Abstract
Temporal and spatial changes in abundance, prosome length, oil sac volume, molting patterns and morphometric parameters were studied in Calanus euxinus from the Black and Marmara Seas. In the south‐western part of the Black Sea and deep shelf zone near Sinop the abundance of C. euxinus was high during the whole studied period (2000–2005), with a maximum 23,400 ind m−2 in March 2004. In the Marmara Sea near the Prince Islands in the deep zone the mean annual abundance of C. euxinus was 47 times lower than in the deep zone of the Black Sea (during 2000–2007). However, this parameter reached a significant magnitude of 12,264 ind m−2 in spring in Izmit Bay. During the warm season, C. euxinus are rare in the Marmara Sea. The high temperature and salinity accelerate development in this species; therefore, preadults and adults possess reduced prosome length and oil sac volume. In the cold period in the Marmara Sea the size and lipid content in late copepodite stages increase, especially in Izmit Bay. Similar size of eggs, prosome length of early copepodite stages in the Black and Marmara Seas indicate that the C. euxinus population in the Marmara Sea originates from the individuals penetrating from the Black Sea through the Bosphorus.
Introduction
Copepods from the genus Calanus are a subject of intense research throughout the temperate‐boreal regions of the world oceans (Marshall & Orr Citation1972; Mauchline Citation1998; Bonnet et al. Citation2005). The representatives of this genus usually dominate the mesozooplankton biomass and play a prominent role in the carbon cycle in the sea, constituting a major link in pelagic food webs. Due to large body size and significant lipid amounts in the oil sac (Marker et al. Citation2003), Calanus makes a substantial contribution to the diet of the juvenile stages of some economically important fish.
In the Black Sea there is the only one Calanus species which is considered to be a phenotypic variation of Calanus helgolandicus widespread in neritic waters of the Seas of the northern Atlantic (Fleminger & Hulsemann Citation1987). Due to very limited connection between the Black and Mediterranean Seas through two narrow straits of the Marmara Sea (Dardanelles and Bosphorus), the Black Sea Calanus population is isolated from the populations of Calanus in the other seas of the Atlantic. Based on some morphometric characteristics, Fleminger and Hulsemann (Citation1987) recognized the Black Sea population as a distinct species. In 1991 a new name – C. euxinus – was given to this species by Hulsemann (Citation1991). Nevertheless, later, Papadopoulos et al. (Citation2005) and Unal et al. (Citation2006) did not find substantial genetic differentiation between C. euxinus and C. helgolandicus from the English Channel, north‐eastern Atlantic and Adriatic Sea.
Bonnet et al. (Citation2005) examined the biology and ecology of Calanus helgolandicus over a wide range of different environments in areas distributed from the northern North Sea to the Aegean and Levantine Seas and showed distinct temperature preferences and specific adaptation of their body size and life cycles to the temperature regime. However, the authors did not include the data on development of this species in the unique environment of the Black and Marmara Seas.
In the Black Sea C. euxinus occur all the year round (Vinogradov et al. Citation1992b). In the Marmara Sea near the Bosphorus, this species was observed during spring–autumn (Tarkan et al. Citation2005) and in winter (our unpublished data). Thus, C. euxinus penetrating into the Marmara Sea through the Bosphorus with the Black Sea water permanently enrich the fauna of the Marmara Sea where the highest abundance of Calanus was found in Izmit Bay (Isinibilir et al. Citation2008). It is unlikely that C. helgolandicus may get into the Marmara Sea through the Dardanelles because in the eastern Mediterranean this species is present only seasonally in the Aegean Sea (Moraitou‐Apostolopoulou Citation1985; Fleminger & Hulsemann Citation1987).
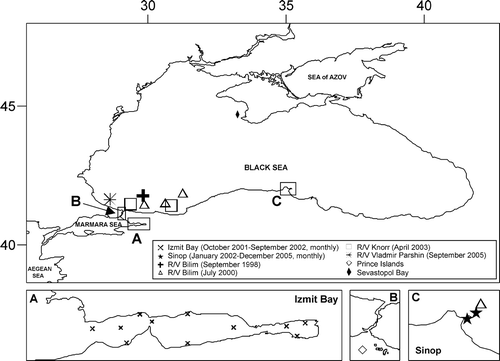
The habitat conditions of C. euxinus in the Black and Marmara Seas differ markedly (Figure ). The salinity in offshore regions of the Black Sea slowly increases with depth from approximately 18 to 22‰. The temperature of the surface layers changes from 6 to 8°C during winter–spring homothermy up to 22–25°C during late spring–autumn stratification. At depths greater than 20–30 m the temperature varies within the limits of 6.5–8.5°C throughout the year (Vinogradov et al. Citation1992b). A sharp decrease in dissolved oxygen above the hydrogen sulfide zone is considered to be a distinctive attribute of the Black Sea. In the oxygen minimum zone (OMZ, 0.5–1.15 mgO2 l−1), the migrating preadults and adults of C. euxinus are aggregated during daytime (Vinogradov et al. Citation1992a). It has been shown that C. euxinus could optimize its life cycle strategy under unique temperature and oxygen conditions of the Black Sea (Svetlichny et al. Citation2006). Due to diurnal vertical migrations to cold hypoxic layers, the Black Sea C. euxinus decrease mean daily energy expenditure and accumulate lipids even during periods of summer low phytoplankton concentration. At low oxygen concentrations lipid catabolism is limited and protein of food consumed near sea surface becomes the main metabolic substrate for synthesis of wax ester reserved in the sac (Sargent & McIntosh Citation1974; Yuneva et al. Citation1997). However, in shallow zones of the Black Sea (where hypoxic layers are absent), the development rate in C. euxinus increases and copepods cannot accumulate high lipid amounts as in deep regions (Svetlichny et al. Citation2006).
Figure 1 The profiles of temperature and salinity typical for winter (1) and summer (2) in the southwestern Black Sea (a, b), in the northeastern Marmara Sea near the Prince Islands (c, d) and in the Izmit Bay (e, f).
The Marmara Sea is considered as a transit basin, providing water exchange between the Aegean and Black Seas. As a result of the positive water balance in the Black Sea, its water masses are transferred into the Marmara Sea through the Bosphorus Strait, forming a brackish upper layer (15–20 m) with a salinity of 22–25‰ and temperature of 7–24°C. Below this layer there is more saline (∼38.5‰) Mediterranean Sea water with constant temperature of ∼15°C throughout the year (Besiktepe et al. Citation1994). Dissolved oxygen (DO) concentrations amount to 7.4–10.7 mg l−1 in the upper layer and 1.1–1.5 mg l−1 in the lower layer (Unluata et al. Citation1990).
Izmit Bay, located in the north‐eastern Marmara Sea, is an elongated semi‐enclosed water body with a length of 50 km and width varying between 2 and 10 km. Izmit Bay is oceanographically an extension of the Marmara Sea with a constant two‐layered water system. The upper layer originates from less saline Black Sea waters (18.0–22.0‰), whereas the lower layer originates from the Mediterranean Sea waters which are more saline (∼38.5‰) (Unluata et al. Citation1990). A permanent stratification occurs at about 25 m in the Marmara Sea (Besiktepe et al. Citation1994); however, it is highly variable in Izmit Bay (Oguz & Sur Citation1986) due to the formation of a dissolved hydrogen sulfide zone in deep layers (Balkis Citation2003). The thickness of the upper layer changes seasonally from 9 to 18 m in spring and autumn, respectively (Oguz & Sur Citation1986; Algan et al. Citation1999). An intermediate layer develops throughout the year in the water column of the Bay with varying thickness (Oguz & Sur Citation1986). The depth profile of DO concentration in Izmit Bay shows a sharp decrease at approximately 20 m below the surface in the western and central basins during late spring to autumn, and a gradual decline of DO occurs at about 30 m water depth in winter (Morkoc et al. Citation2001; Balkis Citation2003).
Therefore, in the Black and Marmara Seas, C. euxinus undergo gradients of temperature, salinity and oxygen concentration during development which are absent in the other seas of the Atlantic Ocean.
The aim of the present study was to analyze seasonal and regional changes in abundance, prosome length, body proportions, lipid content and molting patterns (as an index of development rate) in C. euxinus in relation to environmental parameters in deep and shallow regions of the Black Sea, and in the Marmara Sea near the Prince Islands and in Izmit Bay.
Materials and methods
In the Black Sea zooplankton samples were collected (Figure ) with vertical hauls from the entire oxic layer (σ t = 16.2) by Nansen net with mouth opening diameter of 0.5 m and 210 µm mesh size at the monitoring stations near Sinop with the depth of 50 m and 180 m monthly in 2002–2005 (cruises of R/V “Araştırma 1”), in the south‐western anticyclonic regions with the depths more than 300 m during the cruises of the R/V “Bilim” in July 2000 and June 2001, R/V “Knorr” in April 2003 and R/V “Vladimir Parshin” in October 2005. In the Marmara Sea at the permanent station near the Prince Islands with the depth about 200 m zooplankton samples were collected by vertical hauls from the depth with a Nansen net (opening diameter 0.5 m, mesh size 200 µm) during the cruises of small fishing boat (Hedef‐1) in 2005–2007 and cruises of the R/V “Bilim” in June 2001. In Izmit Bay samples were collected monthly at 11 stations from November 2001 until July 2002 during the cruises of small boat (Altınnal). Samples were collected during the day by a single vertical haul using plankton net with mouth opening diameter of 0.5 m and 157 µm mesh size. Water temperature and salinity were measured by pIONeer 65 multiprobe using the Practical Salinity Scale.
The samples were immediately preserved with 4% borax‐buffered formaldehyde.
In the laboratory, nauplii, copepodite stages and adults of C. euxinus were counted in a Bogorov chamber under a dissecting microscope. Individuals (30–40) of every copepodite stage or adults were selected for the measurements of body size and oil sac volume, and up to 80 individuals of copepodites stage V (CV) were identified according to tooth formation inside the gnathobases of mandibles for determination of molting stages.
Morphological examination of mandibular gnathobases was performed under a light microscope. The left mandible was dissected with needles, transferred to a drop of glycerine on a microscope slide, covered with a cover slip and examined at a magnification of 150×. Five molting phases (postmolt, late postmolt, intermolt, early premolt and premolt) were determined using the morphological characteristics defined for C. finmarchicus by Miller and Nielsen (Citation1988), Marker et al. (Citation2003) (modified from Miller et al. Citation1991) and Arashkevich et al. (Citation2004) and for C. pacificus defined by Johnson (Citation2004).
The females of C. euxinus collected near Sevastopol in autumn 2003 and reared in the laboratory (at 18°C and 39‰) during winter–spring 2003 were also used for morphological analysis.
Length and width of prosome (Lpro and dpr, mm) and oil sac (Ls and ds, mm), length of urosome (Luro) were measured to the nearest 10 µm under a light microscope, fitted with an eyepiece micrometer. Diameters of eggs laid by C. euxinus females in the laboratory were measured under a light microscope at a 300× magnification.
Body volume (Vb, mm3) was calculated as Vb = kLpr dpr 2, where k is the empiric coefficient of 0.64 in males and 0.58 in females and copepodites (Svetlichny Citation1983). The oil sac volume (Vs, mm3) was determined as an ellipsoid volume: Vs = π/6Ls ds 2.
Statistical evaluation of data was conducted by one‐way ANOVA. The values presented in the figures and tables are the means±standard deviation. The relationships between any two variables in the present study were derived using least squares linear regression. The comparisons of the parameters were made using Student's t‐test.
Results
Abundance and molting patterns of Calanus euxinus in the Black and Marmara Seas
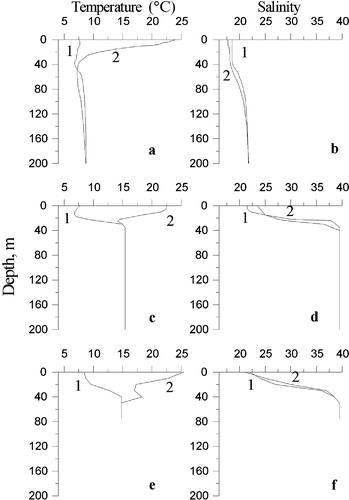
In the deep regions of the south‐western Black Sea the abundance of C. euxinus changed from 1247 ind m−2 (biomass of 0.6 mg m−2) in June 2000 to 12,201 ind m−2 (biomass of 5.7 mg m−2) in April 2003 (Figure ). During 2002–2005 at deep station near Sinop minimum and maximum population densities amounted to 1400 ind m−2 in May 2003 and 23,400 ind m−2 in March 2004, respectively. Average long‐term abundance of C. euxinus constituted 4798±5814 ind m−2. The number of this species at shallow station (50 m) near Sinop changed from 5 to 5200 ind m−2. During 2002–2005, the average abundance of C. euxinus at shallow station was 4.2±2.3 lower than at deep station for the periods of simultaneous sampling.
Figure 3 Abundance (ind m−2) of Calanus euxinus in the Black Sea near Sinop at the permanent inshore (○) and offshore (•) stations during 2002 (a), 2003 (b), 2004 (c) and 2005 (d) years, in the southwestern part of the Black Sea (▪) in April 2003, June 2001, July 2000 and October 2005, in the Marmara Sea (⧫) near the Prince Islands in February 2007, April 2005, June 2001, July 2007, October 2000, 2005 and December 2006, 2007, and in Izmit Bay (![]()
In the Marmara Sea near the Prince Islands in 2001–2007, C. euxinus number varied from 5 to 1055 ind m−2, with a maximum value in April 2005. However, in Izmit Bay, the abundance of C. euxinus increased from 564 to 12,264 ind m−2 in winter–spring and diminished to 2–132 ind m−2 in summer 2002.
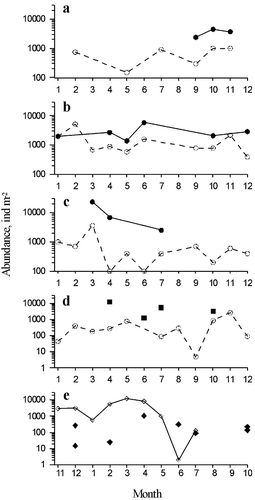
Stage V copepodites made up about 80% of the C. euxinus population in the Black Sea. During the year postmolts constituted 40–70% of CV inhabiting deep waters of the Black Sea (Figure ).
Figure 4 Molting phase frequency distribution in Calanus euxinus copepodites V during sampling in the southwestern Black Sea (a) and in the Marmara Sea near the Prince Islands (b) and in Izmit Bay (c). 1, postmolt; 2, late postmolt; 3, intermolt; 4, early premolt; 5, premolt.
On the contrary, in the Marmara Sea near the Prince Islands, premolts predominated in spring and autumn (Figure ).
In Izmit Bay, frequency distribution of molting phases in CV depended upon the season. In November, intermolts and premolts prevailed and in January only intermolts were found, while during winter–spring period (excepting April) postmolts dominated as in the Black Sea (Figure ).
Morphometry of Calanus euxinus in the Black and Marmara Seas
Morphometric characteristics of C. euxinus from the Black Sea and Marmara Sea (near the Prince Islands and in the Izmit Bay) are typical for C. helgolandicus (Fleminger & Hulsemann Citation1987), including the features of curvature of the toothed border of the inner margin of the basipod of p5 in males and females, and other details of limb morphology.
During the winter–spring period, the average prosome length of C. euxinus collected at deep stations near Sinop and in the south‐western part of the Black Sea increased from 0.69±0.05 in CI to 2.67±0.06 mm in adult females and 2.52±0.08 mm in adult males (Table ). At the summer–autumn period prosome the lengths of CIII, CIV and females were significantly 3.7–5.1% lower (p<0.001). At other stages, the prosome length between the warm and cold periods did not significantly differ.
Table I. Diameter of eggs (D) and prosome length (Lpr) from copepodites 1 (CI) to adults females (CVIF) and males (CVIM) in Calanus euxinus collected in the deep water near Sinop and in the south‐western part of the Black Sea during 2000–2005, in the shallow water near Sevastopol in 2002 and reared in the laboratory, and from the Marmara Sea near the Prince Islands (2000–2005) and in Izmit Bay (2001–2002). G1–G3 size groups, determined according to frequency distribution of the prosome length in different stages of Calanus euxinus.
At the shallow station near Sevastopol prosome length (2.29±0.16 mm) was significantly (p<0.001) 9% lower than at the deep station in males only. Also, this parameter is lower in CIV (13%), CV (21%) and females (22%) reared in winter–spring at 19°C and 39‰ from eggs which were laid by large females from the Black Sea.
In the Marmara Sea near the Prince Island we distinguished two size groups from CIII to adult females and males with significantly differing prosome length in October 2000 and three size groups of C. euxinus in April 2005 (Table ).
In spring mean prosome lengths of the females and males from the first group (G1) amounted to 2.01±0.04 and 1.99±0.06 mm, respectively, and were significantly smaller (p<0.001) by 24.7% and 21.1% than those in C. euxinus from the Black Sea population, while mean prosome lengths in preadults, adult females and males from the G3 were similar to the Black Sea individuals. In autumn the smallest females (even with prosome length of 1.7 mm) were found in the Marmara Sea.
In Izmit Bay, prosome lengths in copepodite stages and adults of C. euxinus were close to CI, CII and CIII of the Black Sea population, whereas in CIV, CV and adults were significantly lower, especially in warm period.
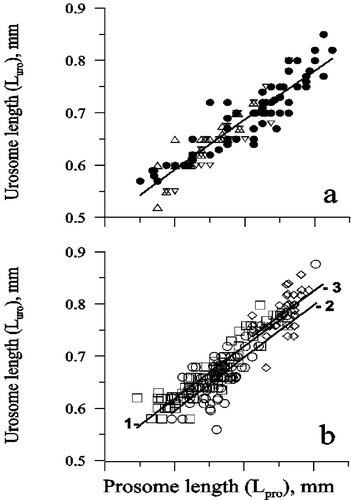
Urosome length (Luro, mm) in C. euxinus females collected in the Marmara and Black Seas during the winter–spring period and reared in the laboratory increased proportionally to prosome length (Lpro, mm) according to the equation Luro = 0.34 Lpro 0.82 (n = 143, r 2 = 0.86) (Figure ).
Figure 5 Urosome length plotted against prosome length in Calanus euxinus females (a) collected in the Black Sea (•) in deep and shallow water and reared in the laboratory, in the Marmara Sea (▵) and Izmit Bay (▿) during winter–spring period, and Calanus helgolandicus females (b) from the North Atlantic (□, 1), Mediterranean Sea (○, 2) and Black Sea (⋄, 3) according to the results of Fleminger and Hulsemann (Citation1987).
The diameters of eggs laid by small females (prosome length of 1.9–2.15 mm, collected in December 2006 in the Marmara Sea near the Prince Islands) and by large females (prosome length of 2.6–2.7 mm, collected in the same period in the Black Sea) were close (174.8±2.9 and 179.2±5.6 µm, respectively) (Table ). Minimum egg diameters of the females from the Black and Marmara Seas were also similar (∼168 µm), whilst maximum egg diameter in the Black Sea population (195 µm) was higher than that in C. euxinus sampled near the Prince Islands (182 µm).
Lipid reserves
In the Black Sea large lipid reserves were formed in C. euxinus CV (Table ). In comparison with CIII and CIV, oil sac volume (OSV, % of body volume) in CV increased dramatically (8–17 times) amounting to 15.7–17.4%. The OSV was lower in females (6.8–7.9%) utilizing lipids during formation of gonads, whereas in males lipid reserves constituted 12.2–15.8% of body volume.
Table II. Oil sac volume (% of body volume) in Calanus euxinus collected in the deep water near Sinop and in the south‐western part of the Black Sea during 2000–2005 and from the Marmara Sea near the Prince Islands (2000–2005) and in Izmit Bay (2001–2002). G1–G3 size groups, determined according to frequency distribution of the prosome length in different stages of Calanus euxinus.
In the Marmara Sea, near the Prince Islands, no lipid‐rich CV was found during spring in the smallest size group (G1). All sampled females either had no sac or its volume did not exceed ∼3% of body volume. On the other hand, larger CV (with body size similar to individuals in the Black Sea) and males had a relatively larger oil sac and the OSV changing from 12.0±6.0 to 11.0±5.9% in spring. In autumn, the OSV was small (3.5±1.9%) even in large CV. During the same seasons the OSV in the Marmara Sea females was found to be smaller than that in the Black Sea females.
The fattest CIII, CIV, CV and males (as in the Black Sea) were observed in Izmit Bay during the winter–spring period. However, the OSV of females in Izmit Bay were usually small (0.7–1.4%), similar to those in the north‐eastern Marmara Sea.
Discussion
Abundance of Calanus euxinus in the Black and Marmara Seas
Our study summarized the data obtained in different regions of the Black and Marmara Seas using Nansen net with the mouth opening diameter of 0.5 m but different mesh size (157, 200 and 210 µm). Nevertheless, during the analysis of our results we did not use correction coefficients for nets with different mesh sizes. Evans and Sell (Citation1985) who had studied collection characteristics of 50‐cm diameter conical plankton nets of 363, 156 and 76 µm mesh size concluded that the 156‐mesh net provided accurate estimates of microcrustacean zooplankton abundances except for nauplii. Ovie et al. (Citation2003) reported that the net with mesh size of 200 µm was effective for collecting microcrustacean zooplankton. Kitain et al. (Citation1995) showed that the ratio of zooplankton biomass derived with the plankton nets with mesh size 178 µm and 330–350 µm was equal to 1.46. During our study we used the nets with lower difference in mesh sizes so we did not have to take into account differing efficiency of these nets in comparison with total zooplankton sampling error.
In the Black Sea C. euxinus is abundant predominantly in deep regions. In the direction to the coast the biomass of this species reduces sharply (Arashkevich at al. Citation2002) due to decrease in the number of late developmental stages. The same tendency was observed at two stations near Sinop (Figure ).
Literature data on seasonal and inter‐annual dynamics of C. euxinus abundance in the Black Sea are fragmental and contradictory. Sazhina (Citation1987) studied the number of C. euxinus developmental stages near Sevastopol, collecting samples every 10 days from June 1965 to June 1966. Taking into account the number of peaks in abundance of this species, the author suggested that during the year C. euxinus can produce 7–8 generations. Nevertheless, according to Sazhina (Citation1987, Fig. 34), there are two pronounced periods of C. euxinus development beginning from the mass appearance of copepodite stages I in late spring and autumn and finishing with peaks of abundance of females and males in summer–autumn and winter–spring.
Vinogradov and Shushkina (Citation1992) reported high biomass (7.9 g m−2) of C. euxinus in summer 1978 and its lower value (5.0 g m−2) in winter and spring 1988 in the southern Black Sea. In autumn 1991 and 1992 the biomass of this species decreased to 1.8 and 1.1 g m−2, respectively. In August 1993 the biomass of C. euxinus varied in limits of 0.6–5.5 g m−2 (580–5600 ind m−2), whilst in November this parameter amounted to 4.3 and 2.2 g m−2 in the northern and southern regions of the Black Sea (Vinogradov et al. Citation1995), respectively. The authors suggested that predator ctenophore Mnemiopsis leidyi invasion in the Black Sea at the end of the 1980s which had undermined the abundance of many neritic copepods should cause a reduction the population density of C. euxinus as well.
According to Niermann et al. (Citation1998), during 1991–1995 in the southern and south‐western Black Sea, the mean biomass of C. euxinus varied in limits of 1.6–5.7 g m−2, with a minimum in July 1992 and a maximum in April 1994.
Zagorodnyaya et al. (Citation2001) observed minimum biomass of C. euxinus in deep regions near the Crimea coast in September 1994 and January 1995 (0.74 and 0.91 g m−2, respectively) and maximum biomass in April and August 1995 (6.68 and 9.78 g m−2, respectively). The latter values are close to the magnitudes of biomass for C. euxinus in the 1980s. After including the data of Gruzov et al. (Citation1994) in the analysis, Zagorodnyaya et al. (Citation2001) concluded that the prognosis about drastic decrease of C. euxinus abundance due to the press of Mnemiopsis leidyi (Vinogradov & Shushkina Citation1992) was not proved. On the contrary, Konsulov and Kamburska (Citation1997) found the trend of stable increase in the number of C. euxinus near the Bulgarian coast in 1991–1995. In the central regions of the Black Sea population density of C. euxinus was lower in summer and autumn 1992 (1.11–1.17 g m−2) (Zagorodnyaya & Skryabin Citation1995) and in November 1993 (1.1 g m−2) (Vinogradov et al. Citation1995). However, in October 1999 in the central part of the Black Sea, the biomass of C. euxinus (9.7 g m−2) was close to that in the north‐eastern deep shelf (Arashkevich et al. Citation2002).
During our study (2000–2005), the maximum abundance and biomass of C. euxinus in deep regions of the Black Sea near the north‐western Turkish coast were found in April 2003 (12,201 ind m−2, 61.0 ind m−3, 5.7 g m−2) and in May 2004 near Sinop (23,400 ind m−2, 130 ind m−3, ∼10 g m−2). Mean annual C. euxinus population density amounted to 30 ind m−3, which is higher than that for C. helgolandicus in the North Sea, close to that in the English Channel and Bay of Biscay, and lower than that near the coast of Spain and in the northern Adriatic (Bonnet et al. Citation2005).
According to the results of Tarkan et al. (Citation2005) and Svetlichny et al. (Citation2006), total zooplankton is less abundant in the Marmara Sea than in the Black Sea all the year round. The number of C. euxinus shows the same tendency. Yuksek et al. (Citation2003) found the decrease in C. euxinus abundance near the Marmara Sea exit of the Bosphorus Strait in December 1997–March 1998. In the Marmara Sea, spring seems to be the most favorable period for C. euxinus development.
Our results showed that mean annual abundance of C. euxinus in the Black Sea was 47 times higher than that in the north‐eastern Marmara Sea (during 2000–2007), and only 1.4 times higher than that in phytoplankton‐rich Izmit Bay.
Size, oil sac volume and molting patterns
According to Bonnet et al. (Citation2005), the mean values of prosome length of C. helgolandicus females in European waters changes from 1.94 mm in the Aegean Sea to 2.6 mm in the North Sea, with larger females at higher latitudes with lower temperature. These authors have stated that “If prosome length and temperature are correlated, this will be a result of the fact that as temperature increases, development time decreases and growth increases. However, development time decreases proportionally faster than growth increases, hence at warmer temperatures animals reach adulthood (or any other fixed stage) at a smaller size”.
In the Black and Marmara Seas, C. euxinus live under various combinations of physical and chemical parameters. Svetlichny et al. (Citation2006) suggested that successful development of C. euxinus in the Black Sea is due to low temperature and presence of the OMZ deeper than approximately 100 m.
During diel vertical migrations to the OMZ the Black Sea late copepodite stages of C. euxinus are affected by low temperature and oxygen concentration. As a result, the development is inhibited and longer duration of growth brings to upsizing (prosome length of 3.0 mm in females) and formation of large lipid reserves in the body. Extremely intensive lipid accumulation takes place during early development period in postmolt CV (Svetlichny et al. Citation2006); consequently, CV in this molting phase prevail in the Black Sea Calanus population (Figure ). Prosome lengths of copepodite stages and adults did not differ significantly from each other in warm and cold periods. Probably, it may be the result of C. euxinus development in deep layers of the Black Sea with low and stable temperatures (6.5–8.5°C) throughout the year.
On the contrary, warmer and more saline lower layers of the Marmara Sea accelerated the growth rates and development in Calanus, therefore, the size and oil sac volume of the individuals maturing in this region (especially in the warm period) were lower than those in the Black Sea. In the Marmara Sea CV were mainly intermolts and premolts. Prosome length in females collected in autumn near the Prince Islands amounted to 1.7 mm, these individuals possessed well‐developed gonads but have no oil sacs.
The presence of the individuals with transitional size in the Marmara Sea indicates that during the development C. euxinus are affected by different combinations of temperature and salinity. In the cold period, the size and lipid content of CV were similar to those in the Black Sea (Table ), particularly in Izmit Bay. During high chlorophyll‐a periods in February and March 2002 (Isinibilir et al. Citation2008), very dense populations of C. euxinus were observed with CV containing extremely large oil sacs. Postmolts were dominant in CV studied in this period, and this pattern should be associated with the hypoxic zone in Izmit Bay, similar to the Black Sea.
In warm periods, Calanus are very rare in the Marmara Sea in both the upper and lower layers. Therefore, we suggest that the winter–spring population of C. euxinus in the Marmara Sea originates from the females having been recruited from the Black Sea through the Bosphorus Strait. This assumption can be supported by close prosome lengths in early copepodite stages, the identity in egg mass densities (1.039±0.007 g cm−3, unpublished data) and similar ranges of egg diameters (168–182 µm) in the small Marmara Sea females and large Black Sea ones, being in accordance with the typical ranges reported for the Black Sea C. euxinus population (Sazhina Citation1987) and C. helgolandicus from the North Atlantic (Guisande & Harris Citation1995; Poulet et al. Citation1995).
The divergence in prosome lengths of Calanus populations in the Black and Marmara Seas can be observed only in CIII becoming more pronounced in late development stages (up to 25% in females).
The Black Sea population of C. helgolandicus has been allocated recently into separate species C. euxinus (Hulsemann Citation1991) basing on the difference in distribution of supernumerary pores on the second and third urosome segments of adult females as well as difference in the prosome to urosome length ratio between females of populations of C. euxinus and C. helgolandicus from the Mediterranean Sea and Atlantic Ocean (Fleminger & Hulsemann Citation1987).
According to these authors, the coefficients of linear regressions describing the relationship between prosome length (Lpr) and urosome length (Luro) in females from the Black Sea (Lpr = 1.513+1.399 Luro, r 2 = 0.34) and from the Atlantic and Mediterranean localities (Lpr = 0.364+2.787 Luro, r 2 = 0.69) were significantly different. We expressed the data of Fleminger and Hulsemann (Citation1987, Fig. 6) on C. euxinus and C. helgolandicus in the form of allometric equation y = axb plotting urosome length against prosome length due to frequent postmortal changes in urosome length. It was calculated that for C. euxinus females from the Black Sea Luro = 0.35Lpr 0.83 (r 2 = 0.36), and for C. helgolandicus females from the Mediterranean Sea and Atlantic Ocean Luro = 0.35Lpr 0.82 (r 2 = 0.85) and Luro = 0.33Lpr 0.87 (r 2 = 0.56), respectively (Figure ). These regression coefficients were close to each other and to regression coefficient of the equation Luro = 0.34Lpr 0.82 (r 2 = 0.86) which we obtained for C. euxinus females collected in the Black Sea and reared in the laboratory (Figure ).
Consequently, the prosome to urosome length ratio is not the criterion to distinguish the Black Sea population as a separate species. There are no other distinctive morphological features (Fleminger & Hulsemann Citation1987). At the same time, larger number of supernumerary pores on the urosome segments of the Black Sea females may be related to lower salinity (18‰) of this sea in comparison with the salinities of the Mediterranean Sea (up to 39‰) and Atlantic Ocean (∼35‰). Papadopoulos et al. (Citation2005) and Unal et al. (Citation2006) showed that genetic differences between these species are exceedingly subtle and typical for conspecific populations. Therefore, we suggest giving back the species name of Calanus helgolandicus to the Black Sea population adding var. euxinus. Knowledge of the reasons of development success of this species under the unique (high gradients of salinity, temperature and oxygen concentration) conditions of the Black and Marmara Seas will improve our understanding of ecology of Calanus helgolandicus (Bonnet et al. Citation2005).
In conclusion, in the Black Sea deep regions containing the cold intermediate layer and oxygen minimum zone Calanus euxinus are present during the whole year and their abundance, prosome length, lipid reserve amount are much higher than in the warm Marmara Sea where local populations of this species develop from the individuals penetrating through the Bosphorus only in cold seasons. However, in Izmit Bay with the hydrosulfide zone lying near the bottom and with the vertical profile of oxygen concentration as in the Black Sea, the condition of Calanus euxinus population in winter–spring is similar to that in the Black Sea. Since Calanus euxinus and Calanus helgolandicus have no significant morphological and genetic differences, we suggest giving back the species name of Calanus helgolandicus to the Black Sea population adding var. euxinus.
Acknowledgments
The present study was partly supported by a TUBITAK (Turkish Scientific Technical Research Council)‐NASU (National Academy of the Ukraine) joint project (107Y001) and a NATO Linkage Grant (EST NUKR CLG 983036); and the Research Found of the Istanbul University (T‐1121) and Ondokuz Mayıs University (S.090) and the Undersecretary of Organization of Planning of State (TAP‐S013). We appreciate the cooperation and help of the staff at the R/V “Bilim” and “Knorr” during the cruises. This study is a cooperating project of the Census of Marine Zooplankton (CMarZ), a field project of the Census of Marine Life.
References
- Algan , O. , Altıok , H. and Yüce , H. 1999 . Seasonal variation of suspended particulate matter in two‐layered Izmit Bay, Turkey. . Estuarine, Coastal and Shelf Science , 49 : 235 – 250 .
- Arashkevich , E. G. , Drits , A. V. , Musaeva , E. I. , Gagarin , V. I. and Sorokin , Yu P. 2002 . “ Mesozooplankton spatial distribution in relation to circulation pattern in the north‐eastern part of the Black Sea. ” . In Multi‐disciplinary investigations of the north‐east part of the Black Sea , Edited by: Zatsepin , A and Flint , M . 257 – 272 (in Russian) . Moscow : NAUKA .
- Arashkevich , E. G. , Tande , K. S. , Pasternak , A. F. and Ellertsen , B. 2004 . Seasonal moulting patterns and the generation cycle of Calanus finmarchicus in the NE Norwegian Sea, as inferred from gnathobase structures, and the size of gonads and oil sacs. . Marine Biology , 146 : 119 – 132 .
- Balkis , N. 2003 . The effect of Marmara (Izmit) earthquake on the chemical oceanography of Izmit Bay, Turkey. . Marine Pollution Bulletin , 46 : 865 – 878 .
- Besiktepe , S. T. , Sur , H. I. , Ozsoy , E. , Latif , M. A. , Oguz , T. and Unluata , U. 1994 . The circulation and hydrography of the Marmara Sea. . Progress in Oceanography , 34 : 285 – 334 .
- Bonnet , D. , Richardson , A. , Harris , R. , Hirst , A. , Beaugrand , G. , Edwards , M. , Ceballos , S. , Diekman , R. , Lopez‐Urrutia , A. Valdes , L. 2005 . An overview of Calanus helgolandicus ecology in European waters. . Progress in Oceanography , 65 : 1 – 53 .
- Evans , M. S. and Sell , D. W. 1985 . Mesh size and collection characteristics of 50‐cm diameter conical plankton nets Great Lakes Research Division, The University of Michigan, Ann Arbor, MI 48109, U.S.A. . Hydrobiologia , 122 : 97 – 104 .
- Fleminger , A. and Hulsemann , K. 1987 . Geographical variation in Calanus helgolandicus s.l. (Copepoda, Calanoida) and evidence of recent speciation of the Black Sea population. . Biological Oceanography , 5 : 43 – 81 .
- Gruzov , L. N. , Lyumkis , P. V. and Napadovsky , G. V. 1994 . “ Studies of space–time structure of planktonic fields of the northern Black Sea in 1992–1993. ” . In Studies of the Black Sea ecosystem , Edited by: Zaitsev , Yu P . 94 – 113 (in Russian) . Odessa : Iren‐poligraf .
- Guisande , C. and Harris , R. 1995 . Effect of total organic content of eggs on hatching success and naupliar survival in the copepod Calanus helgolandicus. . Limnology and Oceanography , 40 : 476 – 482 .
- Hulsemann , K. 1991 . Calanus euxinus, new name, a replacement name for Calanus ponticus Karavaev, 1894 (Copepoda: Calanoida). . Proceedings of the Biological Society of Washington , 104 : 620 – 621 .
- Isinibilir , M. , Kideys , A. E. , Tarkan , A. N. and Yilmaz , I. N. 2008 . Annual cycle of zooplankton abundance and species composition in Izmit Bay (the northeastern Marmara Sea). . Estuarine, coastal and shelf science , 78 : 739 – 747 .
- Johnson , C. L. 2004 . Seasonal variation in the moult status of an oceanic copepod. . Progress in Oceanography , 62 : 15 – 32 .
- Kitain , Ya V. , Rudyakov , Yu A. and Tseitlin , V. B. 1995 . Seston and zooplankton of the Bering Sea and the northwestern Pacific. . Oceanology, English Translation , 35 : 66 – 68 .
- Konsulov , A. and Kamburska , L. 1997 . “ Black Sea zooplankton structural dynamics and variability off the Bulgarian Black Sea coast during 1991–1995. ” . In NATO TU‐Black Sea project: Ecosystem modelling as a management tool for the Black Sea , Edited by: Ivanov , L and Oguz , T . 281 – 292 . Dordrecht : Kluwer Academic Publishers .
- Marker , T. , Andreassen , P. , Arashkevich , E. and Hansen , B. W. 2003 . Lipid deposition and sexual maturation in cohorts of Calanus finmarchicus (Gunnerus) originating from Bergen (60°N), and Tromsø (69°N) reared in Tromsø, Norway. . Marine Biology , 143 : 283 – 296 .
- Marshall , S. M. and Orr , A. P. 1972 . The biology of a marine copepod , 195 New York : Springer‐Verlag .
- Mauchline , J. 1998 . “ The biology of calanoid copepods. ” . In Advances in marine biology , Edited by: Blaxter , J. H. S , Southward , A. J and Tyler , P. A . 710 San Diego : Academic Press .
- Miller , C. B. , Cowles , T. J. , Wiebe , P. H. , Copley , N. J. and Grigg , H. 1991 . Phenology in Calanus finmarchicus; hypotheses about control mechanisms. . Marine Ecology Progress Series , 72 : 79 – 91 .
- Miller , C. B. and Nielsen , R. D. 1988 . Development and growth of large, calanid copepods in the ocean subarctic Pacific, May 1984. . Progress in Oceanography , 20 : 275 – 292 .
- Moraitou‐Apostolopoulou , M. 1985 . “ The zooplankton communities of the eastern Mediterranean (Levantine basin, Aegean Sea): Influence of man‐made factors. ” . In Mediterranean marine ecosystems , Edited by: Moraitou‐Apostolopoulou , M and Kiortsis , V . 303 – 331 . New York : Plenum Press .
- Morkoc , E. , Okay , S. O. , Tolun , L. , Tüfekçi , V. , Tüfekçi , H. and Legoviç , T. 2001 . Towards a clean Izmit Bay. . Environment International , 26 : 157 – 161 .
- Niermann , U. , Bingel , F. , Ergun , G. and Grive , W. 1998 . Fluctuation of dominant mesozooplankton species in the Black Sea, North Sea and the Baltic Sea: Is a general trend recognizable? . Turkish Journal of Zoology , 22 : 63 – 81 .
- Oguz , T. and Sur , H. I. 1986 . A numerical modeling study of circulation in the bay of Izmit: Final Report , 97 Kocaeli Turkey : TUBITAK‐MRC, Chemistry Department Publication, 187 .
- Ovie , S. I. , Azionu , B. C. , Ovie , S. O. and Adepoju , F. 2003 . Comparative evaluation of the effectiveness of some local fabrics for zooplankton harvest. . Journal of Applied Science Environmental Management , 7 : 57 – 60 .
- Papadopoulos , L. N. , Peijnenburg , K. T. C. A. and Luttikhuizen , P. C. 2005 . Phylogeography of the calanoid copepods Calanus helgolandicus and C. euxinus suggests Pleistocene divergences between Atlantic, Mediterranean, and Black Sea populations. . Marine Biology , 147 : 1353 – 1365 .
- Poulet , S. A. , Laabir , M. , Ianora , A. and Miralto , A. 1995 . Reproductive response of Calanus helgolandicus. I. Abnormal embryonic and naupliar development. . Marine Ecology Progress Series , 129 : 85 – 95 .
- Sargent , J. R. and McIntosh , R. 1974 . Studies on the mechanism of biosynthesis of wax esters in Euchaeta norvegica. . Marine Biology , 25 : 271 – 277 .
- Sazhina , L. I. 1987 . Reproduction, growth, productivity of marine Copepoda , 156 Kiev : Naukova Dumka . (in Russian)
- Svetlichny , L. S. 1983 . Calculation of the biomass of planktonic copepods using the coefficients of proportionality between volume and linear dimensions of the body. . Ehkologia Morya , 15 : 46 – 58 (in Russian) .
- Svetlichny , L. S. , Kideys , A. E. , Hubareva , E. S. , Besiktepe , S. and Isinibilir , M. 2006 . Development and lipid storage in Calanus euxinus from the Black and Marmara seas: Variabilities due to habitat conditions. . Journal of Marine System , 59 : 52 – 62 .
- Tarkan , A. N. , Isinibilir , M. and Tarkan , A. S. 2005 . Seasonal variations of the zooplankton composition and abundance in the Istanbul Strait. . Pakistan Journal of Biological Sciences , 8 : 1327 – 1336 .
- Unal , E. , Frost , B. W. , Armbrust , V. and Kideys , A. E. 2006 . Phylogeography of Calanus helgolandicus and the Black Sea copepod Calanus euxinus, with notes on Pseudocalanus elongatus (Copepoda, Calanoida). . Deep‐Sea Research II , 53 : 1911 – 1922 .
- Unluata , U. , Oguz , T. , Latif , M. A. and Ozsoy , E. 1990 . “ On the physical oceanography of the Turkish straits. ” . In The physical oceanography of sea straits , Edited by: Pratt , L. J . 25 – 60 . Dordrecht : Kluwer Academic Publishers .
- Vinogradov , M. E. , Arashkevich , E. G. and Ilchenko , S. V. 1992a . The ecology of the Calanus ponticus population in the deeper layer of its concentration in the Black Sea. . Journal of Plankton Research , 14 : 447 – 458 .
- Vinogradov , M. E. , Sapozhnikov , V. V. and Shushkina , E. A. 1992b . The Black Sea ecosystem , 112 Moskow : Nauka . (in Russian)
- Vinogradov , M. E. , Shiganova , T. A. and Khoroshilov , V. S. 1995 . The state of the main organisms in a plankton community in the Black Sea in 1993. . Oceanology , 35 : 387 – 391 .
- Vinogradov , M. E. and Shushkina , E. A. 1992 . Temporal changes in the zoocoenosis structure in the open regions of the Black Sea. . Oceanology , 32 : 709 – 717 (in Russian) .
- Yuksek , A. , Yilmaz , N. , Okus , E. , Uysal , Z. , Shmeleva , A. A. , Gubanova , A. D. , Altukhov , D. and Polat‐Beken , S. C. 2003 . “ Spatio‐temporal variations in zooplankton communities and influence of environmental factors on them in SW Black Sea and the Sea of Marmara. ” . In Oceanography of the Eastern Mediterranean and Black Sea: Similarities and differences of two interconnected basins , Edited by: Yilmaz , A . 774 – 784 . Ankara, , Turkey : TUBITAK Publishers .
- Yuneva , T. V. , Svetlichny , L. S. and Schepkina , A. M. 1997 . Lipid composition and locomotory activity of diapausing Calanus euxinus (Copepoda). . Hydrobiological Journal , 33 : 74 – 84 (in Russian) .
- Zagorodnyaya , J. A. , Kovalev , A. V. and Ostrovskaya , N. A. 2001 . Quantitative data and seasonal dynamics of Black Sea zooplankton near the Crimean cost in 1994–1995. . Ehkologia Morya , 55 : 17 – 22 (in Russian) .
- Zagorodnyaya , J. A. and Skryabin , V. A. 1995 . “ Modern tendency changes of zooplankton in offshore regions of the Black Sea. ” . In Investigations of the offshore zone of Azovo‐Black Sea basin , Edited by: Eremeev , V. N . 87 – 95 (in Russian) . Sevastopol : MGI NASUk .