Abstract
During the last few decades, Mediterranean epibenthic assemblages have been involved in mass mortality events, in which sea fans were one of the most threatened groups. Explants of the most common Mediterranean gorgonians (Paramuricea clavata, Eunicella cavolinii, E. singularis, and E. verrucosa, 40 explants from each species) were transplanted in the field in order to evaluate the response to transplantation, survival and growth rate of the cuttings during an annual cycle, from February 2003 to March 2004. Colonies of each species overcame transplantation stress and during the first three months they showed a mean survival rate of 98% and an average positive growth rate of 7.65%. In the summer season 2003, during the heatwave that affected the Mediterranean basin, the experimental cuttings suffered, showing a mean negative growth rate and a reduction of survival. At the end of the stress event, P. clavata revealed the worst recovery while all Eunicella species showed a good ability to recover. In particular, E. singularis evidenced the highest resilience among the species. The transplant method described here could be employed to try to recover sea fan populations in the precoralligenous and coralligenous communities in possible future projects of restocking, where anthropic activities (anchoring and/or fishing) and global warming are deeply compromising their survival. Moreover, a species‐specific capability to recover was identified after the stress event concomitant to the increase of the sea temperature.
Introduction
Benthic cnidarians are considered among the most efficient suspension feeders in the world's ocean in extracting and processing energy from water column (Gili & Coma Citation1998). For this reason, the wide variety of disturbances that affect this group should have a deep impact on the entire benthic assemblage (Cerrano et al. Citation2005; Garrabou et al. Citation2009). Some of these disturbances are due to human activities such as pollution, fishing and anchorage, and others are related to climate anomalies that lead to more frequent mass mortality episodes.
Since 1984, when Guzman and Cortes (Citation1984) reported the mortality of Gorgonia flabellum in the Caribbean due to an algal infection, several other episodes were described such as those in 1995, when both Gorgonia ventalina and G. flabellum were affected by the fungus Aspergillus sydowii (Nagelkerken et al. Citation1997), and in 1998, when Briareum asbestinum was damaged by the synergic action of high seawater temperature and a cyanobacterium belonging to the genus Scytonema (Harvell et al. Citation1999, Citation2001)
In the Mediterranean sea fans' mass mortalities, etiological agents have been found only recently (Bally & Garrabou Citation2007): Vibrio coralliilyticus has been considered one of the most likely agents of the diseases affecting Paramuricea clavata, while Vibrio splendidus affected the species Eunicella verrucosa in SW England (Hall‐Spencer et al. Citation2007) and it was also present among the strains isolated by Martin et al. (Citation2002) from diseased tissues of the gorgonians P. clavata and E. cavolinii.
The Ligurian Sea (NW Mediterranean) experienced an unusual warm summer and/or autumn in 1998 and 1999 (Cerrano et al. Citation2000; Perez et al. Citation2000) and from 2003 to 2006 summer seasons, with sponges, gorgonians and several other benthic species showing more or less abundant signs of necrosis (Cerrano et al. Citation2006; Coma et al. Citation2006; Rodolfo‐Metalpa et al. Citation2006; Scinto et al. Citation2007; Cupido et al. Citation2008). The negative impact of high seawater temperature on benthic organisms has also been highlighted by laboratory experiments. The Mediterranean stony coral Cladocora cespitosa suffered temperatures of 26–28°C, showing tissue necrosis after one month (Rodolfo‐Metalpa et al. Citation2006). These data, along with several other field observations, allowed the description of a possible future tropical scenario for the coastal seascape of the Mediterranean Sea (Bianchi Citation2007). Among the consequences is the decrease of the bathymetric upper limit of several boreal species, such as some sponge and gorgonian species.
This overview underlines the need to improve the knowledge about restoration techniques such as transplantation as a possible tool to rehabilitate degraded habitats such as the coralligenous community. Most of the studies focused on restoration of hard bottoms, explored the gardening and replenishment of degraded coral reefs trough scleractinian coral species transplantation (Clark & Edwards Citation1995; Yap et al. Citation1998; Epstein et al. Citation2001). Experiences on black corals suggested that transplants can have good success (Montgomery Citation2002). Only recently have restoration measures been applied to threatened gorgonian assemblages (Linares et al. Citation2008a).
In this article, we describe a field experiment conducted on explants of four common species of sea fans to evaluate the potential of explants in terms of growth, survival and regeneration after a stress event. Health conditions and growth rate were monitored for one year from winter 2003 to winter 2004 together with the water temperature. During the period studied, the Mediterranean experienced an important thermal anomaly (André et al. Citation2004), characterized by warm waters (24°C) reaching unusually 30 m depth (Schiaparelli et al. Citation2007; Scinto et al. Citation2007; Garrabou et al. Citation2009). The summer 2003 heatwave has been related to an anomalous atmospheric high‐pressure condition over western Europe together with exceptionally high air temperature and a lack of winds (Black et al. Citation2004; Schar et al. Citation2004).
The results allowed us to test experimentally species' amenability to transplantation and another critical aspect for survival of the transplant: the ability to regenerate lost tissue after a necrosis event.
Materials and methods
Study site and specimen collection
The study was carried out at Punta Faro in the Marine Protected Area of Portofino (Ligurian Sea, Italy, 44°17′54.54″N 9°13′08.23″E) where four species were tested: Paramuricea clavata, Eunicella cavolinii, E. singularis, and E. verrucosa. Eunicella cavolinii and E. singularis can live from 5 m depth, the former species preferring more vertical cliffs and the latter horizontal substrates, owing to its symbiosis with zooxanthellae that requires a good light exposition. Paramuricea clavata and E. verrucosa can live from 25 m depth, the former preferring hard substrates and the latter detritic bottoms. The distribution of these species partially overlaps in terms of bathymetric range, light and current conditions as occur in the selected area studied, where wide and sympatric populations of these sea fans are common.
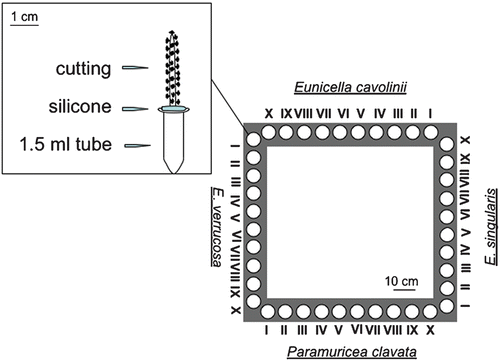
In January 2003, 40 cuttings of each species (8–10 cm long terminal branches for P. clavata and E. singularis, 4–5 cm for E. cavolinii and E. verrucosa) were collected from healthy adult colonies at 25–35 m depth by cutter blades. Only one branch was sampled per colony, to maximize the number of clones in the study. Immediately transported to aquaria, the 160 transplants were glued by silicone on 1.5‐ml plastic tubes (Figure ) and kept in running natural seawater for 2 days for acclimatization.
Field experiment
On February 2003 all colonies were placed at 25 m depth at Punta Faro.
Each set of transplants was mounted on four frames placed on a horizontal surface. In this way all the species were exposed to the same environmental conditions (e.g. water flow, temperature and irradiance). Each frame was 1‐m side and held 10 transplants of each species placed per side (Figure ).
Each colony was photographed with a digital camera (Sony DSC‐P5) using a scale bar (accuracy ±0.05 cm) in each photo for one year, until March 2004. Temperature data at 25 m depth, recorded using a Idronaut probe, were obtained from Schiaparelli et al. (Citation2007).
The digital images were analyzed by ImageJ software to obtain the size of the transplanted colonies as total length of living tissue on branches. For each colony, growth rate between subsequent measures was obtained as the relative size increment or decrement (Figure ).
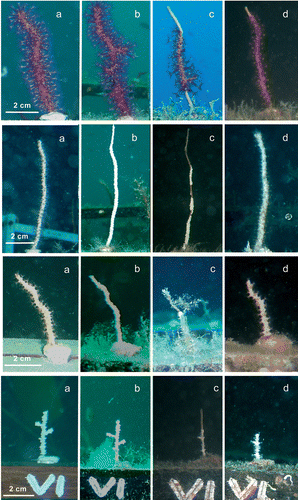
Figure 2 Four different stages of the experiment for each considered species: from the top, Paramuricea clavata, Eunicella singularis, E. cavolinii, and E. verrucosa. A, February; B, May; C, September; D, December. The colour version of this figure is available online.
For each frame, monthly survival rate was estimated as the ratio between the number of living colonies and the colonies transplanted at the beginning of the experiment.
When the coenenchyme revealed signs of damage, the healing rate was also measured.
Statistical analysis
Since the measurements taken on the same experimental unit tend to be correlated with each other, survival and monthly growth rate were analyzed using the one‐way repeated measure analysis of variance (RM‐ANOVA). In the analyses the time is the repeated measure (or within‐subjects) factor, while the species is the between‐groups factor (Winer Citation1971). Cochran's C test was used to check the assumption of homogeneity of variances and Student–Newman–Keuls (SNK) post hoc test was used for multiple comparisons within time and between species (Underwood Citation1997).
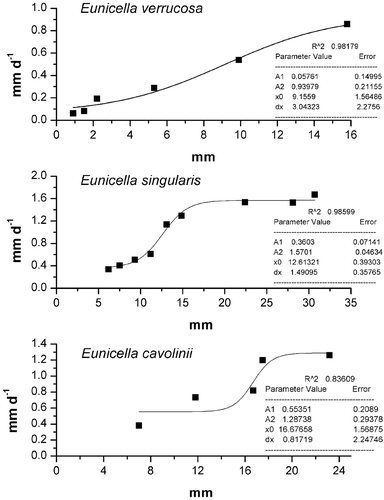
For each species the relationship between the size of wounds and the healing rate has been investigated by the logistic regression model according to the Boltzmann equation: y = A1+(A2–A1)/(1+e(x0–x)/dx) where A1 and A2 are, respectively, the lower and upper values, x0 is the curve center and dx is the curve slope. Data have been fitted using the Levemberg–Marquardt method (Aster et al. Citation2005).
Results
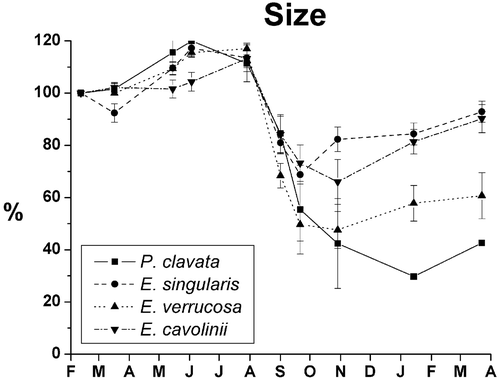
The transplantation procedures did not produce tissue necrosis on the colonies and polyps showed open tentacles 1 h after the positioning, suggesting good health of transplants. No colonies of any of the species studied died in the first three months (Figure ), suggesting a negligible transplant effect.
Figure 3 Survival and growth rate of the four species. The trend of the temperature is also represented.
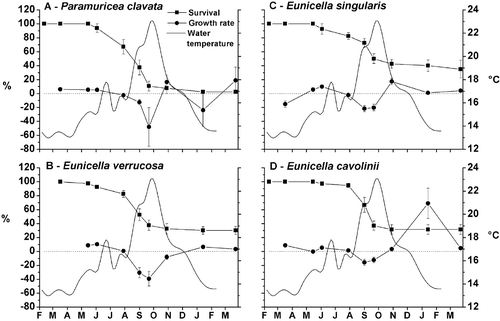
In July, when the water temperature reached 19°C, all the species suddenly experienced a high mortality rate, which increased until September. Regarding the percentage of survival, P. clavata was the most affected species (2.5%) (Figure ), while E. singularis (Figure ) was the most resistant (35%).
The widest variations in survival occurred from July to October, with important differences among species (RM‐ANOVA, P < 0.05). Paramuricea clavata had the lowest survival rate (SNK Test: P < 0.05) than the other species of Eunicella, with significant differences during the year (RM‐ANOVA: P < 0.001). This species showed three main phases of suffering: the first between June and July, the second between July and September and the last one in September–October (SNK Test: P < 0.01).
Significant differences were not detected in survival between the three species of Eunicella. Eunicella verrucosa (Figure ) and E. cavolinii (Figure ) suffered mainly in two periods from July to September (SNK Test: P < 0.01) and from September to October (SNK Test: P < 0.05), while E. singularis (Figure ) showed only one period of significant decrease (SNK Test: P < 0.01).
During the study period, significant variation in growth rate were observed for all the species (RM‐ANOVA: P < 0.001), in particular there was a decrease from August until September (SNK Test: P < 0.05) and an increase from September to November (SNK Test: P < 0.01). All the species showed negative values of growth rate with Paramuricea clavata showing the lowest one (−50% month−1) (Figure ).
The size trends of living colonies reflect that of survival (Figure ). After the transplantation all the species started to grow reaching in June–July an average dimension of about 120% of the initial size; then they showed a decrease of size, 90% in P. clavata, 60–70% in E. cavolinii and E. verrucosa and 50% in E. singularis (Figure ). From July, the size of the living colonies dramatically dropped to 30–60% of the original dimension. The size decrease continued until September in E. singularis, until October in E. cavolinii and E. verrucosa, and until January in P. clavata. During the winter, all the species started to grow again, showing an increase of the size due to a restoration of healthy conditions. During the period studied both survival and growth rate of transplants seemed to follow the water temperature trend inversely. The maximum decrease of these two features coincided with an unusual peak of 23–24°C at the end of September; afterwards, their increase took place jointly with a drop of temperature to 19°C in October and 13–14°C in November.
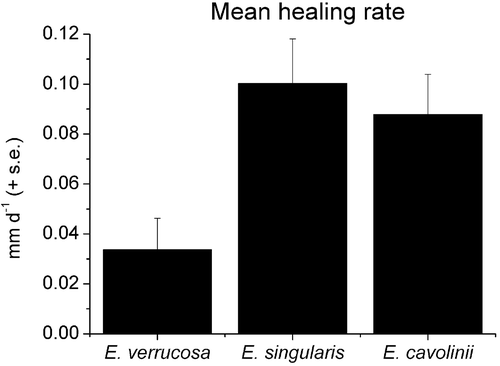
During the summer crisis several colonies showed signs of tissue necrosis, leaving the scleraxis without coenenchyme. In these cases the time required for tissue regeneration was measured, showing that, among explants, the genus Eunicella was able to recover better then Paramuricea after the thermal stress. The average daily healing rate ranged from a minimum of 0.03 mm d−1 in E. verrucosa to a maximum of 0.1 mm d−1 in E. singularis (Figure ).
Plotting the initial size of wounds vs. healing rates (Figure ), it is evident that regenerative capacity increases with the size of the injury, probably up to a maximum value, after which the rate of recovery remains steady in time. According to the Boltzmann equation as reported in the Materials and methods section, E. singularis showed the highest maximum estimated healing rate (A2 = 1.59±0.04se), while E. verrucosa showed the lowest (A2 = 0.93±0.21se).
Discussion
Shallow benthic suspension feeders are among the organisms most threatened by the warming of the Mediterranean Sea, and sea fans are one of the most damaged groups of the coralligenous community (Cerrano et al. Citation2000, Citation2005). Transplantation methods seem to be an effective tool to rehabilitate a population affected by local disturbances, but further research is necessary to better understand how to restore populations damaged by mass mortality events (Linares et al. Citation2008b).
Our results show that the four species tested are suitable for transplant experiments. In the first months after the transplantation and before the thermal stress event all the species showed a survival rate between 80 and 100%. This is in accordance with the results obtained after transplants of Paramuricea clavata by Bavestrello et al. (Citation1999) and Linares et al. (Citation2008b). The methodological approach described here could be employed to try to recover sea fan populations in the precoralligenous and coralligenous communities in possible future restocking projects, even if two critical points still need to be solved: the installation technique of transplants, in particular the attachment of explants to the substratum; and the role of environmental conditions, such as irradiance and water flow intensity.
Although the success of transplant methods was demonstrated by the high survival and growth rate until June, colonies were affected by an unexpected summer heatwave which occurred in the Mediterranean Sea (Garrabou et al. Citation2009). This event allowed comparison of species' resistance and resilience towards this stress, and to confirm the involvement of water temperature anomalies in the necrosis events of Mediterranean sea fans.
The rate of survival is strongly dependent by the period of the year, in fact, from June to July, all the species tested showed signs of suffering and started to die, while from October to November all the remaining colonies survived until the end of the experiment.
The transplant size trend shows that from July all the transplants started to suffer, showing loss of coenenchyme, until November, when the surviving colonies started to grow again. The species that showed the lowest loss of coenenchyme was E. singularis and E. cavolinii, which had the higher size at the end of summer with respect to those recorded at the beginning of the experiment.
As recently reviewed by Henry and Hart (Citation2005), the capacity for an injured modular organism to regenerate from wounds is size‐dependent: the bigger the colony, the faster the regeneration. This could simply be due to the higher amount of healthy tissue bordering the lesion available for regeneration in bigger colonies as reported for sponges (Wulff Citation2006) and hard corals (Meesters et al. Citation1996). In sea fans, there is a different organization: the generally low healing rate we recorded in this experiment can be related to the small dimensions of explants and/or to the general suffering of the colonies owing to thermal stress. In natural colonies, regeneration after mechanical damage is higher, as previous data recorded on Paramuricea clavata suggest (Bavestrello et al. Citation1997). Here we noticed that the bigger the lesion, the faster the recovery. Sanchez and Lasker (Citation2004) showed in Pseudopterogorgia bipinnata that the overcompensation in regenerative processes is related to the original branching network, suggesting a connection between colony organization and regeneration dynamics.
Our results can be better interpreted if we also consider the monthly growth rates for each species. The summer period leads to negative growth for all the species, especially for Paramuricea clavata, but during autumn and winter the growth rates return to positive values, even if somatic growth is often reduced in regenerating modular organisms (Duckworth Citation2003). This trend can be noted especially for E. singularis.
In this case, the use of transplants for comparative long‐term experiments is a good method considering that these species are characterized by a wide bathymetric range of distribution, and the results are not affected by a transplant effect, as experiments performed on corals also suggest (Baker Citation2001).
The actual distribution of sea fan populations on the Portofino Promontory confirms the data on resistance and resilience reported here: in several localities, the upper limit of P. clavata and E. cavolinii decreased to 5–6 m depth (Cerrano & Bavestrello Citation2008), and damaged colonies of E. verrucosa were recorded down to 40 m, although completely dead colonies have never been observed.
The case of E. singularis is the most peculiar. This species can also live very close to the surface, at 4–5 m depth. Since 1999, almost each summer, colonies show various extents of necrosis or completely die; however, the population is regularly renewed and lesions repaired, even if a progressive reduction of colony density occurs. A similar feature is also reported by Coma et al. (Citation2006) in Minorca, where a reduction in recruitment was documented. This high resilience may be related to the symbiosis with zooxanthellae that could help the affected colonies in two ways: (i) providing the oxygen and energy requirements to the host (Muscatine et al. Citation1984) during summer, a season generally characterized by hypoxic conditions and a trophic depletion in the water column (Coma & Ribes Citation2003); (ii) contributing to the healing process as described for the tropical Plexaurella fusifera (Meszaros & Bigger Citation1999).
In conclusion, sea fans' regression from their usual upper bathymetric level (Cerrano & Bavestrello Citation2008) could be a big threat for the dynamics of shallow hard bottom assemblages. The method described here could be a feasible approach for restoration and E. singularis seems to be the most successful species.
Acknowledgments
The authors are indebted to M. Pansini, F. Azzini, and C. Muti (DIPTERIS, University of Genova) for field assistance. M. Ponti (CIRSA, University of Bologna) provided useful suggestions during manuscript preparation. The research was partially supported by RAMOGE (“Alain Vatrican prize” to FF).
References
- André , J. C. , Déqué , M. , Rogel , P. and Planton , S. 2004 . La vague de chaleur de l'été 2003 et sa prévision saisonničre. . Comptes rendus Geoscience , 336 : 491 – 503 .
- Aster , R. C. , Borchers , B. and Thurber , C. H. 2005 . Parameter estimation and inverse problems , New York : Elsevier .
- Baker , A. C. 2001 . Ecosystems – Reef corals bleach to survive change. . Nature , 411 : 765 – 766 .
- Bally , M. and Garrabou , J. 2007 . Thermodependent bacterial pathogens and mass mortalities in temperate benthic communities: A new case of emerging disease linked to climate change. . Global Change Biology , 13 : 2078 – 2088 .
- Bavestrello , G. , Cattaneo‐Vietti , R. , Cerrano , C. , Lanza , S. , Maccarone , M. , Magnino , G. , Sarà , A. and Pronzato , R. 1999 . Distribuzione dei popolamenti di gorgonie dell'Isola di Ustica. . Biologia Marina Mediterranea , 6 : 237 – 239 .
- Bavestrello , G. , Cerrano , C. , Zanzi , D. and Cattaneovietti , R. 1997 . Damage by fishing activities in the gorgonian coral Paramuricea clavata in the Ligurian Sea. . Aquatic Conservation , 7 : 253 – 262 .
- Bianchi , C. N. 2007 . Biodiversity issues for the forthcoming tropical Mediterranean Sea. . Hydrobiologia , 580 : 7 – 21 .
- Black , E. , Blackburn , M. , Harrison , G. , Hoskins , B. and Methven , J. 2004 . Factors contributing to the summer 2003 European heatwave. . Weather , 59 : 217 – 223 .
- Cerrano , C. , Arillo , A. , Azzini , F. , Calcinai , B. , Castellano , L. , Muti , C. , Valisano , L. , Zega , G. and Bavestrello , G. 2005 . Gorgonian population recovery after a mass mortality event. . Aquatic Conservation , 15 : 147 – 157 .
- Cerrano , C. and Bavestrello , G. 2008 . Medium‐term effects of die‐off of rocky benthos in the Ligurian Sea. What can we learn from gorgonians? . Chemistry and Ecology , 24 : 73 – 82 .
- Cerrano , C. , Bavestrello , G. , Bianchi , C. N. , Cattaneo‐Vietti , R. , Bava , S. Morganti , C. 2000 . A catastrophic mass‐mortality episode of gorgonians and other organisms in the Ligurian Sea (Northwestern Mediterranean), summer 1999. . Ecology Letters , 3 : 284 – 293 .
- Cerrano , C. , Totti , C. , Sponga , F. and Bavestrello , G. 2006 . Summer disease in Parazoanthus axinellae (Schmidt, 1862) (Cnidaria, Zoanthidea). . Italian Journal of Zoology , 73 : 355 – 361 .
- Clark , S. and Edwards , A. J. 1995 . Coral transplantation as an aid to reef rehabilitation: Evaluation of a case study in the Maldive islands. . Coral Reefs , 14 : 201 – 213 .
- Coma , R. , Linares , C. , Ribes , M. , Diaz , D. , Garrabou , J. and Ballesteros , E. 2006 . Consequences of a mass mortality in populations of Eunicella singularis (Cnidaria: Octocorallia) in Menorca (NW Mediterranean). . Marine Ecology Progress Series , 327 : 51 – 60 .
- Coma , R. and Ribes , M. 2003 . Seasonal energetic constraints in Mediterranean benthic suspension feeders: Effects at different levels of ecological organization. . Oikos , 101 : 205 – 215 .
- Cupido , R. , Cocito , S. , Sgorbini , S. , Bordone , A. and Santangelo , G. 2008 . Response of a gorgonian (Paramuricea clavata) population to mortality events: Recovery or loss? . Aquatic Conservation , 18 : 984 – 992 .
- Duckworth , A. R. 2003 . Effect of wound size on the growth and regeneration of two temperate subtidal sponges. . Journal of Experimental Marine Biology and Ecology , 287 : 139 – 153 .
- Epstein , N. , Bak , R. P. M. and Rinkevich , B. 2001 . Strategies for gardening denuded coral reef areas: The applicability of using different types of coral material for reef restoration. . Restoration Ecology , 9 : 432 – 442 .
- Garrabou , J. , Coma , R. , Bensoussan , N. , Bally , M. , Chevaldonné , P. Cigliano , M. 2009 . A new large scale mass mortality event in the NW Mediterranean rocky benthic communities: Effects of the 2003 heatwave. . Global Change Biology , 15 : 1090 – 1103 .
- Gili , J. M. and Coma , R. 1998 . Benthic suspension feeders: Their paramount role in littoral marine food webs. . Trends in Ecology and Evolution , 13 : 316 – 321 .
- Guzman , H. M. and Cortes , J. 1984 . Mass death of Gorgonia flabellum L. (Octocorallia: Gorgonidae) on the Carribean coast of Costa Rica. . Revista de Biología Tropical , 32 : 305 – 308 .
- Hall‐Spencer , J. M. , Pike , J. and Munn , C. B. 2007 . Diseases affect cold‐water corals too: Eunicella verrucosa (Cnidaria: Gorgonacea) necrosis in SW England. . Disease of Aquatic Organisms , 76 : 87 – 97 .
- Harvell , C. D. , Kim , K. , Burkholder , J. M. , Colwell , R. R. , Epstein , P. R. Grimes , D. J. 1999 . Review: Marine ecology – Emerging marine diseases – Climate links and anthropogenic factors. . Science , 285 : 1505 – 1510 .
- Harvell , D. , Kim , K. , Quirolo , C. , Weir , J. and Smith , G. 2001 . Coral bleaching and disease: Contributors to 1998 mass mortality in Briareum asbestinum (Octocorallia, Gorgonacea). . Hydrobiologia , 460 : 97 – 104 .
- Henry , L. A. and Hart , M. 2005 . Regeneration from injury and resource allocation in sponges and corals – A review. . International Review of Hydrobiology , 90 : 125 – 158 .
- Linares , C. , Coma , R. , Garrabou , J. , Diaz , D. and Zabala , M. 2008a . Size distribution, density and disturbance in two Mediterranean gorgonians: Paramuricea clavata and Eunicella singularis. . Journal of Applied Ecology , 45 : 688 – 699 .
- Linares , C. , Coma , R. and Zabala , M. 2008b . Restoration of threatened red gorgonians populations: An experimental and modelling approch. . Biological Conservation , 141 : 427 – 437 .
- Martin , Y. , Bonnefort , J. L. and Chancerelle , L. 2002 . Gorgonians mass mortality during the 1999 late summer in French Mediterranean coastal waters: The bacterial hypothesis. . Water Research , 36 : 779 – 782 .
- Meesters , E. H. , Wesseling , I. and Bak , R. P. M. 1996 . Partial mortality in three species of reef‐building coral and the relation with colony morphology. . Bulletin of Marine Science , 58 : 838 – 852 .
- Meszaros , A. and Bigger , C. 1999 . Qualitative and quantitative study of wound healing processes in the coelenterate, Plexaurella fusifera: Spatial, temporal, and environmental (light attenuation) influences. . Journal of Invertebrate Pathology , 73 : 321 – 331 .
- Montgomery , A. D. 2002 . The feasibility of transplanting black coral (Order Antipatharia). . Hydrobiologia , 471 : 157 – 164 .
- Muscatine , L. , Falkowski , P. , Porter , J. and Dubinsky , Z. 1984 . Fate of photosynthetic fixed carbon in light and shade adapted colonies of the symbiotic coral Stylophora pistillata. . Proceedings of the Royal Society, series B, Biological Sciences , 222 : 181 – 202 .
- Nagelkerken , I. , Buchan , K. , Smith , G. W. , Bonair , K. , Bush , P. Garzon‐Ferreira , J. 1997 . Widespread disease in Caribbean sea fans: II. Patterns of infection and tissue loss. . Marine Ecology Progress Series , 160 : 255 – 263 .
- Perez , T. , Garrabou , J. , Sartoretto , S. , Harmelin , J. G. , Francour , P. and Vacelet , J. 2000 . Mass mortality of marine invertebrates: An unprecedented event in the Northwestern Mediterranean. . Comptes Rendus de l'Académie des Sciences – Séries III – Sciences de la Vie , 323 : 853 – 865 .
- Rodolfo‐Metalpa , R. , Richard , C. , Allemand , D. and Ferrier‐Pages , C. 2006 . Growth and photosynthesis of two Mediterranean corals, Cladocora caespitosa and Oculina patagonica, under normal and elevated temperatures. . Journal of Experimental Biology , 209 : 4546 – 4556 .
- Sanchez , J. A. and Lasker , H. R. 2004 . Do multi‐branched colonial organisms exceed normal growth after partial mortality? . Proceedings of the Royal Society, series B, Biological Sciences , 271 : S117 – S120 .
- Schar , C. , Vidale , P. L. , Luthi , D. , Frei , C. , Haberli , C. , Liniger , M. A. and Appenzeller , C. 2004 . The role of increasing temperature variability in European summer heatwaves. . Nature , 427 : 332 – 336 .
- Schiaparelli , S. , Castellano , M. , Povero , P. , Sartoni , G. and Cattaneo‐Vietti , R. 2007 . A benthic mucilage event in North‐Western Mediterranean Sea and its possible relationships with the summer 2003 European heatwave: Short term effects on littoral rocky assemblages. . Marine Ecology – An Evolutionary Perspective , 28 : 1 – 13 .
- Scinto , A. , Benvenuto , C. , Cerrano , C. and Mori , M. 2007 . Seasonal cycle of Jassa marmorata Holmes, 1903 (Crustacea, Amphipoda) in the Ligurian Sea (Mediterranean Sea, Italy). . Journal of Crustacean Biology , 27 : 212 – 216 .
- Underwood , A. J. 1997 . Experiments in ecology , Cambridge : Cambridge University Press .
- Winer , B. J. 1971 . Statistical principles in experimental designs , Kogakusha, Tokyo : McGraw‐Hill . 2nd ed.
- Wulff , J. 2006 . A simple model of growth form‐dependent recovery from disease in coral reef sponges, and implications for monitoring. . Coral Reefs , 25 : 419 – 426 .
- Yap , H. T. , Alvarez , R. M. , Custodio III , H. M. and Dizon , R. M. 1998 . Physiological and ecological aspects of coral transplantation. . Journal of Experimental Marine Biology and Ecology , 229 : 69 – 84 .