Abstract
The bdelloid rotifers represent an intriguing example of organisms displaying an array of unusual ecological and evolutionary features, yet have managed to remain an extremely successful group for more than 35 million years. Some of these unusual features include: strictly parthenogenetic reproduction, degenerate tetraploidy, horizontal gene transfer, and resistance to desiccation, starvation and ionising radiation. This review emphasises these as well as other ecological and evolutionary features of bdelloids, highlighting the current knowledge regarding the patterns and processes governing these organisms. We suggest a unifying framework, with dormancy representing the bdelloids' key feature. We hypothesise that dormancy, and especially the DNA repair mechanisms activated during dormancy recovery, might be responsible for all the unusual features present in the taxon. We propose further work that needs to be performed to test this hypothesis, and recommend further research areas that will help to unravel this “evolutionary scandal”.
Bdelloid rotifers have notoriously been termed “evolutionary scandals” by Maynard Smith (Citation1986), as they have survived and speciated in the absence of sex. The Bdelloidea is the largest, oldest and most diverse multicellular taxon, for which there is compelling morphological, cytological, and molecular evidence for long‐term asexual evolution. The most obvious atypical feature of the bdelloids is their obligate parthenogenesis; however, there are a range of other unusual features in these microscopic animals that make them rather peculiar. Possibly the most unusual feature they display is the remarkable ability to enter metabolic stasis in response to environmental stress, such as desiccation. Desiccated bdelloids can withstand a range of extreme stresses, including very low temperatures, high pressures and ionising radiation. Incredibly they can recover after weeks or months of stasis with no apparent interruption in their lifespan. Recent research indicates that, in some species, mothers that have recovered after desiccation produce daughters of increased fitness, suggesting that some repair processes may be acting during the time of recovery from desiccation, and that this may have a beneficial effect on traits other than just desiccation resistance. Potentially these repair mechanisms may be at the basis of the process that have allowed bdelloids to abandon sexuality, to perform horizontal gene transfer, and to resist exposure to ionising radiation. Moreover, another key factor for their success is that dormant bdelloids can act as propagules, allowing them to be passively dispersed around the globe, producing widespread cosmopolitan populations with enormous numbers of individuals. Here we review the recent literature on the ecological and evolutionary features of bdelloids, summarising the state of the art, which suggests that, rather than a scandal, bdelloids represent a triumph in parthenogenetic evolution. We also suggest further directions of research which need pursuing to understand how this “evolutionary scandal” has managed to persist through evolutionary time.
What is a bdelloid?
Bdelloids are microscopic aquatic metazoans, characterised by an elongate soft body with a superficial subdivision in the head, trunk and foot (Figure ). The head and foot are retracted in the trunk in the characteristic “inching” or “looping” motion of bdelloids on a substrate (and thus their name, from the Greek βδελλα for “leech”). Minute in size (usually much less than 1 mm), with a more or less slender soft body, the “bauplan” of the bdelloids is rather conservative. They have pseudosegments on both cephalic and caudal extremities, trophi (hard jaws) of “ramate” type (Figure ), and head with ciliated corona (Wallace et al. Citation2006). The ciliated corona can follow one of three patterns (Melone & Ricci Citation1995): either arranged into two ciliated discs, the trochi, which can be used for filter‐feeding and for swimming (order Philodinida); shaped as a ventral ciliated field (order Adinetida); or restricted to a rudimentary ciliated field (order Philodinavida). The shape of bdelloid trophi varies very little, but the number of teeth can vary significantly (Melone et al. Citation1998; Fontaneto et al. Citation2004; Melone & Fontaneto Citation2005). Body plan, including trophi shape and corona organisation, seems rather invariant in the whole bdelloid group, and, surprisingly, does not vary even when comparing the different feeding strategies of microphagy and macrophagy (Ricci et al. Citation2001).
Figure 1 Scanning Electron Microscopy pictures of a bdelloid rotifer(Rotaria macrura). a, ventral view; b, jaws (trophi). F, foot; H, head; T, trunk. (Photo courtesy of Giulio Melone.) Scale bars: a, 100 µm; b, 10 µm.
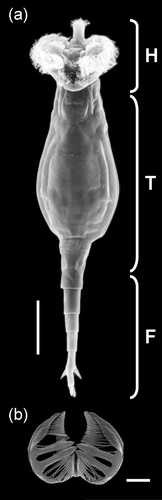
Although bdelloids contain only roughly 1000 nuclei, they possess neurons, musculature, a complete digestive system, and complex paired gonads, each made of an ovary (germarium) embedded in a syncytial nurse gland, the vitellarium (Amsellem & Ricci Citation1982). Bdelloid ovaries contain a specific number of primary oocytes that can develop into eggs (Gilbert Citation1983; Clement & Wurdak Citation1991; Pagani et al. Citation1993). According to Hsu (Citation1956a, Citationb), oocytes develop after two mitotic divisions of the mother cell, each division giving rise to a polar body. However, chromosome pairing or reduction has never been detected. There is both genetic (Mark Welch et al. Citation2004a) and cytogenetic (Mark Welch et al. Citation2004b) evidence to suggest that sexual reproduction was abandoned in an ancient ancestor of bdelloids in favour of obligate parthenogenesis. Most species lay unsegmented eggs, although a few are ovoviviparous. From the unfertilised, parthenogenetic egg a juvenile develops through spiral‐like cleavage, hatching and growing into an adult without additional cell division (Zelinka Citation1891; Remane Citation1929; Gilbert Citation1989; Boschetti et al. Citation2005). Adults live for 30–40 days under laboratory conditions, during which time all or nearly all primary oocytes are utilised (Pagani et al. Citation1993). A relatively long period of senescence is often present in experimental cultures (Ricci Citation1984), and senescent animals, recognisable because of a swollen body and whitish glands, can be occasionally found in nature. In the species that have been studied, the number of chromosomes in a metaphase embryo cell ranges between 8 and 16 (Hsu Citation1956a, Citationb; Mark Welch & Meselson Citation1998b; Pouchkina‐Stantcheva et al. Citation2007). They seem to be degenerate tetraploids (Mark Welch et al. Citation2008), with tetraploidy established before families diverged (Hur et al. Citation2009). The genomic DNA content was thought to be approximately 1000 Mbp (Pagani et al. Citation1993; Mark Welch & Meselson Citation1998a; Mark Welch & Meselson Citation2003), but more recent research suggests bdelloid genomes are less than 200 Mbp (R. Gregory, personal communication cited in Gladyshev & Meselson Citation2008). This is very much in the range of genome sizes which are now easily achievable using modern sequencing techniques. Indeed, at least two bdelloid genome sequencing projects will begin shortly, which will enhance our understanding of these enigmatic creatures enormously.
Bdelloids appear to have existed for at least 35–40 million years, the age of the oldest amber in which bdelloid remains have been identified (Poinar & Ricci Citation1992). Presently, they can be found all around the world in freshwater lakes, ponds, and streams, and in moist terrestrial habitats such as moss, lichen, tree bark, soil and detritus (Bartoš Citation1951; Donner Citation1965; Schmid‐Araya Citation1998; Linhart et al. Citation2002; Wallace & Ricci Citation2002; Devetter Citation2007). Adults of many species survive freezing, and are abundant even in Antarctica and alpine habitats above 4000 m (Sohlenius & Bostrom Citation2005; Fontaneto & Ricci Citation2006). Surprisingly, the marine habitats host very few species of bdelloids (Fontaneto et al. Citation2006a). Consumers of bacteria, unicellular fungi, and algae, they are themselves consumed by oligochaetes, crustaceans, fish fry, and insect larvae, often constituting a relevant part of the biomass of their trophic level (Ricci Citation1984; Linhart et al. Citation2002; Reiss & Schmid‐Araya Citation2008).
There are over 450 described bdelloid species, classified into 18 genera and 4 families (Donner Citation1965; Segers Citation2007). Taxonomic keys to genera are available in English (Koste & Shiel Citation1986; Turner Citation1999; Ricci & Melone Citation2000; Fontaneto & Ricci Citation2004), while the best key for species identification is written in German by Donner (Citation1965). Bartoš (Citation1951) wrote a key in English; however, it is not always reliable and is limited to the fauna of the Czech Republic.
Cladistic and molecular evidence have not yet resolved the phylogenetic relationship within Rotifera, the closest relatives of bdelloids may be either monogononts or seisonids and acanthocephalans (Melone et al. Citation1998; Garcia Varela et al. Citation2000; Mark Welch Citation2000, Citation2005; Herlyn et al. Citation2003; Funch et al. Citation2005; Sørensen & Giribet Citation2006; Witek et al. Citation2008). More work needs to be carried out in this field to more accurately resolve phylogenetic relationships, both within Rotifera and among other taxa within Platyzoa, Gnathostomulida, Cycliophora, Acanthocephala, Gastrotricha and Platyhelminthes (Giribet et al. Citation2000; Funch et al. Citation2005; Passamaneck & Halanych Citation2006; Dunn et al. Citation2008). A better understanding of such relationships can be achieved by adding more genes and morphological markers to the phylogenetic analyses, increasing the number of species utilised in analysis, using slowly evolving species, and choosing better models of sequence evolution (Baurain et al. Citation2007). Recently developed techniques of large‐scale DNA sequencing such as EST or pyrosequencing (Ronaghi Citation2001; Philippe & Telford Citation2006; Brinkmann & Philippe Citation2008) may change our view of the relationships within Platyzoa.
Dormancy: The most distinctive unusual feature of bdelloids
Bdelloids, like other freshwater invertebrates, are capable of surviving conditions that are incompatible with active life by halting activity, lowering metabolism to undetectable levels, remaining like a “living dead”, but still being capable of resuming activity when conditions become favourable again. This capacity of a reversible life suspension is to be considered an extreme form of dormancy. As an example for this phenomenon, most animals inhabiting terrestrial mosses, lichens or soils that dry out totally from time to time respond to drought by desiccating themselves and entering a stage of life suspension. Remarkably, they are able to reabsorb water when available, rehydrating the tissues and cells, regaining motility and, finally, reproduction. Commonly, dormancy in freshwater habitats is cued by water evaporation, and is termed anhydrobiosis (Crowe Citation1971; Crowe et al. Citation1992). Also bdelloids that inhabit mosses and lichens resist habitat desiccation by becoming anhydrobionts. Actually, most bdelloid species are capable of anhydrobiosis, and only few, scattered among the four bdelloid families, are desiccation‐sensitive and seem incapable of anhydrobiosis. Since bdelloids are monophyletic, it is more parsimonious to hypothesise that anhydrobiotic capability is likely apomorphic to the whole taxon, inherited from a common ancestor, and the few species that do not survive desiccation have lost this capacity secondarily (Ricci Citation1998).
Anhydrobiosis is not the only form of dormancy among bdelloids, recently some species have been found to survive prolonged starvation by suspending activity and possibly, reducing metabolic expenditure as well (Ricci & Perletti Citation2006). Under dormancy, starved bdelloids have been found to survive periods that are longer than their regular lifespan, i.e. 30 days. In fact, starvation lasting 40 days was found to be tolerated by about 60% of the tested individuals, all of which resumed reproduction a few days after being regularly fed. Translating this capacity into human terms, it would be the equivalent of finding 60 persons out of 100 that can survive 100 years without any food, and that, when fed again, can live and reproduce as if the starvation had never occurred to them. Also, not only should they survive, but the time spent in starvation or in anhydrobiosis is totally disregarded and not included into their age. This seems to be a trait peculiar to bdelloids and not shared by other animals capable of dormancy.
In fact, nematodes, that resist desiccation surviving in high percentages, do not survive starvation (Ricci et al. Citation2005), and when anhydrobiotic seem to age (Ricci & Pagani Citation1997). In contrast, bdelloid rotifers arrest ageing during dormancy and resume “counting” time when dormancy is broken. This latter condition has been termed the “sleeping beauty” behaviour that consists in deleting the duration of dormancy from age. The nematode behaviour, in contrast, has been termed “The Picture of Dorian Gray” condition, since they appear to age in the absence of activity. This is detectable when age‐specific traits are analysed (Ricci et al. Citation1987; Ricci & Pagani Citation1997; Ricci Citation2001; Ricci & Covino 2004; Ricci & Caprioli Citation2005; Ricci & Perletti Citation2006). A recent study on another group of anhydrobiotic micrometazoans, the tardigrades, has shown that this taxon, as well, behaves like a sleeping beauty (Hengherr et al. Citation2008).
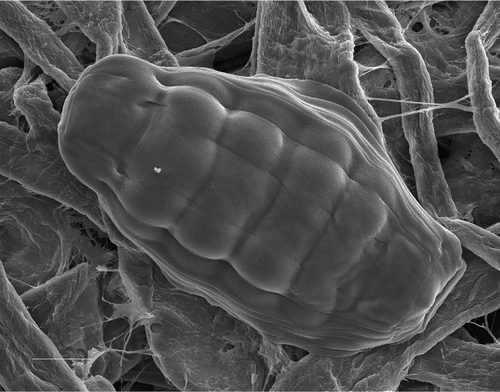
On entering anhydrobiosis, organisms undergo a series of biochemical, physiological and morphological changes. Most of them synthesise disaccharides as osmoprotectant molecules before suspending active life (Crowe Citation1971; Westh & Ramløv Citation1991; Higa & Womersley Citation1993; Clegg Citation2001). In contrast, bdelloid rotifers lack disaccharides as osmoprotectant substances (Lapinski & Tunnacliffe Citation2003; Caprioli et al. Citation2004), and synthesise a protein, LEA (Late Embryogenesis Abundant protein) (Tunnacliffe et al. Citation2005; Pouchkina‐Stantcheva et al. Citation2007), that seems to work as a “molecular shield” (Browne et al. Citation2002; Wise & Tunnacliffe Citation2004; Chakrabortee et al. Citation2007). The most conspicuous modification of desiccating animals is the change of body shape and the reduction of body volume. Bdelloids contract longitudinal muscles, retract head and foot into the trunk (Figure ) and form a compact shape called “tun” (Wright Citation2001; Ricci et al. Citation2003). When desiccated, bdelloids reduce their volume by roughly 60%, and modify internal organisation remarkably (Ricci et al. Citation2008). Tissues, cells and organelles condense, and membranes fold into “myelin figures” (Dickson & Mercer Citation1967; Wharton & Lemmon Citation1998). After this, the ultrastructure of bdelloid tissues changes at the level of all cells, with new junctions established that last as long as the desiccation period (Marotta et al. Citation2008). Internal modifications of the ultrastructure also occur in the starved bdelloids, in which secretory tissues become rich in vesicles (R. Marotta et al. unpublished).
Figure 2 Scanning Electron Microscopy picture of an anhydrobiotic bdelloid rotifer(Macrotrachela quadricornifera) on filter paper, contracted in the typical “tun” shape. (Photo courtesy of Giulio Melone.) Scale bar: 20 µm.
It seems intuitive that, given the severe stresses bdelloids are able to withstand through dormancy, not every individual from the original population will survive. Indeed, the major cost of dormancy is paid in terms of mortality. As not all bdelloids entering dormancy are expected to recover, the fate of the population originating from the survivors stands on their reproductive opportunities. In addition, dormant bdelloids can easily be transported passively and, if successful, become the founders of a new population. Under these restrictive conditions, uniparental reproduction seems the more secure reproductive strategy: no risk of low encounter probability between mates, even a single animal is capable of producing a new population (Ricci Citation1992).
It has been suggested that the unusual ecology of bdelloids, that through dormancy can cause frequent DNA damage and need for repair, may have facilitated adaptations that favoured their long‐term evolutionary survival in the absence of sexual reproduction and of recombination (Dolgin & Charlesworth Citation2006). Evidence for a DNA repair mechanism has been demonstrated by Gladyshev and Meselson (Citation2008), who showed double strand repair occurs in bdelloids after extensive DNA breakage caused by artificial ionising radiation. Recent experimental results also indicate that, for some bdelloid species, mothers that have been through desiccation produce daughters of increased fitness and longevity, suggesting the existence of some repair processes associated with recovery from desiccation which may have a beneficial effect beyond surviving desiccation (Ricci & Covino Citation2005). Furthermore, laboratory experiments have shown that the fitness of bdelloids declines if populations are maintained hydrated for several generations compared to populations that are cyclically desiccated (Ricci et al. Citation2007). This fact is surprising, since it is normally counter‐intuitive to think of desiccation as a positive factor for an organism. Thus, bdelloids are not only efficient in tolerating desiccation, but seem somehow dependent on anhydrobiosis, a circumstance that might represent a beneficial event in their life cycle. If this is true, life in unpredictable habitats should not be seen as the result of competitive exclusion from “easier” habitats, but a requirement for the long‐term survival of these parthenogenetic animals.
The precise mechanism that is capable of restoring fitness after dormancy is still to be determined. However, we can assert that whatever mechanism is responsible, its nature may be epigenetic, because if a fraction of the permanently “hydrated” populations is desiccated only once, it resumes high fitness traits, similar to those of the cyclically desiccated lines (C. Ricci, unpublished). Thus the “mechanism” might be related to some repair system that is activated at recovery.
Genomic consequences of dormancy
It is beyond the aims of this review to provide a detailed account of the prediction of the loss of sexual recombination on genomes due to repair mechanisms acting during recovery from dormancy. Moreover, recent reviews oriented on genetics aspects of bdelloids have been already published. We suggest Normark et al. (Citation2003) for a detailed review of the predicted genomic effects of absence of sexual recombination, and Hillis (Citation2007) and Rice and Friberg (Citation2007) for comments and suggestions on the present state of knowledge for bdelloid genomics. Most of the predicted long‐term effects on genomes because of loss of sex and recombination have been actually found in bdelloids, such as: (1) congruent divergence of alleles at one locus across species (Mark Welch & Meselson Citation2000), (2) inefficient selection against deleterious mutations (Barraclough et al. Citation2007), and (3) loss of activity of most transposable elements (Arkhipova & Meselson Citation2000, but see Arkhipova & Meselson Citation2005 and Gladyshev et al. Citation2007 for more recent contrasting results). One important aspect that has been predicted from theory but has yet to be confirmed is whether the gene trees for any locus show the same topology. In sexual organisms each gene is known to undergo a different coalescent process due to differential parental inheritance (e.g. the extreme case of the Y chromosome and mtDNA in humans; Underhill & Kivisild Citation2007), while in bdelloids, as all the genes are linked to each other and are uniparentally inherited, the phylogenetic and phylogeographic history for any locus should, in theory, be strictly congruent to that of all other genes (Barraclough & Herniou Citation2003; Barraclough et al. Citation2003).
Much more work is still required to increase our understanding of the genome of bdelloids, as all our current knowledge relies on a very low number of genes that have been analysed from few species (Rice & Friberg Citation2007). Even from such limited information, interesting and promising clues have been obtained. For instance, gene copies representing former alleles have been shown to have diverged in function with the proteins they encode playing complementary roles in desiccation resistance (Pouchkina‐Stantcheva et al. Citation2007). The functional divergence of former alleles suggests that lack of sexual recombination could itself be an evolutionary mechanism for the generation of diversity.
Nevertheless, the most important mechanism to generate diversity in bdelloids is offered by the recently discovered shocking capability of horizontal gene transfer (Gladyshev et al. Citation2008). During the DNA repairing mechanisms known to act after an event that causes DNA breakage (Gladyshev & Meselson Citation2008), e.g. desiccation, bdelloids might be capable of incorporating within their own genome genes from the environment. This could be how bdelloids cope with the lack of sexual recombination, by undertaking a very weird form of genetic exchange that Meselson named as one of the most bizarre sexual appetites of all: “necrophilia”. This uptake of foreign genes through horizontal transfer has been shown to happen close to the telomeric regions only (Gladyshev et al. Citation2008), but the possibility of incorporating foreign DNA opens the potential for all possible genetic exchanges and allele‐sharing, making the anhydrobiosis capability a central condition in bdelloid evolutionary success (M. Meselson, personal communication, but see also Maderspacher Citation2008).
Genome projects on more bdelloid species and their sexual relatives are critically needed for evaluating the “scandal” status of bdelloids, disclosing the amount of foreign genes present in their genome, and the molecular bases of dormancy and of the DNA repair mechanism.
Biogeographical consequence of dormancy
The ability to enter dormancy, and thus to become dormant propagules (Cáceres Citation1997; Bohonak & Jenkins Citation2003; Fenchel & Finlay Citation2004), might explain how microscopic eukaryotes, like protists and small invertebrates such as bdelloids, can, in principle, potentially acquire global distributions. The everything‐is‐everywhere hypothesis encapsulates the classical view that microscopic organisms are globally distributed and do not show any geographical patterns in their diversity, and that their distribution and abundance are factors of habitat properties only (Fenchel & Finlay Citation2004; Kellogg & Griffin Citation2006).
Many of the predictions of the everything‐is‐everywhere hypothesis have been confirmed in bdelloids, like high local:global diversity (Fontaneto et al. Citation2006b), absence of distance–decay relationships in species composition (Fontaneto & Ricci Citation2006), and high number of cosmopolitan species (Fontaneto et al. Citation2007a). Nevertheless, the number of endemic species with restricted distributions is high (e.g. Ricci Citation1987; Örstan Citation1995; Koste Citation1996; Ricci et al. Citation2001, Citation2003; Fontaneto & Melone Citation2003b; Segers & Shiel Citation2005). Species with restricted distribution seem to contradict the predictions of the everything‐is‐everywhere hypothesis, but such restricted distributions may be due to the biased faunistic knowledge that is still relatively limited. Almost every survey adds lists of species previously unknown from the area investigated, even in quite well studied European areas (Ricci Citation1987; Braioni & Ricci Citation1995; Yakovenko Citation2000a, Citationb; Fontaneto & Melone Citation2003a; Devetter Citation2007).
The potential ubiquity of protists and bdelloids is supported identifying species using traditional taxonomy based upon morphology. In protists and monogonont rotifers, however, many studies on molecular phylogeny and phylogeography are revealing an opposite pattern, with genetic distance–decay relationships, genetic isolation‐by‐distance and limited distribution of genetic clusters being revealed (Darling et al. Citation2004; Foissner Citation2006; Telford et al. Citation2006; Gómez et al. Citation2007; Mills et al. Citation2007). Phylogeography analyses in bdelloids show a similar relationship between genetic and geographic distances (Birky et al. Citation2005; Fontaneto et al. Citation2008a), suggesting that microscopic animals such as bdelloids are indeed more widespread than larger animals, but still retain a biogeography.
Species reality
Is speciation possible in the absence of sexual reproduction? This question is still an open one, and there is a great amount of theoretical discussion present today regarding species concepts in asexual organisms (e.g. Cohan Citation2001, Citation2002; Barraclough et al. Citation2003; Coyne & Orr Citation2004; de Queiroz Citation2005; Hillis Citation2007). Bdelloid rotifers are an excellent model organism to answer such a fundamental biological question. There are over 450 described bdelloid species, but the accuracy of this number can be called into question. Bdelloid diversity has been established upon morphology alone, as cross‐mating tests are obviously not possible in organisms that do not mate. Nevertheless, different morphological features are congruent in grouping individuals; for example, geometric morphometric analyses of shape and size of trophi in more than 1400 individuals of the genus Rotaria revealed clusters which were consistent with traditional species identification, which is based on body traits other than trophi (Fontaneto et al. Citation2004, Citation2007b). Moreover, molecular phylogenetic analyses showed that entities equivalent to species actually exist in bdelloids. Birky et al. (Citation2005) first showed that bdelloids have undergone diversification in mtDNA with patterns similar to sexually reproducing organisms, as already proposed on a theoretical basis (Barraclough et al. Citation2003). Fontaneto et al. (Citation2007c) have also confirmed that genetic clusters of bdelloids correspond well to traditional species as identified on a morphological basis, providing evidence for independent evolution of clusters and ecological divergence by natural selection. Bdelloid rotifers demonstrate that very similar patterns of genetic and morphological variation exist among asexuals, and that reproductive isolation is not the only explanation for the existence of species (Fontaneto et al. Citation2007c; Hillis Citation2007).
Species do seem to exist in bdelloids; however, as in many other microscopic organisms, molecular phylogenetic analyses show a high degree of cryptic genetic diversity (e.g. Knowlton Citation1993; Derycke et al. Citation2005; Casu & Curini‐Galletti Citation2006; Suatoni et al. Citation2006), which is largely unexpected when considering morphology alone. Indeed it has been hypothesised that many species of bdelloids may in fact be species complexes (Ricci Citation2001; Fontaneto et al. Citation2008b; Kaya et al. Citation2009).
In summary, the take‐home message of this review is that dormancy can be considered the key feature of the bdelloids' evolutionary and ecological success. Other organisms may produce dormant stages, but only the bdelloids have the capacity of dormancy as an apomorphic trait accompanied by obligate parthenogenesis. The ability to recover after dormancy in bdelloids seems to promote efficient DNA repair mechanisms, which possibly allow genetic exchange through horizontal gene transfer; thus allowing genetic exchange and variability to be maintained without the “burden of males”. The parthenogenetic reproduction mode utilised by bdelloids also allows the production of a huge number of individuals in each population, and dormant propagules allow dispersal to reach many different habitats, enhancing the chances of encountering new genetic material in the environment. The absence of asexual recombination provides the basis for allelic divergence in function, generating diversity and promoting adaptation. Also, the phenomenon of degenerate tetraploidy provides genetic redundancy, further contributing to the ability of bdelloids to adapt to their environment. The diversity found in bdelloids is structured in patterns similar to those found in other sexual animals, with ecological adaptation as the main process shaping the evolutionary pathway. We are starting to unravel some of the mysteries surrounding bdelloids, but much more work needs to be performed at all levels to gain a better understanding of the importance of the processes involved in their success. It is also likely that this research combined with the unusual features of bdelloids may provide insights into other important research areas such as the role of epigenetic mechanisms, and the processes of ageing and senescence.
Acknowledgements
We thank Giulio Melone for providing scanning electron microscopy pictures, three anonymous reviewers for their comments on the original manuscript, and Shane Donnelly for improving the English text.
References
- Amsellem , J. and Ricci , C. 1982 . Fine structure of the female genital apparatus of Philodina (Rotifera, Bdelloidea). . Zoomorphology , 100 : 89 – 105 .
- Arkhipova , I. and Meselson , M. 2000 . Transposable elements in sexual and ancient asexual taxa. . Proceedings of the National Academy of Sciences USA , 97 : 14,473 – 14,477 .
- Arkhipova , I. R. and Meselson , M. 2005 . Diverse DNA transposons in rotifers of the class Bdelloidea. . Proceedings of the National Academy of Sciences USA , 102 : 11,781 – 11,786 .
- Barraclough , T. G. , Birky , C. W. and Burt , A. 2003 . Diversification in sexual and asexual organisms. . Evolution , 57 : 2166 – 2172 .
- Barraclough , T. G. , Fontaneto , D. , Ricci , C. and Herniou , E. A. 2007 . Evidence for inefficient selection against deleterious mutations in cytochrome oxidase I of asexual bdelloid rotifers. . Molecular Biology and Evolution , 24 : 1952 – 1962 .
- Barraclough , T. G. and Herniou , E. A. 2003 . Why do species exist? Insights from sexuals and asexuals. . Zoology , 106 : 275 – 282 .
- Bartoš , E. 1951 . The Czechoslovak Rotatoria of the order Bdelloidea. . Vestník Ceskoslovenské Zoologické Spolecnosti , 15 : 241 – 500 .
- Baurain , D. , Brinkmann , H. and Philippe , H. 2007 . Lack of resolution in the animal phylogeny: Closely spaced cladogeneses or undetected systematic errors? . Molecular Biology and Evolution , 24 : 6 – 9 .
- Birky , C. W. , Wolf , C. , Maughan , H. , Herbertson , L. and Henry , E. 2005 . Speciation and selection without sex. . Hydrobiologia , 546 : 29 – 45 .
- Bohonak , A. J. and Jenkins , D. G. 2003 . Ecological and evolutionary significance of dispersal by freshwater invertebrates. . Ecology Letters , 6 : 783 – 796 .
- Boschetti , C. , Ricci , C. , Sotgia , C. and Fascio , U. 2005 . The development of a bdelloid egg: A contribution after 100 years. . Hydrobiologia , 546 : 323 – 331 .
- Braioni , M. G. and Ricci , C. 1995 . “ Rotifera. ” . In Checklist delle specie animali italiane , Edited by: Minelli , A , Ruffo , S and La Posta , S . Vol. 8 , Bologna : Calderini .
- Brinkmann , H. and Philippe , H. 2008 . Animal phylogeny and large‐scale sequencing: Progress and pitfalls. . Journal of Systematics and Evolution , 46 : 274 – 286 .
- Browne , J. , Tunnacliffe , A. and Burnell , A. 2002 . Anhydrobiosis – Plant desiccation gene found in a nematode. . Nature , 416 : 38
- Cáceres , C. E. 1997 . Dormancy in invertebrates. . Invertebrate Biology , 116 : 371 – 383 .
- Caprioli , M. , Katholm , A. K. , Melone , G. , Ramlov , H. , Ricci , C. and Santo , N. 2004 . Trehalose in desiccated rotifers: A comparison between a bdelloid and a monogonont species. . Comparative Biochemistry and Physiology A – Molecular & Integrative Physiology , 139 : 527 – 532 .
- Casu , M. and Curini‐Galletti , M. 2006 . Genetic evidence for the existence of cryptic species in the mesopsammic flatworm Pseudomonocelis ophiocephala (Rhabditophora: Proseriata). . Biological Journal of the Linnean Society , 87 : 553 – 576 .
- Chakrabortee , S. , Boschetti , C. , Walton , L. J. , Sarkar , S. , Rubinsztein , D. C. and Tunnacliffe , A. 2007 . Hydrophilic protein associated with desiccation tolerance exhibits broad protein stabilization function. . Proceedings of the National Academy of Sciences USA , 104 : 18,073 – 18,078 .
- Clegg , J. S. 2001 . Cryptobiosis – A peculiar state of biological organization. . Comparative Biochemistry and Physiology B – Biochemistry & Molecular Biology , 128 : 613 – 624 .
- Clement , P. and Wurdak , E. 1991 . “ Rotifera. ” . In Microscopic anatomy of the invertebrates , Edited by: Harrison , F. W and Ruppert , E. E . 219 – 297 . New York : Wiley‐Liss . Volume 4: Aschelminthes
- Cohan , F. M. 2001 . Bacterial species and speciation. . Systematic Biology , 50 : 513 – 524 .
- Cohan , F. M. 2002 . What are bacterial species? . Annual Review of Microbiology , 56 : 457 – 487 .
- Coyne , J. A. and Orr , H. A. 2004 . Speciation , Sunderland, MA : Sinauer Associates .
- Crowe , J. H. 1971 . Anhydrobiosis – Unsolved problem. . American Naturalist , 105 : 563 – 573 .
- Crowe , J. H. , Hoekstra , F. A. and Crowe , L. M. 1992 . Anhydrobiosis. . Annual Review of Physiology , 54 : 579 – 599 .
- Darling , K. F. , Kucera , M. , Pudsey , C. J. and Wade , C. M. 2004 . Molecular evidence links cryptic diversification in polar planktonic protists to quaternary climate dynamics. . Proceedings of the National Academy of Sciences USA , 101 : 7657 – 7662 .
- de Queiroz , K. 2005 . Ernst Mayr and the modern concept of species. . Proceedings of the National Academy of Sciences USA , 102 : 6600 – 6607 .
- Derycke , S. , Remerie , T. , Vierstraete , A. , Backeljau , T. , Vanfleteren , J. , Vincx , M. and Moens , T. 2005 . Mitochondrial DNA variation and cryptic speciation within the free‐living marine nematode Pellioditis marina. . Marine Ecology Progress Series , 300 : 91 – 103 .
- Devetter , M. 2007 . Soil rotifers (Rotifera) of the Kokorinsko Protected Landscape Area. . Biologia , 62 : 222 – 224 .
- Dickson , M. R. and Mercer , E. H. 1967 . Fine structural changes accompanying desiccation in Philodina roseola (Rotifera). . Journal of Microscopy , 6 : 331 – 348 .
- Dolgin , E. S. and Charlesworth , B. 2006 . The fate of transposable elements in asexual populations. . Genetics , 174 : 817 – 827 .
- Donner , J. 1965 . Ordnung Bdelloidea. Bestimmungsbücher zur Bodenfauna Europas 6 , Berlin : Akademie Verlag .
- Dunn , C. W. , Hejnol , A. , Matus , D. Q. , Pang , K. , Browne , W. E. Smith , S. A. 2008 . Broad phylogenomic sampling improves resolution of the animal tree of life. . Nature , 452 : 745 – 750 .
- Fenchel , T. and Finlay , B. J. 2004 . The ubiquity of small species: patterns of local and global diversity. . BioScience , 54 : 777 – 784 .
- Foissner , W. 2006 . Biogeography and dispersal of microorganisms: A review emphasizing protists. . Acta Protozoologica , 45 : 111 – 136 .
- Fontaneto , D. , Barraclough , T. G. , Chen , K. , Ricci , C. and Herniou , E. A. 2008a . Molecular evidence for broad‐scale distributions in bdelloid rotifers: Everything is not everywhere but most things are very widespread. . Molecular Ecology , 17 : 3136 – 3146 .
- Fontaneto , D. , Boschetti , C. and Ricci , C. 2008b . Cryptic speciation in ancient asexuals: Evidences from the bdelloid rotifer Philodina flaviceps. . Journal of Evolutionary Biology , 21 : 580 – 587 .
- Fontaneto , D. , De Smet , W. H. and Ricci , C. 2006a . Rotifers in saltwater environments, re‐evaluation of an inconspicuous taxon. . Journal of the Marine Biological Association of the United Kingdom , 86 : 623 – 656 .
- Fontaneto , D. , Ficetola , G. F. , Ambrosini , R. and Ricci , C. 2006b . Patterns of diversity in microscopic animals: Are they comparable to those in protists or in larger animals? . Global Ecology and Biogeography , 15 : 153 – 162 .
- Fontaneto , D. , Herniou , E. A. , Barraclough , T. G. and Ricci , C. 2007a . On the global distribution of microscopic animals: New worldwide data on bdelloid rotifers. . Zoological Studies , 46 : 336 – 346 .
- Fontaneto , D. , Herniou , E. A. , Barraclough , T. G. , Ricci , C. and Melone , G. 2007b . On the reality and recognisability of asexual organisms: Morphological analysis of the masticatory apparatus of bdelloid rotifers. . Zoologica Scripta , 36 : 361 – 370 .
- Fontaneto , D. , Herniou , E. A. , Boschetti , C. , Caprioli , M. , Melone , G. , Ricci , C. and Barraclough , T. G. 2007c . Evidence for independently evolving species in bdelloid rotifers. . PLoS Biology , 5 : 914 – 921 .
- Fontaneto , D. and Melone , G. 2003a . On some rotifers new for the Italian fauna. . Italian Journal of Zoology , 70 : 253 – 259 .
- Fontaneto , D. and Melone , G. 2003b . Redescription of Pleuretra hystrix, an endemic alpine bdelloid rotifer. . Hydrobiologia , 497 : 153 – 160 .
- Fontaneto , D. , Melone , G. and Cardini , A. 2004 . Shape diversity in the trophi of different species of Rotaria (Rotifera, Bdelloidea): A geometric morphometric study. . Italian Journal of Zoology , 71 : 63 – 72 .
- Fontaneto , D. and Ricci , C. 2004 . “ Rotifera: Bdelloidea. ” . In Freshwater invertebrates of the Malaysian region , Edited by: Yule , C. M and Hoi , Sen Yong . 121 – 126 . Kuala Lumpur : Academy of Sciences Malaysia .
- Fontaneto , D. and Ricci , C. 2006 . Spatial gradients in species diversity of microscopic animals: The case of bdelloid rotifers at high altitude. . Journal of Biogeography , 33 : 1305 – 1313 .
- Funch , P. , Sorensen , M. V. and Obst , M. 2005 . On the phylogenetic position of Rotifera – have we come any further? . Hydrobiologia , 546 : 11 – 28 .
- Garcia Varela , M. , Perez‐Ponce de Leon , G. , de la Torre , P. , Cummings , M. P. , Sarma , S. S. S. and Laclette , J. P. 2000 . Phylogenetic relationships of Acanthocephala based on analysis of 18S ribosomal RNA gene sequences. . Journal of Molecular Evolution , 50 : 535 – 540 .
- Gilbert , J. J. 1983 . “ Rotifera. ” . In Reproductive biology of the invertebrates , Edited by: Adiyodi , K. G and Adiyodi , R. G . Vol. 1 , 181 – 209 . New York : John Wiley and Sons .
- Gilbert , J. J. 1989 . “ Rotifera. ” . In Reproductive biology of the invertebrates , Edited by: Adiyodi , K. G and Adiyodi , R. G . Vol. 4 , 179 – 199 . New York : John Wiley and Sons .
- Giribet , G. , Distel , D. L. , Polz , M. , Sterrer , W. and Wheeler , W. C. 2000 . Triploblastic relationships with emphasis on the acoelomates and the position of Gnathostomulida, Cycliophora, Plathelminthes, and Chaetognatha: A combined approach of 18S rDNA sequences and morphology. . Systematic Biology , 49 : 539 – 562 .
- Gladyshev , E. and Meselson , M. 2008 . Extreme resistance of bdelloid rotifers to ionizing radiation. . Proceedings of the National Academy of Sciences USA , 105 : 5139 – 5144 .
- Gladyshev , E. A. , Meselson , M. and Arkhipova , I. R. 2007 . A deep‐branching clade of retrovirus‐like retrotransposons in bdelloid rotifers. . Gene , 390 : 136 – 145 .
- Gladyshev , E. A. , Meselson , M. and Arkhipova , I. R. 2008 . Massive horizontal gene transfer in bdelloid rotifers. . Science , 320 : 1210 – 1213 .
- Gómez , A. , Montero‐Pau , J. , Lunt , D. H. , Serra , M. and Campillo , S. 2007 . Persistent genetic signatures of colonization in Brachionus manjavacas rotifers in the Iberian Peninsula. . Molecula Ecology , 16 : 3228 – 3240 .
- Hengherr , S. , Brummer , F. and Schill , R. O. 2008 . Anhydrobiosis in tardigrades and its effects on longevity traits. . Journal of Zoology , 275 : 216 – 220 .
- Herlyn , H. , Piskurek , O. , Schmitz , J. , Ehlers , U. and Zischler , H. 2003 . The syndermatan phylogeny and the evolution of acanthocephalan endoparasitism as inferred from 18S rDNA sequences. . Molecular Phylogenetics and Evolution , 26 : 155 – 164 .
- Higa , L. M. and Womersley , C. Z. 1993 . New insights into the anhydrobiotic phenomenon – The effects of trehalose content and differential rates of evaporative water‐loss on the survival of Aphelenchus avenae. . Journal of Experimental Zoology , 267 : 120 – 129 .
- Hillis , D. M. 2007 . Asexual evolution: Can species exist without sex? . Current Biology , 17 : R543 – R544 .
- Hsu , W. S. 1956a . Oogenesis in Habrotrocha tridens (Milne). . Biological Bulletin , 111 : 364 – 374 .
- Hsu , W. S. 1956b . Oogenesis in the Bdelloidea rotifer Philodina roseola Ehrenberg. . Cellule , 57 : 283 – 296 .
- Hur , J. H. , Van Doninck , K. , Mandigo , M. L. and Meselson , M. 2009 . Degenerate tetraploidy was established before bdelloid rotifer families diverged. . Molecular Biology and Evolution , 26 : 375 – 383 .
- Kaya , M. , Herniou , E. A. , Barraclough , T. G. and Fontaneto , D. 2009 . Inconsistent estimates of diversity between traditional and DNA taxonomy in bdelloid rotifers. . Organisms, Diversity and Evolution , 9 : 3 – 12 .
- Kellogg , C. A. and Griffin , D. W. 2006 . Aerobiology and the global transport of desert dust. . Trends in Ecology and Evolution , 21 : 638 – 644 .
- Knowlton , N. 1993 . Sibling species in the sea. . Annual Review of Ecology and Systematics , 24 : 189 – 216 .
- Koste , W. 1996 . On soil rotatoria from a lithotelma near Halali Lodge in Etosha National Park in N‐Namibia, South Africa. . Internationale Revue der Gesamten Hydrobiologie , 81 : 353 – 365 .
- Koste , W. and Shiel , R. J. 1986 . Rotifera from Australian inland waters. I. Bdelloidea (Rotifera: Digononta). . Australian Journal of Marine and Freshwater Research , 37 : 765 – 792 .
- Lapinski , J. and Tunnacliffe , A. 2003 . Anhydrobiosis without trehalose in bdelloid rotifers. . FEBS Letters , 553 : 387 – 390 .
- Linhart , J. , Fiuraskova , M. and Uvira , V. 2002 . Moss‐ and mineral substrata‐dwelling meiobenthos in two different low‐order streams. . Archiv fur Hydrobiologie , 154 : 543 – 560 .
- Maderspacher , F. 2008 . Sex and the drought. . Current Biology , 18 : R983 – R985 .
- Mark Welch , D. B. 2000 . Evidence from a protein coding gene that acanthocephalans are rotifers. . Invertebrate Biology , 111 : 17 – 23 .
- Mark Welch , D. B. 2005 . Bayesian and maximum likelihood analysis of rotifer–acanthocephalan relationships. . Hydrobiologia , 546 : 47 – 54 .
- Mark Welch , D. B. , Cummings , M. P. , Hillis , D. M. and Meselson , M. 2004a . Divergent gene copies in the asexual class Bdelloidea (Rotifera) separated before the bdelloid radiation or within bdelloid families. . Proceedings of the National Academy of Sciences USA , 101 : 1622 – 1625 .
- Mark Welch , D. B. , Mark Welch , J. L. and Meselson , M. 2008 . Evidence for degenerate tetraploidy in bdelloid rotifers. . Proceedings of the National Academy of Sciences USA , 105 : 5145 – 5149 .
- Mark Welch , D. B. and Meselson , M. 1998a . Measurements of the genome size of the monogonont rotifer Brachionus plicatilis and of the bdelloid rotifers Philodina roseola and Habrotrocha constricta. . Hydrobiologia , 387/388 : 395 – 402 .
- Mark Welch , J. L. and Meselson , M. 1998b . Karyotypes of bdelloid rotifers from three families. . Hydrobiologia , 387/388 : 403 – 407 .
- Mark Welch , D. B. and Meselson , M. 2000 . Evidence for the evolution of bdelloid rotifers without sexual reproduction or genetic exchange. . Science , 288 : 1211 – 1215 .
- Mark Welch , D. B. and Meselson , M. 2003 . Oocyte nuclear DNA content and GC proportion in rotifers of the anciently asexual Class Bdelloidea. . Biological Journal of the Linnean Society , 79 : 85 – 91 .
- Mark Welch , J. L. , Mark Welch , D. B. and Meselson , M. 2004b . Cytogenetic evidence for asexual evolution of bdelloid rotifers. . Proceedings of the National Academy of Sciences USA , 101 : 1618 – 1621 .
- Marotta , R. , Ricci , C. and Melone , G. 2008 . Morphological changes accompanying anhydrobiosis in the rotifer Macrotrachela quadricornifera (Rotifera Bdelloidea). . International Congress on Invertebrate Morphology , : 78
- Maynard Smith , J. 1986 . Contemplating life without sex. . Nature , 324 : 300 – 301 .
- Melone , G. and Fontaneto , D. 2005 . Trophi structure in bdelloid rotifers. . Hydrobiologia , 546 : 197 – 202 .
- Melone , G. and Ricci , C. 1995 . Rotatory apparatus in Bdelloids. . Hydrobiologia , 313 : 91 – 98 .
- Melone , G. , Ricci , C. , Segers , H. and Wallace , R. L. 1998 . Phylogenetic relationships of phylum Rotifera with emphasis on the families of Bdelloidea. . Hydrobiologia , 387/388 : 101 – 107 .
- Mills , S. , Lunt , D. H. and Gómez , A. 2007 . Global isolation by distance despite strong regional phylogeography in a small metazoan. . BMC Evolutionary Biology , 7 : 225
- Normark , B. B. , Judson , O. P. and Moran , N. A. 2003 . Genomic signatures of ancient asexual lineages. . Biological Journal of the Linnean Society , 79 : 69 – 84 .
- Örstan , A. 1995 . A new species of bdelloid rotifer from Sonora, Mexico. . Southwestern Naturalist , 40 : 255 – 258 .
- Pagani , M. , Ricci , C. and Redi , C. A. 1993 . Oogenesis in Macrotrachela quadricornifera. I. Germarium eutely, karyotype, and DNA content. . Hydrobiologia , 255/256 : 225 – 230 .
- Passamaneck , Y. and Halanych , K. M. 2006 . Lophotrochozoan phylogeny assessed with LSU and SSU data: Evidence of lophophorate polyphyly. . Molecular Phylogenetics and Evolution , 40 : 20 – 28 .
- Philippe , H. and Telford , M. J. 2006 . Large‐scale sequencing and the new animal phylogeny. . Trends in Ecology & Evolution , 21 : 614 – 620 .
- Poinar , G. O. Jr and Ricci , C. 1992 . Bdelloid rotifers in Dominican amber: Evidence for parthenogenetic continuity. . Experientia , 48 : 408 – 410 .
- Pouchkina‐Stantcheva , N. N. , McGee , B. M. , Boschetti , C. , Tolleter , D. , Chakrabortee , S. , Popova , A. V. , Meersman , F. , Macherel , D. , Hincha , D. K. and Tunnacliffe , A. 2007 . Functional divergence of former alleles in an ancient asexual invertebrate. . Science , 318 : 268 – 271 .
- Reiss , J. and Schmid‐Araya , J. M. 2008 . Existing in plenty: abundance, biomass and diversity of ciliates and meiofauna in small streams. . Freshwater Biology , 53 : 652 – 668 .
- Remane , A. 1929 . Rotatorien. Bronn's Klassen und Ordnungen des Tier‐Reichs , Leipzig : Akademische Verlagsgesellschaft .
- Ricci , C. 1984 . Culturing of some bdelloid rotifers. . Hydrobiologia , 112 : 45 – 51 .
- Ricci , C. 1987 . Ecology of bdelloids: How to be successful. . Hydrobiologia , 147 : 117 – 127 .
- Ricci , C. 1992 . “ Rotifera: parthenogenesis and heterogony. ” . In Sex origin and evolution. Selected symposia UZI , Edited by: Dallai , R . 329 – 341 . Modena : Mucchi .
- Ricci , C. 1998 . Anhydrobiotic capabilities of bdelloid rotifers. . Hydrobiologia , 387/388 : 321 – 326 .
- Ricci , C. 2001 . A reconsideration of the taxonomic status of Macrotrachela quadricornifera (Rotifera, Bdelloidea). . Journal of Zoology , 255 : 273 – 277 .
- Ricci , C. and Caprioli , M. 2005 . Anhydrobiosis in bdelloid species, populations and individuals. . Integrative and Comparative Biology , 45 : 759 – 763 .
- Ricci , C. , Caprioli , M. and Fontaneto , D. 2007 . Stress and fitness in parthenogens: Is dormancy a key feature for bdelloid rotifers? . BMC Evolutionary Biology , 7 : S9
- Ricci , C. , Caprioli , M. , Fontaneto , D. and Melone , G. 2008 . Volume and morphology changes of a bdelloid rotifer species (Macrotrachela quadricornifera) during anhydrobiosis. . Journal of Morphology , 269 : 233 – 239 .
- Ricci , C. and Covino , C. 2005 . Anhydrobiosis of Adineta ricciae: Costs and benefits. . Hydrobiologia , 546 : 307 – 314 .
- Ricci , C. and Melone , G. 2000 . Key to the identification of the genera of Bdelloid Rotifers. . Hydrobiologia , 418 : 73 – 80 .
- Ricci , C. , Melone , G. and Walsh , E. J. 2001 . A carnivorous bdelloid rotifer, Abrochtha carnivora n. sp. . Invertebrate Biology , 120 : 136 – 141 .
- Ricci , C. and Pagani , M. 1997 . Desiccation of Panagrolaimus rigidus (Nematoda): Survival, reproduction and the influence on the internal clock. . Hydrobiologia , 347 : 1 – 13 .
- Ricci , C. and Perletti , F. 2006 . Starve and survive: Stress tolerance and life‐history traits of a bdelloid rotifer. . Functional Ecology , 20 : 340 – 346 .
- Ricci , C. , Perletti , F. and Cornelli , G. 2005 . “ May stress arrest ageing? ” . In 10th ESEB Congress , 219 Krakow : ESEB .
- Ricci , C. , Shiel , R. J. , Fontaneto , D. and Melone , G. 2003 . Bdelloid rotifers recorded from Australia with description of Philodinavus aussiensis n.sp. . Zoologischer Anzeiger , 242 : 241 – 248 .
- Ricci , C. , Vaghi , L. and Manzini , M. L. 1987 . Desiccation of rotifers (Macrotrachela quadricornifera) – Survival and reproduction. . Ecology , 68 : 1488 – 1494 .
- Rice , W. R. and Friberg , U. 2007 . Genomic clues to an ancient asexual scandal. . Genome Biology , 8 : 232
- Ronaghi , M. 2001 . Pyrosequencing sheds light on DNA sequencing. . Genome Research , 11 : 3 – 11 .
- Schmid‐Araya , J. M. 1998 . Small‐sized invertebrates in a gravel stream: Community structure and variability of benthic rotifers. . Freshwater Biology , 39 : 25 – 39 .
- Segers , H. 2007 . Annotated checklist of the rotifers (Phylum Rotifera), with notes on nomenclature, taxonomy and distribution. . Zootaxa , 1564 : 3 – 104 .
- Segers , H. and Shiel , R. J. 2005 . Tale of a sleeping beauty: A new and easily cultured model organism for experimental studies on bdelloid rotifers. . Hydrobiologia , 546 : 141 – 145 .
- Sohlenius , B. and Bostrom , S. 2005 . The geographic distribution of metazoan microfauna on East Antarctic nunataks. . Polar Biology , 28 : 439 – 448 .
- Sørensen , M. V. and Giribet , G. 2006 . A modern approach to rotiferan phylogeny: Combining morphological and molecular data. . Molecular Phylogenetics and Evolution , 40 : 585 – 608 .
- Suatoni , E. , Vicario , S. , Rice , S. , Snell , T. and Caccone , A. 2006 . An analysis of species boundaries and biogeographic patterns in a cryptic species complex: The rotifer Brachionus plicatilis. . Moleculal Phylogenetics and Evolution , 41 : 86 – 98 .
- Telford , R. J. , Vandvik , V. and Birks , H. J. B. 2006 . Dispersal limitations matter for microbial morphospecies. . Science , 312 : 1015
- Tunnacliffe , A. , Lapinski , J. and McGee , B. 2005 . A putative LEA protein, but no trehalose, is present in anhydrobiotic bdelloid rotifers. . Hydrobiologia , 546 : 315 – 321 .
- Turner , P. N. 1999 . A simple generic key to the bdelloid rotifers. . Quekett Journal of Microscopy , 38 : 351 – 356 .
- Underhill , P. A. and Kivisild , T. 2007 . Use of Y chromosome and mitochondrial DNA population structure in tracing human migrations. . Annual Review of Genetics , 41 : 539 – 564 .
- Wallace , R. L. and Ricci , C. 2002 . “ Rotifera. ” . In Freshwater meiofauna: Biology and ecology , Edited by: Rundle , S. D , Robertson , A. L and Schmid‐Araya , J. M . 15 – 44 . Leiden : Backhuys Publishers .
- Wallace , R. L. , Snell , T. W. , Ricci , C. and Nogrady , T. 2006 . “ Rotifera: Volume 1 Biology, ecology and systematics. 2nd ed. ” . In Guides to the identification of the microinvertebrates of the continental waters of the world , Edited by: Segers , H . Vol. 23 , Leiden : Kenobi Productions, Ghent, and Backhuys Publishers .
- Westh , P. and Ramlov , H. 1991 . Trehalose accumulation in the tardigrade Adorybiotus coronifer during anhydrobiosis. . Journal of Experimental Zoology , 258 : 303 – 311 .
- Wharton , D. A. and Lemmon , J. 1998 . Ultrastructural changes during desiccation of the anhydrobiotic nematode Ditylenchus dipsaci. . Tissue & Cell , 30 : 312 – 323 .
- Wise , M. J. and Tunnacliffe , A. 2004 . POPP the question: what do LEA proteins do? . Trends in Plant Science , 9 : 13 – 17 .
- Witek , A. , Herlyn , H. , Meyer , A. , Boell , L. , Bucher , G. and Hankeln , T. 2008 . EST based phylogenomics of Syndermata questions monophyly of Eurotatoria. . BMC Evolutionary Biology , 8 : 345
- Wright , J. C. 2001 . Cryptobiosis 300 years on from van Leuwenhoek: What have we learned about tardigrades? . Zoologischer Anzeiger , 240 : 563 – 582 .
- Yakovenko , N. S. 2000a . New for the fauna of Ukraine rotifers (Rotifera, Bdelloidea) of Adinetidae and Habrotrochidae families. . Vestnik Zoologii , 34 : 11 – 19 .
- Yakovenko , N. S. 2000b . New for the fauna of Ukraine rotifers (Rotifera, Bdelloidea) of Philodinidae family. . Vestnik Zoologii Supplement , 14 : 26 – 32 .
- Zelinka , C. 1891 . Studien uber Raderthiere. III Zur Entwicklungsgeschichte der Raderthiere nebst Bemerkungen uber ihre Anatomie und Biologie. . Zeitschrift für wissenschaftliche Zoologie , 53 : 1 – 159 .