vegetated [the mean amount of macrophyte vegetation cover of the ponds being up to 30% (Hartel et al. Citation2006)], the ponds without predatory fish are still in high number [41% (Hartel et al. 2007a)], the permanent ponds are closely situated to the forests [average distance is 232 m (Hartel et al. Citation2006)], the forest coverage is large (Table ), and the land use is, in large part, traditional (Mountford & Akeroyd Citation2005). In this generally less‐fragmented area, the dispersion of juveniles and a quick colonization of the newly created ponds is still possible.
The most probable explanation for the negative relationship between the altitude and the pond occupancy in P. fuscus, H. arborea, R. esculenta complex and R. dalmatina is their preference for ponds that are situated at lower altitudes (along the river and its tributaries). These ponds tend to be sunny, vegetated and permanent. The values of the AUC further suggest that the preference for low altitude ponds (that are predominantly permanent) is high in the R. esculenta complex and P. fuscus (Table ). However, the relatively low values of the AUC for H. arborea and R. dalmatina in the pond occupancy–altitude relationship suggest that other variables have more influential effect than the pond altitude alone. Such variables are, for example, the reed cover of the ponds and the presence of predatory fish (Hartel et al. 2007a), but in the case of H. arborea it may also be the light conditions (and implicit the temperature) of the ponds (Pellet & Hoehn Citation2004). The lack of pond occupancy–altitude relationship for other amphibian species that prefer permanent ponds such as T. cristatus (Gustafson et al. Citation2006; Skei et al. Citation2006) and B. bufo (Scribner et al. Citation2001) may be explained by the larger ecological plasticity of these species in the altitudinal range and environmental conditions of this area. These species were found to be influenced by reed cover and predatory fish (Hartel et al. 2007a), and in the case of B. bufo by landscape composition and configuration (Hartel et al. Citation2008). The presence of B. variegata in permanent ponds is generally not constant during the years, and is linked to seasons when the water level increases due to precipitations, when small areas are flooded (Hartel Citation2004). Moreover, its presence in permanent ponds depends on the existence of temporary ponds in the surroundings, with successful reproduction of the species (Hartel, personal observations).
All species identified in the permanent ponds were also present in the temporary ponds. Our personal observations in this area show that some amphibian species use the temporary ponds near the permanent ones when the later becomes inhospitable (i.e. due to fish introductions) (Hartel, unpublished results). Bombina variegata, R. temporaria and R. dalmatina are the most common species in temporary ponds, including the short duration ones. This is possible because the species have several adaptations to temporary pond‐breeding, such as short larval periods; high phenotypic plasticity, and in the case of B. variegata, the multiple breeding during a season in synchronization with rainfall (Barandun & Reyer Citation1997a,Citationb, Citation1998; Loman Citation1999; for local studies see Hartel et al. Citation2005, 2007b). Despite the considerable similarity in reproductive mechanisms the parental species of the Rana esculenta complex show a great degree of general morpho‐anatomical and functional differentiation which results in a different susceptibility to environmental factors (Bucci et al. Citation2000; Marracci & Ragghianti Citation2008).
The scarcity of B. viridis in this area is most probably due to the lack of suitable habitats. The landscape has large deciduous forests and mesophilic meadows and pastures (Mountford & Akeroyd Citation2005) that maintain a relatively high level of humidity not preferred by this toad. This species prefers open, more xerophilic habitats and breeds in temporary ponds with high risk of desiccation (Sinsch et al. Citation1999). It is able to quickly adjust its development rate according to the presence of competitors and pond desiccation (Katzmann et al. Citation2003). Its presence in the study area is mainly tied to human settlements that assure open and xerophilic areas for this toad. A long‐term study carried out in a pond system in this area (Hartel Citation2004) shows a high rate of “gains and losses” (sensu Hecnar & M'Closkey Citation1996), meaning that the species could not be detected every year, despite the high sampling effort.
Rana arvalis was historically rare along the Târnava Mare Valley, to our knowledge being only mentioned by Méhelÿ (Citation1894) and Fuhn (Citation1960). A reason for its historical scarcity may be represented by the geomorphologic features of the Târnava basin, a hilly area with very narrow (up to 100–150 m) adjacent valleys. In these conditions larger marshy areas cannot be formed. The Moor Frog was found in wide flatlands and plains (northwestern Transylvania; Sas et al. Citation2006) or in mountain basins (southeastern Transylvania; Demeter & Mara Citation2006).
In conclusion, the cultural landscape studied by us still holds species‐rich amphibian communities. The data gathered by this multi‐year inventory allow us to track the consequences of land‐use changes and to provide conservation management recommendations. Although the distribution and the habitat factors that influence the distribution of amphibian species in this area were identified, the pond monitoring and inventories should continue in the future as well, and the distribution maps should be permanently updated. The present database is already useful for testing hypotheses regarding the spatial dynamic of amphibian populations.
Acknowledgements
Our studies in this area were financially supported by the Declining Amphibian Populations Task Force (grant to Tibor Hartel, Citation2004), the British Ornithologists Union (grant to Cosmin Ioan Moga, Citation2005), the Swedish Biodiversity Centre (grant to Kinga Öllerer, 2006) and the Mihai Eminescu Trust (2005–2007). We thank three anonymous reviewers for their comments on the manuscript.
References
- Barandun , J. and Reyer , H‐U. 1997a . Reproductive ecology of Bombina variegata: Characterisation of spawning ponds. . Amphibia–Reptilia , 18 : 143 – 154 .
- Barandun , J. and Reyer , H‐U. 1997b . Reproductive ecology of Bombina variegata: Development of eggs and larvae. . Journal of Herpetology , 31 : 107 – 110 .
- Barandun , J. and Reyer , H‐U. 1998 . Reproductive ecology of Bombina variegata: Habitat use. . Copeia , 2 : 407 – 500 .
- Beebee , T. J. C. and Griffiths , R. A. 2005 . The amphibian decline crisis: A watershed for conservation biology? . Biological Conservation , 25 : 271 – 285 .
- Bucci , S. , Ragghianti , M. and Guerrini , F. 2000 . Negative environmental factors and biodiversity: The case of the hybridogenetic green frog system from Lake Trasimeno. . Italian Journal of Zoology , 67 : 365 – 370 .
- Cogălniceanu , D. , Hartel , T. and Plăiaşu , R. 2006 . “ Establishing an amphibian monitoring program in two protected areas of Romania. ” . In Herpetologia Bonnensis II. Proceedings of the 13th Congress of the Societas Europaea Herpetologica , Edited by: Vences , M , Köhler , J , Ziegler , T and Böhme , W . 31 – 34 . Bonn, Germany : SEH .
- Colwell , R. K. 2005 . EstimateS. Statistical estimation of species richness and shared species from samples. Version 7.5. User's Guide and application. . Available: http://purl.oclc.org/estimates
- Crochet , P. A. , Chaline , O. , Cheylan , M. and Guillaum , C. P. 2004 . No evidence of general decline in an amphibian community of Southern France. . Biological Conservation , 119 : 297 – 301 .
- Demeter , L. , Hartel , T. and Cogălniceanu , D. 2006 . Notes on the spatial distribution and conservation status of amphibians in the Ciuc basin. . Zeitschrift für Feldherpetologie, Supplement , 10 : 217 – 224 .
- Demeter , L. and Mara , Gy. 2006 . The distribution and population size of the moor frog (Rana arvalis) in the Ciuc basin. . A Csíki Székely Múzeum Évkönyve , 2005 : 439 – 450 .
- Dodd , C. K Jr. , Barichivich , W. J. , Johnson , S. A. and Staiger , J. S. 2007 . Changes in a Northwestern Florida Gulf Coast herpetofaunal community over a 28‐year period. . American Midland Naturalist , 158 : 29 – 48 .
- Faraway , J. 2005 . Linear Models with R. Chapman & Hall/CRC
- Frost , D. R. , Grant , T. , Faivovich , J. , Bain , R. H. , Haas , A. , Haddad , C. F. B. , de Sá , R. O. , Channing , A. , Wilkinson , M. Donnellan , S. C. 2006 . The amphibian tree of life. . Bulletin of the American Museum of Natural History , 297 : 1 – 370 .
- Fuhn , I. E. 1960 . Fauna R.P.R. Amphibia. Editura, Academiei Republicii Socialiste Romania
- Ghira , I. , Venzel , M. , Covaciu‐Marcov , S. D. , Mara , Gy. , Ghile , P. , Hartel , T. , Török , Zs. , Rácz , T. , Farkas , Z. and Brad , T. 2002 . Mapping of Transilvanian herpetofauna. . Nymphaea , 29 : 145 – 201 .
- Gustafson , D. , Journath Petterson , C. and Malmgren , J. C. 2006 . Great crested newts (Triturus cristatus) as indicators of aquatic plant diversity. . Herpetological Journal , 16 : 347 – 352 .
- Hartel , T. 2004 . The long term trend and the distribution of amphibian populations in a semi‐natural pond in the middle section of the Târnava‐Mare Valley (Romania). . Biota – Journal of Biology and Ecology , 5 : 25 – 36 .
- Hartel , T. , Demeter , L. , Cogălniceanu , D. and Tulbure , M. 2006 . “ The influence of habitat characteristics on amphibian species richness in two river basins of Romania. ” . In Herpetologia Bonnensis II. Proceedings of the 13th Congress of the Societas Europaea Herpetologica , Edited by: Vences , M , Köhler , J , Ziegler , T and Böhme , W . 31 – 34 . Bonn, Germany : SEH .
- Hartel , T. , Moga , C. I. and Nemes , Sz. 2005 . Use of temporary ponds by amphibians in a wood pasture, Romania. . Biota – Journal of Biology and Ecology , 6 : 21 – 28 .
- Hartel , T. , Nemes , Sz. , Cogălniceanu , D. , Öllerer , K. , Schweiger , O. , Moga , C. I. and Demeter , L. 2007a . The effect of fish and habitat complexity on amphibians. . Hydrobiologia , 583 : 173 – 182 .
- Hartel , T. , Nemes , Sz. , Demeter , L. and Öllerer , K. 2008 . Pond and landscape characteristics: which are more important for the common toad (Bufo bufo)? A case study from central Romania. . Applied Herpetology , 5 : 1 – 12 .
- Hartel , T. , Nemes , Sz. and Mara , Gy. 2007b . Spatial and temporal dynamic of pond use by a hybrid fire‐bellied toad (Bombina variegata) population: The importance of pond availability and duration. . Acta Zoologica Lituanica , 17 : 56 – 63 .
- Hartel , T. and Öllerer , K. 2009 . Local turnover and factors influencing the persistence of amphibians in permanent ponds from the Saxon Landscapes of Transylvania. . North‐Western Journal of Zoology , 5 : 40 – 52 .
- Hecnar , S. J. and M'Closkey , R. T. 1996 . Regional dynamics and the status of amphibians. . Ecology , 77 : 2091 – 2097 .
- Katzmann , S. , Waringer‐Löschenkohl , A. and Waringer , A. 2003 . Effects of inter‐ and intraspecific competition on growth and development of Bufo viridis and Bufo bufo tadpoles. . Limnologica , 33 : 122 – 130 .
- Lasko , T. A. , Bhagwat , J. G. , Zou , K. H. and Ohno‐Machado , L. 2005 . The use of receiver operating characteristic curves in biomedical informatics. . Journal of Biomedical Informatics , 38 : 404 – 415 .
- Loman , J. 1999 . Early metamorphosis in common frog Rana temporaria tadpoles at risk of drying: An experimental demonstration. . Amphibia–Reptilia , 20 : 421 – 430 .
- Maracci , S. and Ragghianti , M. 2008 . Rana (Pelophylax) esculenta complex studied in a molecular context. . Italian Journal of Zoology , 75 : 109 – 112 .
- Méhelÿ , L. 1894 . Magyarország barna békái (Ranae fuscae Hungariae). . A. M. T. Akad. Math. és Term. Tud. Közlemények. , 25 : 1 – 64 .
- Mountford , O. J. and Akeroyd , J. R. 2005 . A biodiversity assessment of the Saxon villages region of Transylvania. . Transylvanian Review of Systematical and Ecological Research , 2 : 31 – 42 .
- Nyström , P. , Birkedal , L. , Dahlberg , C. and Brönmark , K. C. 2002 . The declining spadefoot toad Pelobates fuscus: Calling site choice and conservation. . Ecography , 25 : 488 – 498 .
- Nyström , P. , Hansson , J. , Mansson , J. , Sundstedt , M. , Reslow , C. and Broström , A. 2007 . A documented amphibian decline over 40 years: Possible causes and implications for species recovery. . Biological Conservation , 138 : 399 – 411 .
- Palang , H. , Printsmann , A. , Gyuró , É. K. , Urbanc , M. , Skowronek , E. and Woloszyn , W. 2004 . The forgotten landscapes of Central and Eastern Europe. . Landscape Ecology , 21 : 347 – 357 .
- Pellet , J. and Hoehn , S. 2004 . Characterization of tree frog (Hyla arborea) calling ponds in western Switzerland. In: Glandt D, Kronshage A, editors. Der Europaïsche Laubfrosch (Hyla arborea): Biologie, Schutzmassnahmen und Effizienzkontrollen. . Zeitschrift für Feldherpetologie Supplement , 5 : 159 – 164 .
- Petranka , J. W. , Smith , C. K. and Scott , A. F. 2004 . Identifying the minimal demographic unit for monitoring pond‐breeding amphibians. . Ecological Applications , 14 : 1065 – 1078 .
- Pop , G. P. 2001 . Depresiunea Transilvaniei , Cluj‐Napoca : Presa Universitarã Clujeană .
- Puky , M. 2006 . “ Amphibian road kills: A global perspective. ” . In Proceedings of the 2005 International Conference on Ecology and Transportation, Center for Transportation and the Environment , Edited by: Irwin , C. L , Garrett , P and McDermott , K. P . 325 – 338 . Raleigh, NC : North Carolina State University .
- R Development Core Team . 2006 . R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. . Available: http://www.R‐project.org
- Sas , I. , Covaciu‐Marcov , S. D. , Kovács , É. H. , Radu , N. R. , Tóth , A. and Popa , A. 2006 . The populations of Rana arvalis Nills. 1842 from the Ier Valley (The Western Plain, Romania): present and future. . North‐Western Journal of Zoology , 2 : 1 – 16 .
- Scribner , K. T. , Arntzen , J. W. , Cruddace , N. , Oldham , R. S. and Burke , T. 2001 . Environmental correlates of toad abundance and population genetic diversity. . Biological Conservation , 98 : 201 – 210 .
- Sing , T. , Sander , O. , Beerenwinkel , N. and Legauer , T. 2005 . ROCR: Visualizing classifier performance in R. . Bioinformatics , 21 : 3940 – 3941 .
- Sinsch , U. 1990 . Migration and orientation of anuran amphibians. . Ethology Ecology and Evolution , 2 : 65 – 79 .
- Sinsch , U. , Höfer , S. and Keltsch , M. 1999 . Syntope Habitatnutzung von Bufo calamita, Bufo viridis und Bufo bufo in einem Rheinischen Auskiesungsgebiet. . Zeitschrift für Feldherpetologie , 6 : 43 – 64 .
- Skei , J. K. , Dolmen , D. , Rønning , L. and Ringsby , T. R. 2006 . Habitat use during the aquatic phase of the newts Triturus vulgaris (L.) and T. cristatus (Laurenti) in central Norway: proposition for a conservation and monitoring area. . Amphibia–Reptilia , 27 : 309 – 327 .
- Skelly , D. K. , Yurewicz , K. L. , Werner , E. E. and Relyea , R. A. 2003 . Estimating decline and distributional change in amphibians. . Conservation Biology , 17 : 744 – 751 .
- Stuart , S. N. , Chanson , I. S. , Cox , N. A. , Young , B. E. , Rodrigues , A. S. L. , Fishman , D. L. and Waller , R. W. 2004 . Status and trends of amphibian declines and extinctions worldwide. . Science , 3 : 1783 – 1785 .
- Thompson , G. G. and Withers , P. C. 2003 . Effect of species richness and relative abundance on the shape of the species accumulation curve. . Austral Ecology , 28 : 355 – 360 .
- Vos , C. C. and Stumpel , A. H. P. 1995 . Comparison of habitat isolation parameters in relation to fragmented distribution patterns in the tree frog (Hyla arborea). . Landscape Ecology , 11 : 203 – 214 .