Abstract
The Iberian phasmid species Leptynia attenuata and L. hispanica (Pantel, Citation1890) are actually species‐complexes, each embodying a few specific taxa. Morphological, karyological and molecular investigations concordantly revealed that considerable differences exist between the two complexes, each representing a monophyletic group. Significant differentiating body traits are: the presence in the “attenuata” males of a well‐developed subanal vomer, which is lacking in those of the hispanica‐complex; the sharply pointed abdomen tip in the “hispanica” females, quite different from the rounded abdomen ending of the attenuata‐complex females. Also the egg structure and SEM chorionic pattern differ neatly. Karyotypes of the “attenuata” taxa mainly differ by chromosome repatternings entraining number reductions, while those of the hispanica‐complex maintain the same chromosome number in sexual taxa and add similar haploid sets in polyploid parthenogens. Also molecular investigations neatly separate the complexes and support specific differentiations of taxa within each complex; therefore, a splitting of the genus Leptynia is felt necessary. Caudell wrongly reported the designation by Pantel of L. hispanica as the type species of the genus, while Kirby validly indicated Leptynia attenuata as the type species; therefore, the subsequent Leptyniella proposition for the Leptynia attenuata complex by Bolìvar, is not valid either. Thus in the genus splitting, the Leptynia designation must be maintained to the “attenuata” complex, while the new name Pijnackeria is here given to the former “hispanica” taxa. This splitting is in full accordance with the seminal description by Pantel for the occurrence of the subanal vomer in the L. attenuata males and the presence of the pointed female abdomen in “hispanica” taxa, plainly settling a rather confusing nomenclatural situation. It also significantly updates the Iberian phasmid systematics. The formal description of taxa belonging to the reshaped Leptynia and to the new genus Pijnackeria will be dealt with in papers to follow.
Introduction
Phasmida (Otte & Brock Citation2005) have attracted the attention of entomologists because of their mimetic body structure and colour, matching environmental traits, particularly twigs and leaves of their food plants, so that their mimicry is often astonishing. Furthermore, the prolonged motionless habit may make the perception of their presence quite sudden, providing them with the main traits of a ghost ( = phasma): a silent and unexpected apparition.
In the early description by Bolìvar (Citation1878), Iberian stick insects were referred to as Bacillus hispanicus, with the main goal of separating them from the hitherto known Bacillus species. Quite convincingly, 12 years later, Pantel (Citation1890) reinforced their distinctness from Bacillus, erecting for them the genus Leptynia. At the same time he also split the Iberian stick insects into two species: L. attenuata and L. hispanica, without decision about the type species. He based such a distinction on a series of characters: the pointed ending of the anal segment of the female abdomen in the latter species appeared the most obvious, but to further support his genus splitting operation, he pointed out the occurrence of a wholly membranous subanal vomer in L. attenuata males, notably distinct from the hard, chitinous vomer of Bacillus (Pantel 1890). Caudell (Citation1903) wrongly reported that Pantel had indicated L. hispanica as the type species of Leptynia, but Kirby (Citation1904) validly designated L. attenuata instead; nonetheless, Bolìvar (Citation1926) proposed the genus name Leptyniella for L. attenuata, since he erroneously believed the designation of L. hispanica as type species to be valid.
The confused nomenclatural status of the subsequent attributions made it necessary to inspect the original specimens collected by Bolìvar and Pantel by visiting the Natural History Museums of Madrid, Paris and Vienna personally. In those museum collections, I could confirm that Bolìvar really did not designate the type, being that all specimens were reported as syntypes or co‐types, and that in central Spain Pantel attributed the local males of L. attenuata (now L. montana Scali) obviously provided with a subanal vomer, to the parthenogenetic females of L. hispanica, the only syntopic phasmid of that area (see below, sections on Materials and methods and Description of genera). It could be then understood why Pantel wrongly assumed that both L. attenuata and L. hispanica males possessed a similar subanal vomer and why L. hispanica of central Spain had been taken as a bisexual species. This early mistake has been maintained, because it led subsequent phasmid students to take into account the female abdomen tips exclusively, while overlooking the subanal vomer trait in males of real bisexual “hispanica” taxa that had been collected at different locations meanwhile.
Over the years, the collections of Leptynia, while substantiating a clear distinction between L. attenuata and L. hispanica, and directly demonstrating the existence of bisexual populations in both species, also evidenced the occurrence of all‐female, parthenogenetic populations for L. hispanica (see Brock Citation1991, Citation1993 for reviews). In more detail, L. attenuata is to be found both in Portugal and western Spain, whereas L. hispanica mainly spreads on Eastern Spain, with some overlapping zones in central Spain (Sierra de Guadarrama, Sistema Central); all‐female populations of L. hispanica also reach the Mediterranean French coasts spreading up to several districts, such as Var, Herault, Basses Alpes.
Furthermore, Nascetti et al. (Citation1983) proposed that both L. attenuata and L. hispanica could actually represent species‐complexes and that the all‐female populations were triploid and tetraploid forms related to bisexual L. hispanica; they also suggested that the parthenogenetic tetraploids originated by heterospecific hybridization. These hypotheses, based on preliminary electrophoretic gene–enzyme investigations, were supported through karyological findings by Bianchi (Citation1992); she also provided evidence that the triploid parthenogenetic populations should also have originated by hybridization. Thus, the new collections led to a much better definition of range, population structure and ecology of the two species‐complexes. It was ascertained that these stick insects feed on the broom Sarothamnus scoparius Koch, Dorycnium suffruticosum Vill., Cytisus spp., Ulex spp. and Rosa canina L., with the broom much preferred by L. attenuata species and the Dorycnium by L. hispanica taxa. The increased eto‐ecological knowledge also made Leptynia collections easier (reviewed in Brock Citation1991, Citation1993; Lelong Citation1992), so that substantial specimen samples could be collected from several places. However, as far as the diagnostic characters between them was concerned, no progress was actually achieved since Pantel's paper: females looked so alike within each complex while conspicuously distinct between the two that no investigation on males was felt necessary: the real L. hispanica males were not characterized further and the Pantel's mistake was long maintained.
Multidisciplinary investigations of Iberian stick insects by means of body and egg SEM, karyotype reconstructions and allozyme analyses, allowed Scali (Citation1996) and Passamonti et al. (Citation1999) to define a number of differential characters within the L. attenuata complex so that, in addition to the nominal species L. attenuata of Portugal, two new species, namely L. caprai of the Toledo Mountains and L. montana of the Sierra de Guadarrama, were described; in addition, a putative new species of southern Spain (Sistema Panibetico) with a distinct karyotype (2n = 40/39, XX/XO) (Bianchi & Meliado Citation1998) was acknowledged and confirmed on a molecular basis (Passamonti et al. Citation1999). Most importantly, in the males of all these taxa the occurrence of a well‐developed membranous subanal vomer, fully respondent to Pantel's original description, was constantly observed.
The findings within the L. attenuata complex also prompted deeper research within L. hispanica. To find out the phylogenetic relationships among taxa of the L. hispanica species‐complex, including the polyploids, allozyme analyses could not be utilized further, since the electrophoretic gene–enzyme patterns, reported as simple by Nascetti et al. (Citation1983), in the early samples were not confirmed in the new ones and, as a matter of fact, proved too complex for their unambiguous interpretation (Scali and co‐workers, unpublished). Therefore, to investigate the differentiation and the likely phyletic relationships of taxa within L. hispanica comprehensively, mitochondrial cox2 gene sequencing was utilized (Figure ).
Figure 1 Minimum Evolution (ME) tree based on the mitochondrial cox2 gene sequences describing the likely relationships among taxa of the unsplitted genus Leptynia: the “attenuata” and “hispanica” clades are clearly separated; for the “hispanica” complex the significant (>60) bootstrap values obtained from ME, Maximum Parsimony and Maximum Likelihood are reported in the order. Medaura scabriusculus has been chosen as a well‐differentiated taxon from the Mediterranean genera (from Ghiselli et al. Citation2007).
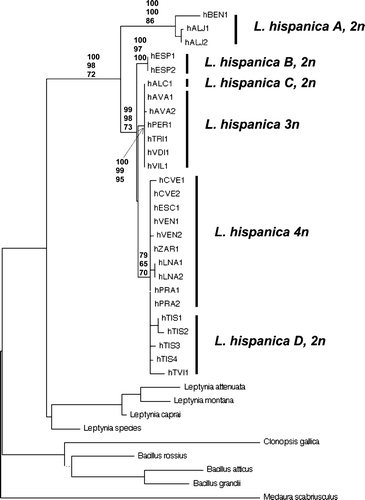
From the cox2 haplotypes, it was apparent that the sequences derived from triploids were identical to those obtained from the diploid bisexuals of Alcoceber: the L. hispanica C (Figure ), which was therefore taken as the maternal ancestor of the triploids. Similarly, the haplotypes derived from tetraploid L. hispanica s. s. were clearly linked to those obtained from the bisexual diploid L. hispanica D of Tiscar (Sierra de Cazorla), which was therefore deemed to be the maternal ancestor of tetraploids (Ghiselli et al. Citation2007).
On the whole, the conclusion was reached that the two complexes, referred to as Pantel's originally described species, should be recognized as distinct genera, each encompassing a variety of specific and subspecific taxa. This splitting is here defined taking into account both the experimental findings and the nomenclature rules.
Materials and methods
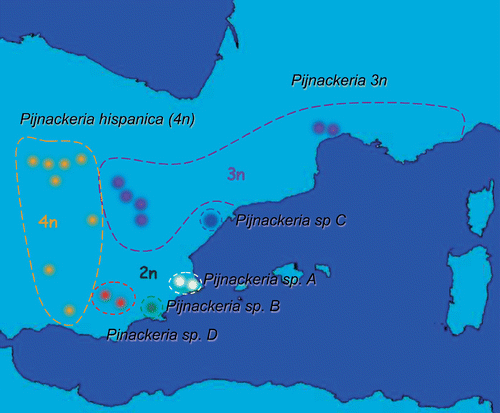
From new samples, amounting to more than 700 specimens, 174 were examined: 59 tetraploid females, 67 triploid females and 48 bisexuals (35 females and 13 males). The specimens came from 14 distinct populations: either French (Abbey Valmagne) or Spanish (Alcoceber, Alcoy, Benissa, several sites in the El Escorial area, Las Navas del Marques, Peralbece, Puerto Cruz Verde, Sierra de Espugna, Trillo, Ventana Diablo, Ventorillo, Villalba de Cuenca, Zarzalejo), all good representatives of the species‐complex range (Figure ). All these specimens were compared with those of the L. attenuata‐complex, analysed electrophoretically and karyologically (224, all together) by Passamonti et al. (Citation1999). It should also be pointed out that Bolìvar collected in the El Escorial area, referred among type localities, and that the examined samples from Las Navas, Puerto Cruz Verde, Ventorillo and Zarazalejo are close to El Escorial itself; furthermore, none of these locations are far from the Madrid area, where several of Bolìvar's original specimens came from.
Figure 2 Ranges of the Pijnackeria taxa, reporting the locations of the samples analysed for body morphology, ootaxonomy, karyotypes and the mitochondrial gene cox2.
SEM preparations were obtained after at least 3 days fixation of bodies and eggs of field collected specimens in 70% ethanol and an ultrasonication for 30 s to eliminate or reduce surface debris, followed by a complete dehydration in a graded series of ethanol solutions. Specimens were then air‐dried and attached to specimen‐holders with a suitable double‐sided adhesive tape; bodies or eggs were afterwards coated with gold in a Bio‐Rad SC 502 sputter‐coater evaporator and observed in a Jeol JSM 5200 scanning electron microscope. The terminology adopted for body description is the one commonly used for stick insects (see for instance Beier Citation1957; Bradley & Galil Citation1977), whereas egg traits were indicated according to Clark (Citation1976, Citation1979) and Scali and Mazzini (1981).
Chromosome plates, from which the different karyotypes were prepared, were obtained from testes or ovariole tips after 30 min fixation in Carnoy solution, a short hypotonic shock in 1% sodium citrate solution, teasing in 45% acetic acid on a hot (60°C) plate and Giemsa staining. Mitotic or meiotic plates were then either photographed under a Zeiss FOMI II microscope on Agfa Ortho films and printed on Ilford paper or directly recorded from the microscope through an electronic apparatus.
Total genomic DNA was obtained according to the methods described in Preiss et al. (Citation1988), while the partial sequence of the mitochondrial cox2 gene, corresponding to the region sequenced in several insect orders, was analysed according to Mantovani et al. (Citation2001). Sequencing covered 639‐bp coding for 213 amino acids of the cox2. All sequences were aligned using MEGA 3.1 (Kumar et al. Citation2004) and phylogenetic analyses were performed as in Passamonti et al. (Citation2004), and Ghiselli et al. (Citation2007). The tree in Figure was obtained with Mr Bayes 3.1 (10,000,000 generations; Huelsenbeck & Ronquist Citation2003).
Taxonomic accounts
General observations
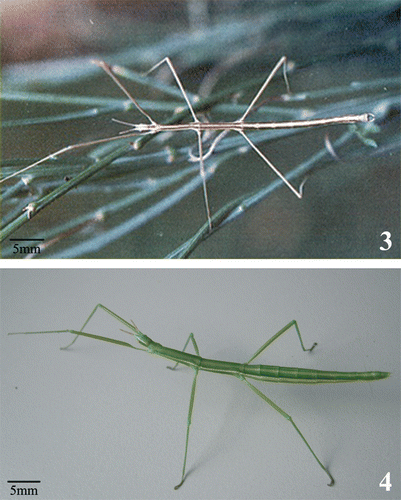
Iberian phasmids have the typical slender general aspect of most wingless stick insects, with males much thinner than females (Figures ). In the 2nd and 3rd leg pairs no area apicalis is found at the distal apex of the tibial inferior carina of specimens of both complexes; therefore, their systematic position is within the suborder Anareolatae. They also share the general features of the Diapheromeridae, subfamily Pachymorphinae, tribe Gratidiini (Otte & Brock Citation2005).
Figure 3 Male of Leptynia montana showing the general body features shared by all taxa of the genus Leptynia; particularly evident are the very slender apterous body, the long antennae and the backward projecting claspers. Figure 4. Female of Pijnackeria hispanica (ex Leptynia hispanica): this parthenogenetic tetraploid taxon is the nominal species of the new genus, since it has been found to correspond to the originally described L. hispanica species by Pantel.
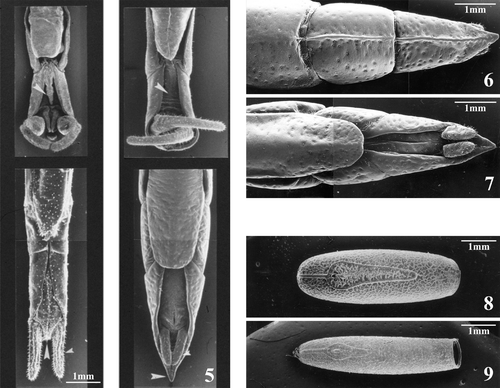
The newly gathered samples of Iberian stick insects (several hundreds altogether), without a single exception, supported the presence of the subanal vomer only in the L. attenuata‐species complex (Figure ).
Figure 5 Comparison between male and female terminalia pairs of Leptynia and Pijnackeria. The presence on the ventral side of the subanal vomer (arrowhead) is clearly seen in the Leptynia s.s male specimen (upper left), whereas the Pijnackeria male specimen (upper right) lacks it (arrowhead). In the Leptynia male the claspers are perpendicular to the photographic plane and their structure can be hardly seen, while those of the Pijnackeria male are mostly parallel to the photo plane, so that their size and bending can be better appreciated. The ventral aspect of terminalia of female Leptynia (bottom left) shows the tapered abdomen tip (large arrowhead) and the straight cerci (small arrowhead) going well beyond the anal segment; in the Pijnackeria female terminalia (bottom right), the pointed last tergite (large arrowhead) completely conceals the shorter cylindrical cerci (small arrowhead). Figure 6. Dorsal side of the Pijnackeria hispanica terminalia showing the sparse round pits centred with a sensory hair; the acute termination of the last tergite is also clearly visible. Figure 7. Ventral aspect of the Pijnackeria hispanica terminalia clearly showing, in addition to the highly characteristic abdomen tip shared by all Pijnackeria taxa, the subgenital valve of the ovipositor, which always goes beyond the basal articulation of the 10th sternite. Figure 8. Egg capsule of Pijnackeria: its shape and chorionic sculpturing are similar in all taxa of the genus and quite distinct from the corresponding traits of Leptynia. Figure 9. Egg capsule of Leptynia: its elongated, thin shape varies slightly among the generic taxa, but it is always diagnostic when compared with the Pijnackeria egg capsule. The ootaxonomic distinction between the two genera is reinforced by the sharply different chorionic pattern.
From the taxonomical characterization viewpoint, the presence/absence of the subanal vomer clearly affects an acknowledged feature of supra‐specific level, even proposed as a “guide‐character” at higher ranks (Key Citation1970).
In addition to this clear‐cut male feature, the other major differentiating trait obviously is the marked different abdominal tip structure of the “hispanica” females, affecting both the segments and the cerci appearance. Female abdominal segments, particularly last two, are pierced with small round pits, each centred by a sensory hair (Figure ). The same segments appear laterally compressed and, if viewed from the side, look notably convex; they are hardened and scarcely articulated to form a sort of stiff termination, where the anal tergite realizes a very peculiar structure by having its lateral borders extended proximally downwards to approach each other on the ventral side and being prolonged dorsally into a sclerotic, acute tip, completely concealing the anal valve. The peculiar prolongation of the anal segment conceals almost completely the short, cylindrical cerci (Figures , ).
Another major differentiating set of characters between the two complexes resides in egg‐laying mode as well as in both gross egg structure and fine pattern at SEM level. All “hispanica” females lay their eggs loosely anchored to the soil, while “attenuata” species firmly glue them on food‐plant twigs. Major “hispanica” ootaxonomical features are: the dark‐sand colour, a moderately elongated complexion (about three times longer than wide or high, Figure ), dorsal convexity but straight ventral side; an outstanding chorionic pattern of ribbons, forming an irregular network all throughout, including the shallow‐domed, downwards‐looking operculum (negative angle). The major distinguishing features of “attenuata” eggs are the clearly elongate complexion (from four to six times longer than wide or high, Figure ) and the very dark grey chorion. The pattern of the almost cylindrical capsule is given by scattered thin ribbons superimposed to a finer background of small mamelons; on the contrary, the opercular pattern is characterized by an irregular net of stout ribbons, whose variable size confers the operculum a more or less convex appearance according to species. The ribbon network is sharply different from their own capsule sculpturing and from the hispanica‐complex pattern; also species‐specific is the degree of inclination of the decidedly upwards looking operculum (positive angle, see Scali Citation1996). To fully appreciate the relevance of those egg features, it should be recalled that they constitute a quite reliable set of taxonomic traits for both eumendelian and parthenogenetic species, so that a systematic arrangement of stick insect taxa on an ootaxonomic basis has been successfully proposed (Scali & Mazzini Citation1987 and references therein; Sellick Citation1998).
Also, the karyotype features and reproductive modes appear to have followed different evolutionary pathways in the two species‐complexes: in the attenuata‐complex chromosome number and structure variations – even affecting the sex formula – have been realized repeatedly while keeping a strict bisexual population structure and reproduction mode (Figures ), whereas in the hispanica‐complex the same haploid chromosome number (n = 19) has been constantly maintained: all bisexual taxa show 2n = 38 and even the polyploid unisexual parthenogens (3n = 57; 4n = 76) followed a series of chromosome increases by haploid sets of 19 elements (Figures ).
Figure 10 Male karyotype of Leptynia sp. with 2n = 40/39, XX,XO. This southern taxon is basal to all other Leptynia species and together with L. caprai shows the highest chromosome number. Figure 11. Male karyotype of Leptynia attenuata (2n = 36, XX,XY) from Saõ Fiel. The nominal species of the revised Leptynia has the lowest chromosome number of the genus; chromosome re‐patterning have also involved the sex‐chromosome formula, shifting it from the usual XX/XO to the XX/XY one. Figure 12. Female karyotype of Pijnackeria species D (Sierra de Cazorla), with 2n = 38/37, XX/XO. All bisexual species of Pijnackeria have the same number of chromosomes and rather similar karyotypes. Figure 13. The tetraploid karyotype (4n = 76, XXXX) of the parthenogenetic Pijnackeria hispanica s.s.: within each quartet all elements are very similar, so that the hybrid derivation of this parthenogen cannot be recognized from chromosomes.

On the whole, the clear‐cut differences for relevant morphological traits, together with the characterizing karyotype features, safely support the splitting of the Iberian genus Leptynia into two, by separating the already acknowledged species‐complexes.
Taking into account the taxonomic history sketched in the Introduction, it is here proposed to maintain the name Leptynia to the “attenuata” Artenkreis, in view of Pantel's subanal vomer description when erecting the genus and Kirby's (Citation1904) designation, while choosing the name Pijnackeria (see the name derivation below) for the former L. hispanica complex, disregarding the untenable mention of L. hispanica as a type species operated by Caudell (Citation1903) (Figures 5–, –).
Description of Pijnackeria, gen. n. and comparison to Leptynia Pantel
The type species of the new genus is Bacillus hispanicus Bolívar, Citation1878 [i.e. now Pijnackeria hispanica], here designated, for the reasons specified below.
Derivatio nominis: the new genus is dedicated to the distinguished phasmid student Laas Pier Pijnacker of Groningen University. The attribution is particularly intended to acknowledge his pioneering and skilful work in investigating the intriguing maturation processes of parthenogenetic phasmid eggs.
As it happens with most stick insects, in Pijnackeria a sharp sexual dimorphism is present, the males being shorter and much thinner than females. The total body length of males (excluding antennae and cerci) ranges from 35 to 46 mm, whereas that of the females goes from 47 to 58 mm. Specimens of Leptynia, as newly defined, have similar size, although they are slightly larger on average (length range 34–50 mm for males, 46–61 mm for females; Brock Citation1991; Scali Citation1996).
To further characterize Pijnackeria specimens, it can be mentioned that males can be darker in colour than females for the frequent occurrence of two lateral brown bands all along the pleurae. Female body coloration is highly diversified, going from yellowish‐green to ash‐grey or brown, although light green is by far the commonest coloration. Male antennae most commonly show 16 articles and are proportionately longer than female antennae due to their allometric growth; on the other hand, female antennae have a rather variable number of articles (from 10 to 16), mainly depending on the species.
Male length ratio of mesonotum to metanotum is about 1, as it is in females. Both dorsal and ventral aspects of male mesothorax and metathorax are smooth, while in females they are constantly covered by a conspicuous granulation given by hemispherical sense structures. Ratios of the two last abdominal segments differ among sexes: in males, the 10th anal tergite is slightly shorter than 9th, with a ratio value of about 0.9, while in females it is longer with ratio values going from 1.1 to 1.4, depending on the taxon.
On the ventral aspect of male abdomen, the genital operculum extends as much as the 9th segment and has a more or less tapered posterior border, which in vivo constantly approaches the base of 10th segment. Most importantly, no subanal vomer is to be found (Figure ). The last abdominal tergite of males is constantly incised; cerci are prominent, mostly circular in contour, distally bent to acquire the typical clasper shape with a round apex. Each cercus is provided on its medial aspect with a sizable finger‐like tooth (0.25 mm in length), located at about one‐third from cercus base and forming a mildly acute angle with its distal branch; the basal third of cercus is only slightly thicker than average distal part (ratio of 1.1–1.2).
In Pijnackeria females, in addition to the peculiar abdominal tip, another characterizing feature of their terminalia is the development of the genital operculum, which constantly goes beyond the articulation of the 10th sternite with its smooth, rounded or slightly indented margin, whereas in Leptynia females it never reaches the distal end of the 9th sternite although it may have a similar shape (Figures , ).
The fore leg pair of Pijnackeria males shows an apical tooth on their ventral side, while the remaining two are muticous; the opposite condition appears to be realized in Leptynia males, where the fore femora are muticous, while the two others show 1–5 small teeth; in Pijnackeria females the three leg pairs are muticous, while some tiny teeth may be shown in in Leptynia females. In Pijnackeria, the femur to tibia ratio of both sexes is 1 or just lower; when the 2nd leg pair (the shortest) is directed towards the abdomen tip, its femur, approximately reaches one‐third of the 2nd abdominal tergite, whereas the femur of the 3rd pair (intermediate in length) shows a variable reach, namely from the distal half of the 4th abdominal tergite to the posterior articulation of the 5th. On the other hand, the mid and hind femurs of Leptynia are comparatively longer since the 2nd pair reaches half of the 3rd abdominal segment, while the 3rd leg femur may attain half of the 7th segment.
The new genus Pijnackeria, comprises four bisexuals and two all‐female polyploids (Figures –): among them, the thelytokous tetraploid form becomes the nominal species P. hispanica since the original specimens housed in Bolìvar's collections clearly correspond in every morphological detail, including the relative stoutest body complexion and thickest antennae, to the freshly collected 4n samples. To be as sure as possible that Bolivar's L. hispanica specimens were the parthenogenetic tetraploids, targeted collecting campaigns were dedicated also to the Madrid surroundings and El Escorial area: it invariably turned out that, although the original collecting sites in Madrid suburbs no longer existed having become built up areas, all nearest samples fully supported the identity of Bolìvar's appointed specimens with the 4n P. hispanica. Furthermore, it was possible to ascertain that only 4n Pijnackeria (formerly Leptynia) hispanica females are syntopic with L. montana bisexuals in several places on the Sistema Central mountains: this overlapping of “attenuata” and “hispanica” ranges appears to be the most likely cause of the above described mistaken male attribution.
The other polyploid taxon of the genus is provisionally reported as Pijnackeria 3n, whereas the bisexuals are here indicated as Pijnackeria species A, B, C, D, respectively (see Figure ): all will be formally described in a specifically dedicated paper. Pijnackeria forms a monophyletic group of species, although the hybrid P. hispanica, could be, in theory, polyphyletic in origin owing to mitochondrial haplotype's diversification (Ghiselli et al. Citation2007). Among bisexuals, P. species A is the most differentiated form and appears to be basal to the other three eumendelian taxa: they show a lower but still neat differentiation and, also in view of their isolated ranges, can be considered at least as incipient species. Types and paratypes of the new genus Pijnackeria will be listed and described in a specific paper to follow.
Figure 14 Phylogenetic tree, obtained from cox2 mitochondrial gene sequences (Mr Bayes 3.1, 10,000,000 generations), giving an updated systematic status of the presently defined Iberian taxa.
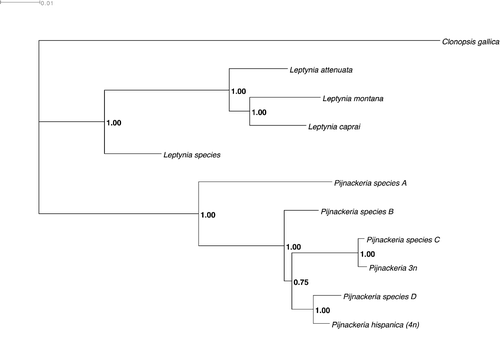
To better characterize the re‐shaped genus Leptynia (type species, Leptynia attenuata Pantel Citation1890: 402) in addition to the constant presence of the membranous subanal vomer – which is the major differentiating feature between Leptynia and Pijnackeria males (Figure ) – it can be pointed out that Leptynia males are uniformly smooth and glabrous (excepting for cerci), while females show a diffuse fine granulation and sparse short hairs, which become more dense on the subgenital plate and cerci (Figure ). The posterior border of the last abdominal tergite of Leptynia males is variably incised: as far as the already‐described species is concerned, the last tergite notching is narrow in L. caprai, intermediate in L. montana and wider in L. attenuata. Male cerci are well developed and have the typical clasper conformation; their most peculiar trait appears the variable size of a tooth, placed near the cercus base: it is smallest in L. caprai and largest in L. attenuata s. s. (Scali Citation1996). In males of this genus, the length proportions of the 9th to 10th abdominal segments are similar to those reported for Pijnackeria, but the shape of the 10th tergite of females is sharply different: although laterally compressed its posterior rim is rounded and does not conceals the straight cerci, which therefore go well beyond the abdomen tip (Figure ).
At present, the genus Leptynia embodies only bisexual taxa; furthermore, the phylogenetic and phylogeographic scenarios coherently supports that the genus is a monophyletic group of species (Figure ). The southern L. species found at Grazalema and Ojen (Sistema Panibetico mountains) with 2n = 39/40, XO/XX, is the basal and most differentiated, while L. caprai with L. montana are intermediate and L. attenuata the most recent (see Passamonti et al. Citation2004). Furthermore, the chromosomal repatternings – mainly translocations, even affecting the sex chromosome structure – appear to have had a major role in speciation: starting from species with higher chromosome number, namely L. species and L. caprai (2n = 40/39, XX/XO; Figure ), a trend of chromosome reduction appears to have occurred in L. montana (2n = 38/37, XX/XO) and, particularly, in L. attenuata (2n = 36, XY; Figure ). Furthermore, in addition to the above‐mentioned species, allozymic relationships' pattern as well as molecular analyses of the mitochondrial cox2 gene suggest a specific rank of differentiation for at least two additional XY eumendelian taxa, to be specifically listed and formerly described in a forthcoming paper.
Conclusive remarks
Evaluating the taxonomic relevance of morphological traits is a difficult task; however, if within a given group several taxa are analysed and compared both among themselves and with related groups, a reasonably sound knowledge can be acquired about their systematic value. For instance, the egg chorion differences described in Orxines macklotti and Sipyloidea sipylus appear to be of similar magnitude, whereas those observed among Clonopsis or Bacillus species appear lower (Scali & Mazzini Citation1981, Citation1987; Scali & Milani 2009; Scali in press). Furthermore, when a significant morphological differentiation is backed by the findings obtained through additional, independent approaches, such as karyology and molecular analyses (mtDNA, nuclear genes), then much safer conclusions can be reached. In the case of Iberian phasmids, all those kinds of data sets have been obtained and found coherent in suggesting a supra‐specific differentiation level: therefore, the splitting of the former genus Leptynia into Leptynia s. s. – which now includes all Iberian Gratidiini taxa whose males are provided with a membranous subanal vomer and females with a tapered, rounded adbomen tip – and the new Pijnackeria genus – embodying all additional Gratidiini, whose males lack the subanal vomer and females show an abdomen tip with compressed, stiff and acute terminalia – appears to be well supported.
On the whole, the splitting operation appears to recognize two natural groups of species, each with a monophyletic origin and a well‐characterized evolutionary history from morphological, karyological and reproductive points of view. Each genus likely encompasses both fully differentiated species, such as the still undescribed southern Leptynia sp. of the Grazalema mountains, and incipient species, such as L. montana and L. caprai or the three branches of the L. attenuata clade. Furthermore, the revision of the former Leptynia genus and its splitting also appear to plainly settle the complex and confused nomenclatural condition.
The marked karyotype variations noticed in Leptynia are apparently shared by the Australian Didymuria‐species complex (Craddock Citation1975), whereas the maintenance of steady chromosome numbers and the formation of polyploid hybrids has been encountered in the holomediterranean genus Bacillus (reviewed in Scali in press); on the other hand, the North African genus Clonopsis (Milani et al. 2009) and the Australian Sipyloidea (John et al. Citation1987) have apparently followed both karyotype variation pathways. On the whole, stick insects appear to have followed a variety of modes in changing their karyotypes so that it is often difficult, or even impossible, to disentangle the actual reticulate pattern of phyletic relationships on a cytotaxonomical basis (Scali in press); however, by adding, as pointed out above, reproductive, morphological and molecular findings, it has been possible to gain significant insights on the phyletic and systematic issues of phasmids.
As a consequence of the present revision and splitting, the systematics assessment of the involved historical museum collections of the former Leptynia should be updated. Finally, owing to the still largely unsearched areas of Spain and Portugal, it seems likely that additional taxa await discovery.
Acknowledgements
I wish to express my gratitude to my co‐workers Marco Passamonti, Fabrizio Ghiselli and Liliana Milani for their skilful suggestions and help in writing and editing the paper.
References
- Beier , M. 1957 . Klassen und Ordnungen des Tierreichs: Arthropoda, Orthopteroidea. . Akademische Verlagsgesellschat, Geest & Portig K.‐G., Leipzig , 5 : 305 – 454 .
- Bianchi , A. P. 1992 . Karyological studies of Mediterranean stick‐insects belonging to the genera Clonopsis and Leptynia (Insecta Phasmatodea). . Caryologia , 45 : 1 – 19 .
- Bianchi , A. P. and Meliado , P. 1998 . Analysis of the karyotypes of four species of the Leptynia attenuata complex (Insecta Phasmatodea). . Caryologia , 51 : 207 – 219 .
- Bolìvar , I. 1878 . Analecta Orthopterologica. . Anales de Historia Natural , 7 : 423 – 424 .
- Bolìvar , I. 1926 . Datos complementarios sobre los Ortòpteros de la Peninsula Ibérica. . Bolletin Real Sociedad Española de Historia Natural , 26 : 98 – 102 .
- Bradley , R. D. and Galil , D. S. 1977 . The taxonomic arrangement of the Phasmatodea with keys to the subfamilies and tribes. . Proceedings of the Entomological Society, Washington , 79 : 176 – 208 .
- Brock , P. D. 1991 . Stick‐insects of Britain, Europe and the Mediterranean , 50 London : Fitzgerald Publishing .
- Brock , P. D. 1993 . Studies on stick‐insects of the genus Leptynia in Spain. . Amateur Entomologist's Society Bulletin , 52 : 165 – 172 .
- Caudell , A. N. 1903 . Note on Phasmidae. . Entomological News , 14 : 314 – 315 .
- Clark , J. T. 1976 . The eggs of stick insects (Phasmida): A review with a description of the eggs of eleven species. . Systematic Entomology , 1 : 95 – 105 .
- Clark , J. T. 1979 . A key to the eggs of stick and leaf insects (Phasmida). . Systematic Entomology , 4 : 325 – 331 .
- Craddock , E. M. 1975 . Intraspecific karyotype differentiation in the Australian phasmatid Didymuria violescens (Leach). . Chromosoma (Berlin) , 53 : 1 – 24 .
- Ghiselli , F. , Milani , L. , Scali , V. and Passamonti , M. 2007 . The Leptynia hispanica species complex (Insecta Phasmida): Polyploidy, parthenogenesis, hybridization and more. . Molecular Ecology , 16 : 4256 – 4268 .
- Huelsenbeck , J. P. and Ronquist , F. 2003 . Mr Bayes 3: Bayesian phylogenetic inference under mixed models. . Bioinformatics , 19 : 1572 – 1574 .
- John , B. , Rentz , D. C. F. and Contreras , N. 1987 . Extensive chromosome variation in the stick insect genus Sipyloidea Brunner von Wattenwyl (Phyllidae: Necrosciinae) within Australia, and descriptions of three new species. . Invertebrate Taxonomy , 1 : 603 – 630 .
- Key , K. H. L. 1970 . “ Phasmatodea (Stick‐insects). ” . In Insects of Australia , 348 – 359 . Melbourne : C.S.I.R.O./Melbourne University Press .
- Kirby , W. F. 1904 . “ A synonymic katalogue of Orthoptera. 1. ” . In Orthoptera Euplexoptera, Cursoria et Gressoria (Forficulidae, Hemimeridae, Blattidae, Mantidae, Phasmidae) , 501 London : Longmans & Co. .
- Kumar , S. , Tamura , K. and Nei , M. 2004 . MEGA 3: Integrated software for molecular evolutionary genetic analysis and sequence alignment. . Briefings in Bioinformatics , 5 : 150 – 163 .
- Lelong , P. 1992 . Le genre Leptynia dans la peninsule Iberique. . Le Monde des Phasmes , 17 : 10 – 15 .
- Mantovani , B. , Passamonti , M. and Scali , V. 2001 . The mitochondrial cytochrome oxidase II gene in Bacillus stick insects: Ancestry of hybrids, androgenesis and phylogenetic relationships. . Molecular and Phylogenetic Evolution , 19 : 157 – 163 .
- Milani , L. , Scali , V. and Passamonti , M. 2009 . The Clonopsis gallica puzzle: Mendelian species, polyploid parthenogens with karyotype re‐diploidisation and clonal androgens in Moroccan stick insects. . Journal of Zoological Systematics and Evolutionary Research , 47 : 132 – 140 .
- Nascetti , G. , Bianchi Bullini , A. P. and Bullini , L. 1983 . Speciazione per ibridazione nei fasmidi del bacino mediterraneo (Cheleutoptera: Bacillidae). Atti XIII Congresso Nazionale Italaliano di Entomologia. . Sestriere, Torino , : 475 – 478 .
- Otte , D. and Brock , P. D. 2005 . Phasmida species file. Catalog of the stick and leaf insects of the world , 414 Philadelphia : The Insect Diversity Association at the Academy of Natural Sciences . 2nd ed
- Pantel , P. J. 1890 . Notes Orthoptérologiques. II Les Phasmides d'Europe et des pays limitrophes. . Anales Sociedad Española de Historia Natural , 19 : 371 – 404 .
- Passamonti , M. , Mantovani , B. and Scali , V. 1999 . Karyotype and allozyme characterization of the Iberian Leptynia attenuata species complex (Insecta Phasmatodea). . Zoological Science , 16 : 675 – 684 .
- Passamonti , M. , Mantovani , B. and Scali , V. 2004 . Phylogeny and karyotype evolution of the Iberian Leptynia attenuata species complex (Insecta Phasmatodea). . Molecular Phylogenetics and Evolution , 30 : 87 – 96 .
- Preiss , A. , Hartley , D. A. and Artavanis‐Tsakonas , S. 1988 . Molecular genetics of henancer of split, a gene required for embryonic neural development in Drosophila. . EMBO Journal , 12 : 3917 – 3927 .
- Scali , V. 1996 . Descrizione di due specie incipienti di insetti stecco (Phasmatodea) del complesso Leptynia attenuata Pantel: L. montana n. sp. e L. caprai n. sp. . Redia , 79 : 123 – 136 .
- Scali , V. In press . “ Metasexual stick insects: model pathways to losing sex and bringing it back. ” . In Lost sex , Edited by: Schön , I , Martens , K and Dijk , P. V . Dordrecht, , Netherlands : Springer .
- Scali , V. and Mazzini , M. 1981 . The eggs of stick insects Sipyloidea sipylus (Westwood) and Orxines macklotti De Haan (Phasmatodea, Heteronemiidae): A scanning electron microscopic study. . International Journal of Invertebrate Reproduction , 4 : 25 – 38 .
- Scali , V. and Mazzini , M. 1987 . “ Ootaxonomy of stick insects (Phasmatodea). ” . In 1st International Symposium on Stick‐Insects: Phylogeny and Reproduction , Edited by: Mazzini , M and Scali , V . 157 – 168 . Siena : Centrooffset .
- Scali , V. and Milani , L. 2009 . New Clonopsis stick insects from Morocco: The amphigonic C. felicitatis sp.n., the parthenogenetic C. soumiae sp.n., and two androgenetic taxa. . Italian Journal of Zoology , 76 : 291 – 305 .
- Sellick , J. T. C. 1998 . The micropylar plate of the eggs of Phasmida, with a survey of the range of plate form within the order. . Systematic Entomology , 23 : 203 – 228 .