Abstract
The main aim of this work is to provide basic data on the aspects of pro-oxidant and antioxidant processes in different life stages of Eudrilus eugeniae. The levels of antioxidant enzymes, superoxide dismutase (SOD) and catalase (CAT), along with the byproducts of lipid and protein oxidation, malondialdehyde (MDA) and protein carbonyl (PC), content were determined in the whole body of juvenile, sub-adult and adult earthworms. The SOD activity significantly (P < 0.05) decreased during development from juvenile to adult, while CAT activity showed an increase with advancing age. The MDA content significantly increased in the adult compared to other age groups. The protein carbonyl content showed insignificant changes with respect to age. Our results suggest that the decline in SOD activity coupled with increased levels of MDA implicate the susceptibility of older animals to oxidative stress.
Introduction
Under normal physiological conditions, there is a critical balance in the generation of oxygen free radicals and a complex web of antioxidant defence systems, used by organisms to deactivate and protect themselves against free radical toxicity (Halliwell & Gutteridge Citation1989). Prominent among these are antioxidant enzymes such as superoxide dismutase (SOD) and catalase (CAT). Impairment in the oxidant/antioxidant equilibrium creates a condition known as oxidative stress, which is known to be a component of molecular and cellular tissue damage in a wide spectrum of human diseases (Klaunig & Kamendulis Citation2004). Extreme levels of oxidative stress may induce apoptotic and necrotic cell death, a pathological process that has been considered a passive process (Samali et al. Citation1999). A hypothesis may be constructed implicating oxidative stress as a cause of tissue damage involving different physiological processes and environmental factors (Finkel & Holbrook Citation2000).
Antioxidant enzyme activities of few annelids have been investigated. Very few studies have been done related to oxidative stress and antioxidant defences in Oliogochaete and other classes of annelids (Stenersen et al. Citation1979). Induction of oxidative stress in Laeonereis acuta has been investigated by Geracitano et al. (Citation2004).
Very few studies exist concerning antioxidant defence systems in earthworms. Stenersen and Oien (Citation1981) showed the existence of a glutathione system and glutathione-S-transferase (GST) in earthworms. Stokke and Stenersen (Citation1993) and Hans et al. (Citation1993) studied GST activities, which play a key role in glutathione metabolism and also in cellular redox status. The ability of Eisenia fetida andrei to accumulate metals or organic contaminants has been highlighted by Ville et al. (Citation1997) and Labrot et al. (Citation1996). An operative system involving glutathione and GST exists in earthworms (Stenersen et al. Citation1979; Stenersen & Oien Citation1981). The effects of metals on the CAT and glutathione peroxidase (GPX) activities in Eisenia fetida andrei have also been studied by Labrot et al. (Citation1996) both in vivo and in vitro. A study on the activities of the CAT, GPX and glutathione reductase (GR) involved in the antioxidant defences of Eisenia fetida andrei has been conducted by Saint-Denis et al. (Citation1998). These results suggest that the antioxidant system of earthworms should be interesting to study as a potential biomarker.
These findings therefore prompted us to obtain basic data on the antioxidant defences in Eudrilus eugeniae and, prior to investigating their use as potential biomarkers, the present study was designed to investigate the age-associated changes in antioxidant enzymes such as SOD and CAT, as well as accumulation of by products of lipid and protein oxidation, such as MDA and PC content.
Materials and methods
Chemicals
Epinephrine, thio-barbituric acid, pyridine, 1,1,3,3-tetramethoxypropane, 2,4 dinitrophenyl hydrazene, and bovine serum albumin (BSA) were obtained from Sigma (St. Louis, MO). All organic solvents were of spectral grade and general chemicals of reagent grade.
Culturing of earthworms
The earthworm species Eudrilus eugeniae was obtained from the Entomology Department, University of Agricultural Sciences, Bangalore, India and were maintained in our laboratory in plastic containers (28 cm high and 30 cm deep) with a pierced lid for aeration. The worms were cultured according to the method described by Bhattacharjee and Chaudhuri (Citation2002) on decomposed organic material (cow dung mixed with leaf litter in ratio of 1:3). The moisture level of the containers was maintained at about 60–70% throughout the study period by sprinkling an adequate quantity of water. To prevent moisture loss, the containers were covered with gunny bags and placed in a humid and dark room at a temperature 27°C. Animals were grouped into adult, sub-adult and juveniles according to scheme of Spurgeon and Hopkin (Citation1999).
Tissue preparation
The whole earthworms were homogenized in ice-cold 50 mM phosphate buffer pH (7.0) containing 0.1 mM EDTA. The resulting homogenate was centrifuged at 1000 rpm for 15 min at 4°C (Rota 4R-V/FM superspin, Plastocrafts, India). The supernatant was used for analytical procedures.
Enzyme assays
Superoxide dismutase (SOD, EC 1.15.1.1)
SOD activity was determined at room temperature (RT) according to the method of Misra and Fridovich (Citation1972). Tissue extract (100 μl) was added to 880 μl carbonate buffer of 0.05 M, pH (10.2), 0.1 mM EDTA. Twenty microlitres of 30 mM epinephrine in 0.05% acetic acid were added to the mixture, and the absorbance was followed for 4 min at 480 nm in a spectrophotometer (Model BL 158, ELICO). The amount of enzyme that results in 50% inhibition of epinephrine auto-oxidation is defined as one unit.
Catalase (CAT, EC 1.11.1.6)
CAT activity was determined by the method of Aebi (Citation1984). Briefly, 100 μl of tissue extract with an equal volume of absolute alcohol was incubated for 30 min in an ice bath for degradation of the inactive CAT–H2O2 complex II to release active CAT enzyme. After 30 min on ice, the tubes were brought back to RT and then 10 μl of Triton X-100 was added. In a cuvette containing 200 μl of phosphate buffer, 50 μl of tissue extract and 250 μl of 0.066 M H2O2 in phosphate buffer were added and the decrease in absorbance was read at 240 nm for 30 s in a spectrophotometer. A molar absorptivity of 43.6 M cm–1 was used to determine CAT activity, one unit of which is equal to the μmoles of hydrogen peroxide degraded per minute per mg of protein.
Lipid peroxidation (LP)
MDA assay was analysed by the method of Ohkawa et al. (Citation1979) using 1,1,3,3-tetramethoxypropane (TMP) as the standard. Briefly, 0.1 ml of tissue extract was taken and 50 μl of 8.1% sodium dodecyl sulphate (SDS) was added and incubated for 10 min at RT. Three hundred and seventy-five microlitres of 20% acetic acid (pH 3.5) and 375 μl of 0.6% TBA were added and then placed in a boiling water bath for 60 min. The sample was then allowed to cool, and 1.25 ml of butanol:pyridine (15:1v/v) mixture was added, vortexed and centrifuged at 1000 rpm for 5 min. Absorbance of the coloured layer was measured at 532 nm and the concentration was expressed in terms of n moles MDA per mg protein.
Protein oxidation
PC content was measured according to the procedure of Levine et al. (Citation1994). Briefly, 10 mmol dinitrophenylhydrazine (DPNH) in 2.5 M HCl was added to the tissue and incubated in the dark for 60 min at RT. This was followed by vortex mixing, addition of 20% trichloro acetic acid (TCA) and subsequently washing thrice with ethanol:ethyl acetate (1:1 v/v) mixture. Precipated protein was then re-dissolved in 6 M guanidine hydrochloride in 20 mM phosphate buffer pH (6.5). Insoluble substances were removed by centrifugation and absorbance of the supernatant was read at 370 nM. An extinction coefficient of 22,000 M–1cm–1 was used to determine the protein carbonyl content and was expressed as mM mg–1 protein.
Protein estimation
Protein concentration was estimated by the method of Lowry et al. (Citation1951) using BSA as a standard.
Statistical analysis
All data were expressed as mean ± SE and were analysed by one-way analysis of variance (ANOVA). When significant F ratios were found, Tukey's multiple comparison test was used to assess the difference between group means. Probability values (P) < 0.05 were considered significant (Snedecor & Cochran Citation1994).
Results
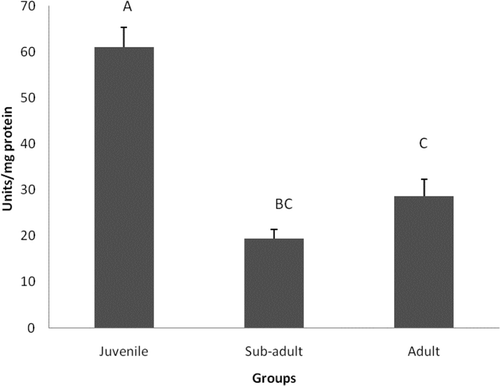
SOD activity
The SOD activity decreased with age, as shown in . The activity was significantly (P < 0.05) decreased in sub-adults and adults by 34% and 26%, respectively, when compared to the juveniles. However, the changes were insignificant between the sub-adult and adult groups.
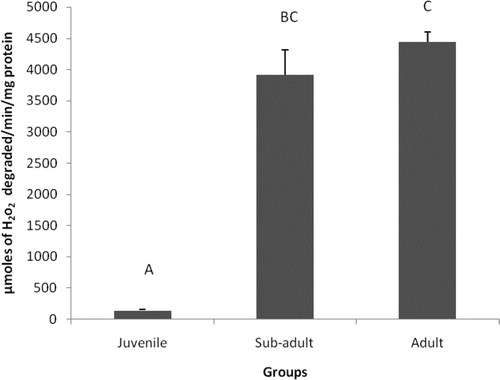
CAT activity
shows the changes in CAT activity between different age groups. Significant (P< 0.05) increases in CAT activity by 94% were observed in sub-adult and adult groups when compared to juvenile.
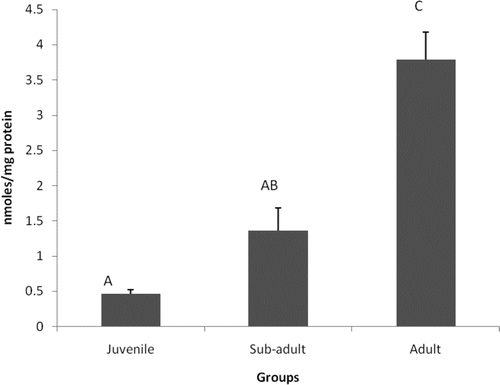
Lipid peroxidation
Levels of MDA, a marker of LP, were increased significantly (P < 0.05) in adults by 88% and 64% in comparison to juveniles and sub-adults, respectively. Changes were insignificant between the juvenile and sub-adult groups ().
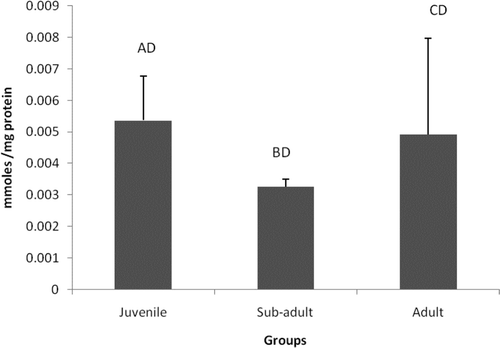
Protein oxidation
The protein oxidation measured in terms of protein carbonyl content is represented in . PC content showed insignificant changes between the age groups.
Discussion
The production of free radicals in the organism, and the imbalance between the concentrations of these and the antioxidant defences, may be related to processes such as ageing and several diseases. The ageing process has been described by various theories. In particular, the free radical theory of ageing has received widespread attention, which proposes that deleterious actions of free radicals are responsible for the functional deterioration associated with ageing. It attributes the ageing process to non-specific damage of macromolecules such as DNA, lipids, and proteins by free radicals. The theory predicts that the ability of cell to resist or prevent oxidative stress is a key determinant of longevity (Harman Citation1956).
In the present study, the SOD which functions as a primary defence to catalyse the conversion of superoxide to H2O2 has been analysed as a function of age. The juveniles showed higher SOD activity compared to other groups. Considering the fact that metabolic rate is higher in juvenile than in adult earthworms, the former require higher SOD activities to protect against increased amount of ROS (Saint-Denis et al. Citation1998).
Catalase activity, which plays an important role in detoxifying H2O2, was found to be increased with age. Significant increase in the CAT activity has been observed between juveniles and adult worms. Our results are in agreement with those of Saint-Denis et al. (Citation1998) on earthworm species Eisenia fetida, wherein catalase activity increased with age, and also in Arenicola, where CAT activity is higher in older compared to younger lugworms (Buchner et al. Citation1996). Previous studies by Di Giulio et al. (Citation1989) indicated that CAT activity could be induced under conditions of oxidative stress. However, the CAT activity is altered by a number of other conditions, including changing nutritional status or oxygen status (Livingstone et al. Citation1992).
An important factor affecting the potential for oxidative damage is the level of target molecules, e.g. polyunsaturated fatty acids (PUFA), which are readily oxidized by ROS to lipid peroxides (Di Giulio et al. Citation1995). The degree of lipid peroxidation may depend upon both the levels of antioxidant enzymes and the composition of fatty acids in the organisms.
To investigate the correlation between oxidative stress and ageing, we have determined the levels of MDA and PC content, which are byproducts of lipid and protein oxidation. A significant increase in the levels of MDA has been observed from juvenile to adult groups and which in part agrees with findings of Corriea et al. (2003) and Ravi Kiran et al. (Citation2006) in invertebrates and vertebrates. A negative correlation has been observed, with increasing MDA levels and decreasing SOD activities, in all the earthworm age groups. It signifies that the free radical scavenging ability of antioxidant enzymes has decreased with age, and there is thereby an increase in the accumulation of LP products.
Protein oxidation contributes to the pool of damaged enzymes, which increases in size during ageing and in various pathological states. The age-related increase in amounts of oxidized protein may reflect the accumulation of unrepaired DNA damage that, in a random manner, affects the concentrations or activities of numerous factors that govern the rates of protein oxidation and the degradation of oxidized protein (Stadtman Citation1992).
Protein carbonyl content showed insignificant changes between age groups. While the relationship between protein oxidation and ageing has been investigated extensively, the studies have shown conflicting results (Pratico Citation2002).
The level of oxidative stress increasing with age, as evidenced by elevations in MDA, cannot entirely be attributed to a decrease in the activities of antioxidant defence system and probably to various factors which may contribute to this process, such as exogenous factors playing a role in induction of lipid peroxidation in Gammarus locusta (Correia et al. Citation2003).
In conclusion, the decline in SOD activity, coupled with increased levels of MDA, as organisms grow older, may be suggestive of the fact that the adults are more prone to oxidative stress. These results appear to justify the definition of ageing as “the progressive accumulation of changes that are responsible for the decreased ability of organism to maintain physiological homeostasis, which may eventually lead to functional impairment and even death” (Yu Citation1994).
Acknowledgements
This work was supported by grants from Maharani Lakshmi Ammanni College for Women, Bangalore. We thank Mr Sushil Kumar Middha and Mr Manju for their technical support. We wish to thank Professor M.V.V. Subramanyam and Dr Sreepriya who reviewed this paper before submission.
References
- Aebi , H . 1984 . Catalase . Methods in Enzymology , 105 : 121 – 126 .
- Bhattacharjee , G and Chaudhuri , PS . 2002 . Cocoon production, morphology, hatching pattern and fecundity in seven tropical species – a laboratory based investigation . Journal of Biosciences , 27 : 283 – 294 .
- Buchner , T , Abele-Oechger , D and Theede , H . 1996 . Aspect of antioxidant status in polychaete Arenicola marina: Tissue and subcellular distribution, and reaction to environmental hydrogen peroxide and elevated temperatures . Marine Ecology Progress Series , 143 : 141 – 150 .
- Correia , AD , Helena Costa , M , Orlando , JL and Livingstone , DR . 2003 . Age related changes in antioxidant enzyme activities, fatty acid composition and lipid peroxidation in whole body Gammarus locusta (Crustacea: Amphipoda) . Journal of Experimental Marine Biology and Ecology , 4112 : 1 – 19 .
- Di Giulio , RT , Benson , WH , Sanders , BM and Van Veld , PA . 1995 . “ Biochemical mechanisms: Metabolism, adaptation and toxicity ” . In Fundamentals of aquatic toxicology, effects, environmental fate and risk assessment , 1st , Edited by: Rand , G . 523 – 561 . London : Taylor & Francis .
- Di Giulio , RT , Washburn , PC , Wenning , RJ , Winston , GW and Jewell , CS . 1989 . Biochemical responses in aquatic animals: A review of determinants of oxidative stress . Environment Toxicology Chemistry , 8 : 1103 – 1123 .
- Finkel , T and Holbrook , NJ . 2000 . Oxidants, oxidative stress and the biology of ageing . Nature , 408 : 239 – 247 .
- Geracitano , LA , Monserrat , JM and Bianchini , A . 2004 . Oxidative stress in Laeonereis acuta (Polychaeta, Nereididae): Environmental and seasonal effects . Marine Environmental Research , 58 : 625 – 630 .
- Halliwell , B and Gutteridge , JMC . 1989 . Free radicals in biology and medicine , 2nd , 139 – 187 . Oxford : Clarendon Press .
- Hans , RK , Khan , MA , Farooq , M and Beg , MU . 1993 . Glutathione-S-transferase activity in an earthworm (Pheretima posthuma) exposed to 3 insecticides . Soil Biology Biochemistry , 25 : 509 – 511 .
- Harman , D . 1956 . Aging: A theory based on free radical and radiation chemistry . Journal of Gerontology , 211 : 298 – 300 .
- Klaunig , JE and Kamendulis , LM . 2004 . The role of oxidative stress in carcinogenesis . Annual Review of Pharmacology and Toxicology , 44 : 239 – 267 .
- Labrot , F , Ribera , D , Saint-Denis , M and Narbonne , JF . 1996 . In vitro and in vivo studies in potential biomarkers of lead and uranium contamination: Lipid peroxidation, acetyl cholinesterase, catalase and glutathione peroxidase activities in three non-mammalian species . Biomarkers , 1 : 21 – 28 .
- Levine , RL , Williams , JA , Stadtman , ER and Shacter , E . 1994 . Carbonyl assays for determination of oxidatively modified proteins . Methods in Enzymology , 233 : 346 – 357 .
- Livingstone , DR , Archbald , S , Chipman , JK and Marsh , JW . 1992 . Antioxidant enzymes in liver of dab Limanda limanda from North Sea . Marine Ecology Progress Series , 91 : 97 – 104 .
- Lowry , OH , Rosebrough , NJ , Farr , AL and Randall . 1951 . Protein measurement with the Folin phenol reagent . Journal of Biological Chemistry , 193 : 265 – 275 .
- Misra , HP and Fridovich , I . 1972 . The role of superoxide anion in the auto oxidation of epinephrine and a simple assay for superoxide dismutase . Journal of Biological Chemistry , 247 : 3170 – 3175 .
- Ohkawa , H , Ohishi , N and Yagi , K . 1979 . Assay for lipid peroxidation in animals and tissues by thiobarbituric acid test . Analytical Biochemistry , 95 : 351 – 358 .
- Pratico , D . 2002 . Lipid peroxidation and the aging process . Science of Aging Knowledge Environment , 50 : 5
- Ravi Kiran , T , Subramanyam , MVV , Prathima , S and Asha Devi , S . 2006 . Blood lipid profile and myocardial superoxide dismutase in swim-trained young and middle-aged rats: Comparison between left and right ventricular adaptations to oxidative stress . Journal of Comparative Physiology B , 176 : 749 – 762 .
- Saint-Denis , M , Labrot , F , Narbonne , JF and Ribera , D . 1998 . Glutathione, glutathione-related enzymes, and catalase activities in the earthworm Eisenia fetida andrei . Archives of Environmental Contamination and Toxicology , 35 : 602 – 614 .
- Samali , A , Nordgren , H , Zhivotovsky , B , Peterson , E and Orrenius , S . 1999 . A comparative study of apoptosis and necrosis in HepG2 cells: Oxidant-induced caspase inactivation leads to necrosis . Biochemistry Biophysics Research Communication , 255 : 6 – 11 .
- Snedecor , GW and Cochran , WG . 1994 . Statistical methods , 503 AMES : Iowa State University Press .
- Spurgeon , DJ and Hopkin , SP . 1999 . Life history patterns in reference and metal exposed earthworm populations . Ecotoxicology , 8 : 133 – 141 .
- Stadtman , ER . 1992 . Protein oxidation and aging . Science , 257 : 1220 – 1224 .
- Stenersen , J , Guthenberg , C and Mannervik , B . 1979 . Glutathione-S-transferases in earthworms (Lumbricidae) . Biochemistry , 181 : 47 – 50 .
- Stenersen , J and Oien , N . 1981 . Glutathione-S-transferase in earthworms (Lumbricidae). Substrate specificity, tissue and species distribution and molecular weight . Comparative Biochemistry and Physiology C , 69 : 243 – 252 .
- Stokke , K and Stenersen , J . 1993 . Non-inducibility of the glutathione transferases of the earthworm Eisenia andrei . Comparative Biochemistry and Physiology C , 106 : 753 – 756 .
- Ville , P , Rochm , P , Cooperm , EL and Narbonne , JF . 1997 . Immuno-modulator effects of carbaryl and 2,4D in the earthworm Eisenia fetida andrei . Archives of Environmental Contamination and Toxicology , 32 : 291 – 297 .
- Yu , BP . 1994 . Cellular defenses against damage from reactive oxygen species . Physiological Reviews , 74 : 139 – 162 .