Abstract
The garden dormouse Eliomys quercinus is a polytypic species that shows remarkable chromosomal variation (2n = 48, 50, 52, 54). Furthermore, among 2n = 48 populations of the species, distributed mainly in the circum-Mediterranean area, different populations may exhibit distinctive karyotypes. Here we analyse samples of E. quercinus from Sicily, the insular population so far barely studied, compared to the samples from the Central Apennines. The two populations show the same cytotype (2n = 48, NFa = 86), matching G- and C- chromosome banding patterns, and identical chromosome locations of major ribosomal genes (rDNA) and interstitial telomeric sequences (ITS). The samples from the two Italian populations show low genetic divergence (1.2%) based on mitochondrial cytochrome b gene sequence analysis, which suggests that both populations belong to the same taxon – E. quercinus pallidus. In addition, we compare the present results to karyological data reported in other 2n = 48 populations and identify possible chromosomal rearrangements.
Introduction
Among the three currently recognized species of garden dormice (Sciuromorpha: Gliridae) of the genus Eliomys Wagner, 1840 – E. melanurus Wagner, 1840 (2n = 46 and 48), E. munbyanus Pomel, 1856 (2n = 46), and E. quercinus Linnaeus, 1766 – the latter shows remarkable chromosomal variation. Four different karyotypes with four different diploid numbers (2n = 48, 50, 52, and 54) have been described in this species (reviewed in Zima et al. Citation1994; Moreno Citation2002).
Eliomys quercinus is largely confined to western Europe, including the Mediterranean islands and North Africa, with eastern populations having become reduced and fragmented into populations of Finland, Baltic Republics, Central Russia, Romania and a few others. The lowest diploid number, 2n = 48, characterizes the populations of the circum-Mediterranean area and the major isles, excluding Sardinia (2n = 50) (Cristaldi & Canipari Citation1976). Moreover, an additional 48-chromosome karyotype has been reported in north-western Russia (Graphodatsky & Fokin Citation1993). Furthermore, in several regions of a wide geographic range of E. quercinus, karyotypes, which conserve an identical diploid number (2n = 48 or, in other cases, 2n = 50), may still differ in chromosome morphology (NF, fundamental number) (). Thus, the 2n = 48 karyotypes of the Croatian E. quercinus dalmaticus (Vujošević et al. Citation1993) and the insular E. q. liparensis (Godena et al. Citation1978) have been reported to differ in the morphology of two pairs of autosomes from E. q. lusitanicus described from Spain (Diaz de la Guardia & Ruiz Girela Citation1979). Substantial differences have been also reported in the 48-chromosome karyotype from Romania (Murariu et al. Citation1985).
Table I. Cytogenetic data in 2n = 48 populations of Eliomys quercinus.
The 2n = 48 karyotype has been reported in southern and central Italian populations of the Italian garden dormouse E. quercinus pallidus (Filippucci et al. Citation1988a), while the standard karyotype from a single male individual from Sicily was described as “corresponding to E. q. pallidus” (Cristaldi & Canipari Citation1976). On the other hand, the Sicilian dormouse was considered a distinct subspecies, E. quercinus dichrurus Rafinesque, 1814, by some authors (Sarà & Casamento Citation1994; McKenna & Bell Citation1997).
While the continuing existence of E. quercinus in Central Italy from the beginning of Würm (70–15 Kya) is evident from fossil data (Kotsakis Citation1991), not as much is known regarding the Sicilian dormouse. For a number of vertebrates, the Sicilian populations have been found to be differentiated compared to the northern populations (Santucci et al. Citation1996; Lenk et al. Citation1999; Michaux et al. Citation2005; Castiglia et al. Citation2008). Nonetheless, there is no biochemical or genetic data available regarding the insular E. quercinus and its genetic status remains uncertain. Whether the Sicilian population of E. quercinus is a distinct taxonomic unit and/or has a distinct cytotype still needs to be elucidated. To approach the subject, we carried out a comparative study of individuals from Sicily and the Central Apennines by analysing DNA sequences of the mitochondrial gene for cytochrome b and metaphase chromosomes by means of G- and C-banding and fluorescence in situ hybridization (FISH) of telomeric repeats and major ribosomal genes (rDNA). The data are discussed in relation to the remarkable chromosomal variation and to the evolutionary dynamics of chromosomal rearrangements in the species.
Materials and methods
Animal samples
Specimens of Eliomys quercinus were collected at Maletto (37° 49’N 14° 51’E) and Nicolosi (37° 49’N 14°51’E) (Catania, Sicily, Italy) (two males QU5, QU8 and one female QU1) and Campo Felice (42°06’N 13°35’E) (l'Aquila, Abruzzo, Italy) (QU4, a female). The animals were handled according to the European Code of Practice for the housing and care of animals used in scientific procedures (Council of Europe Citation1986).
DNA extraction, purification, PCR amplification and analysis
Total genomic DNA was obtained from muscle preserved in 80% ethanol. DNA extraction was performed using the DNeasy tissue kit (Qiagen). A fragment encompassing the entire cytochrome b sequence was PCR-amplified with universal primers L14723 and H15915 (Irwin et al. Citation1991). Each PCR mix (50 μl) contained 50–500 ng of template DNA, 200 ng of each primer, 0.2 mM of each dNTP and 2U of Taq polymerase (Promega). The procedure was performed in an MJ MiniCycler as follows: initial denaturation at 94°C for 2 min; 35 cycles with denaturation at 94°C for 1 min, annealing at 52°C for 1 min, and extension at 72°C for 1 min; final 10 min extension at 72°C. Amplified products were purified using the Qiagen QIAquik purification kit and sequenced in both strands (Macrogen). DNA sequences were aligned using Clustal X and genetic divergence between haplotypes was calculated by Mega 4 (Tamura et al. Citation2007) software using Kimura's two-parameter model (Kimura Citation1980).
Chromosome preparation and analysis
Metaphases were obtained from a primary culture of fibroblasts prepared from skin biopsy or from bone marrow using a standard air-drying technique. One milligram per millilitre of Vinblastin sulphate (Velbe, Lilly) was used as a mitostatic agent in both protocols. Regular G- (Sumner et al. Citation1971) and C-banding (Sumner Citation1972) protocols were applied after necessary adjustment of incubation times.
Fluorescence in situ hybridization: FISH
Two probes were used for FISH: a fragment of human rDNA repeat containing a 1800 base-pair 18S rRNA gene biotin-labelled by random priming (Invitrogen, Life technologies) and telomeric complementary oligonucleotides (GGGTTA)7/(TAACCC)7 3'-end-labelled with biotin (M-Medical, Genenco). Standard procedures for hybridization of repetitive sequences were carried out (Lichter et al. Citation1992), followed by high-stringency post-hybridization washes and three-round signal amplification by Avidin-FITC/biotinylated anti-Avidin (Vector). Metaphases were counterstained with 4',6-diamidino-2-phenylindole (DAPI) and propidium iodide. Digital images were acquired and elaborated by IPLab software (Scanalytics Inc.) and processed in Photoshop CS (Adobe Systems Inc.) (see ).
Results
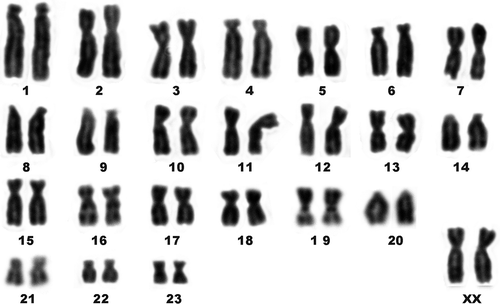
All the individuals studied showed the same standard Giemsa karyotype, 2n = 48, NFa = 86 (). The karyotype includes five pairs of large subtelocentric chromosomes (1, 4, 6, 8 and 9), two pairs of medium-sized acrocentric chromosomes (14, 20), a gradual series of 14 pairs of meta- and submetacentric chromosomes including the large X chromosome, and the three smallest biarmed chromosomes (21, 22, and 23). The chromosomes 16 and 19 show evident secondary constrictions and bear NORs, as revealed by FISH-mapping of 18S rDNA (). No interpopulation differences in the number and position of NORs were detected. Individuals of E. quercinus from Sicily and Central Italy also show matching FISH patterns of telomeric repeats. Telomeric repeats are present interstitially on the largest chromosome pair, and, occasionally, at the centromeres of the X chromosomes (). In both populations, chromosomes 1 show two moderately heterochromatic regions, subcentromeric and telomeric, on the long arms (), while the overall constitutive heterochromatin pattern is remarkably scarce: C-bands are present chiefly at the centromeres of acrocentric chromosomes. No visible disparity between the two populations under examination was evidenced by C- (not shown) and G-banding ().
Figure 2. Selective patterns of chromosome locations of (a) 18S rDNA and (b) interstitial telomeric sequences, detected by FISH, and (c) constitutive heterochromatin detected by C-banding. In (a), arrows indicate rDNA/NOR-bearing chromosomes in a metaphase plate counterstained with DAPI; the insert shows the same chromosomes after FISH. In (b), telomeric signals on chromosomes 1 in the populations of Central Italy (upper row) and Sicily (middle row), additional signals on the X chromosomes (lower row); in (c), corresponding C-banded chromosomes 1.
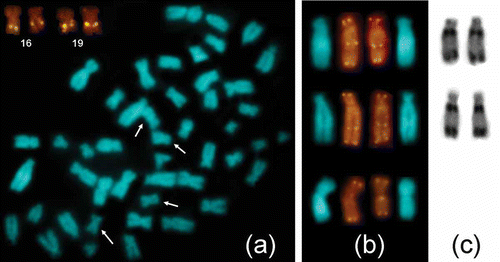
Figure 3. G-banded karyotypes of E. quercinus from Central Italy (upper row, female) and Sicily (lower row, male).
The entire sequence of the cytochrome b gene was obtained for one Sicilian and one central Italian specimen (GenBank accession number GQ453668-9). Genetic divergence between the two Italian haplotypes is low (1.2%), while it is distinctly higher (4.1–4.7%) between the samples examined in this study and a single haplotype accessible in GenBank (AJ225030) presumably obtained from Southern France (Bentz & Montgelard Citation1999).
Discussion
Based on the present, albeit limited, DNA sequencing and cytogenetic data, we found no important differences between the samples of Eliomys quercinus from the Central Apennines and Sicily. The genetic homogeneity between peninsular and Sicilian samples confirms that these individuals belong to the same taxon – E. quercinus pallidus. In fact, the level of genetic divergence in the samples studied is of the same magnitude commonly found between geographical populations of the same species (Baker & Bradley Citation2006) and may indicate a recent time of isolation of the Sicilian dormouse. On the contrary, the single specimen from a trans-Alpine population shows evidently higher level of genetic divergence.
The two populations presently analysed show the same cytotype and no cryptic cytogenetic differences in distribution of interstitial telomeric sequences and rDNA. If compared to other data from literature (Sánchez et al. Citation1989; Zurita et al. Citation1999), the rDNA sites are most likely generally conserved in the species. Furthermore, we revealed no important differences in chromosome banding patterns between presently examined samples and the ones previously obtained from Southern Italy (Pollino Massif, Basilicata) (Filippucci et al. Citation1988a) and Dalmatia (ex-Yugoslavia) (Vujošević et al. Citation1993). The particular C-banding pattern of chromosomes 1 may represent a distinct cytogenetic trait characterising only Italian populations, since it has not been revealed in other populations of E. quercinus so far studied.
FISH also detected interstitial telomeric sequences (ITS) in the long arms of chromosomes 1. A cytogenetic co-localization of ITSs and fragile sites has been reported in rodents (Bertoni et al. Citation1996; Camats et al. Citation2006), suggesting that the regions they are inserted in may be prone to chromosomal rearrangement. The repetitive nature of telomeric sequences, in fact, promotes rearrangements through their own misalignment and subsequent non-allelic homologous recombination (Stankiewicz & Lupski Citation2002). On the other hand, the intercalary position of ITS on chromosomes 1 may be indicative of a past chromosomal break accompanied by insertion of telomeric repeats (Ruiz-Herrera et al. Citation2008). According to this hypothesis, short stretches of telomeric repeats distributed at internal (not centromeric) sites of the chromosomes represent the remnants of evolutionary chromosomal rearrangements (Ruiz-Herrera et al. Citation2008). A paracentric inversion inferred from the G-banding pattern of the largest chromosome pair of another species of Eliomys, the Israeli dormouse E. melanurus (Filippucci et al. Citation1988a) reinforces this supposition.
Different hypotheses of chromosomal evolution in E. quercinus have been proposed. One postulates that the most ancient populations of E. quercinus are those of southern Europe (2n = 48), wherefrom they spread northward since Holocene. The higher diploid numbers of the northern populations of the species, in this case, are believed to be due to Robertsonian rearrangements, such as centric fissions of biarmed chromosomes (Arroyo Nombela et al. Citation1982; Filippucci et al. Citation1988a; Filippucci & Capanna Citation1996). In this context, the presence of a karyotype with 2n = 48 in north-western Russia (Graphodatsky & Fokin Citation1993) is interesting and could indicate that the process of chromosomal divergence have taken place after the post-glacial dispersal from Mediterranean refugia.
The separate glacial refugial survival is now seen as a principal factor, which caused recent genetic divergence and speciation (Hewitt Citation1999). Sicily, the major Mediterranean island proximal to the Italian peninsula, displayed natural conditions that could have provided hospitality to a variety of species during the climatic extremes of the Pleistocene ice ages (Bonfiglio et al. Citation2002). An early isolation of Sicilian populations of many terrestrial species allowed population divergence and speciation (i.e. Fritz et al. Citation2005). On the other hand, several connections between Sicily and Italy during the late Pleistocene could have allowed certain faunal exchange between the island and the mainland (Bonfiglio et al. Citation2002).
Analysing our cytogenetic data together with available comparable karyotypes from other 2n = 48 populations (), we also noticed certain discrepancies in using chromosome nomenclature (Diaz de la Guardia & Ruiz Girela Citation1979; Arroyo Nombela et al. Citation1982; Filippucci et al. Citation1988a; Sánchez et al. Citation1991). Notwithstanding this problem, differences between the Italian and the Iberian and Iberian-derived populations are evident. The 2n = 48 G-banded karyotypes of the Spanish E. quercinus (Diaz de la Guardia & Ruiz Girela Citation1979; Arroyo Nombela et al. Citation1982; Sánchez et al. Citation1991) include two “biarmed” chromosomes, which correspond to “uniarmed” chromosomes 9 and 14 in our study. The comparison of the C- and G-banding patterns confirms the presumed pericentric inversions in these chromosome pairs. This is also true for the one of the largest garden dormouse, E. quercinus ophiusae from the island of Formentera (Ramalhinho & Libois Citation2001). It is worth to be noted that a similar rearrangement has been already observed in the chromosome pair 6 (original numbering) by comparing E. q. quercinus and E. mumbyanus (Sánchez et al. Citation1991).
Chromosome polymorphisms generated by pericentric inversions are common in mammals, but their evolutionary role is not fully understood. It is generally thought that they do not reduce fertility, but a high number of inversions in a heterozygous state may interfere with the correct pairing of homologous chromosomes in meiosis (Volobouev et al. Citation2001) and may accelerate genetic isolation (Volobouev et al. Citation2002). In addition, pericentric inversions of acrocentric chromosomes, which revert them to metacentric chromosomes, may re-potentiate the karyotype for subsequent centric fissions (Imai et al. Citation2001) – the pattern of chromosomal divergence hypothesized for E. quercinus (Arroyo Nombela et al. Citation1982; Filippucci et al. Citation1988a).
Acknowledgement
This work was supported by “Finanziamento Ricerca di Ateneo Federato (2007)” to R.C. We wish to thank E. Capanna for his permission to use part of his funds for our work, M. Cristaldi for his encouragement and help in the field, F. Annesi for technical support and A. Bezerra for her competent help in the field.
References
- Arroyo Nombela , J , Rodriguez-Murcia , C , Delibes , M and Hiraldo , F. 1982 . Comparative karyotype studies between Spanish and French populations of Eliomys quercinus L . Genetica , 59 : 161 – 166 .
- Baker , RJ and Bradley , RD. 2006 . Speciation in mammals and the genetic species concept . Journal of Mammalogy , 87 : 643 – 662 .
- Bentz , S and Montgelard , C. 1999 . Systematic position of the African dormouse Graphiurus (Rodentia, Gliridae) assessed from cytochrome b and 12S rRNA mitochondrial genes . Journal of Mammalian Evolution , 6 : 67 – 83 .
- Bertoni , L , Attolini , C , Faravelli , M , Simi , S and Giulotto , E . 1996 . Intrachromosomal telomere-like DNA sequenze in Chinese hamster . Mammal. Genome , 7 : 853 – 855 .
- Bonfiglio , L , Mangano , G , Marra , AC , Masini , F , Pavia , M and Petruso , D. 2002 . Pleistocene Calabrian and Sicilian bioprovinces . Geobios , 35 : 29 – 39 .
- Camats , N , Ruiz-Herrera , A , Parrilla , JJ , Acien , M Paya , P . 2006 . Genomic instability in rat: Breakpoints induced by ionising radiation and interstitial telomeric-like sequences . Mutation Research , 605 : 157 – 166 .
- Castiglia , R , Annesi , F , Aloise , G and Amori , G. 2008 . Systematics of the Microtus savii complex (Rodentia, Cricetidae) via mitochondrial DNA analyses: Paraphyly and pattern of sex chromosome evolution . Molecular Phylogenetics and Evolution , 3 : 1157 – 1164 .
- Council of Europe . 1986 . “ European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes ” . In Strasbourg: Council of Europe , Obtainable London : HMSO .
- Cristaldi , M and Canipari , R. 1976 . À propos de la caryologie du lérot (Eliomys quercinus) . Mammalia , 40 : 475 – 488 .
- Diaz de la Guardia , RS and Ruiz Girela , M . 1979 . The chromosomes of three Spanish subspecies of Eliomys quercinus (Linnaeus) . Genetica , 51 : 107 – 109 .
- Filippucci , MG and Capanna , E. 1996 . Allozyme variation and differentiation among chromosomal races and species in the genus Eliomys (Rodentia, Myoxidae) . Proceedings of the I European congress of mammalogy, Museu Bocage, Lisboa, pp , : 259 – 270 .
- Filippucci , MG , Civitelli , MV and Capanna , E. 1988a . Evolutionary genetics and systematics of the garden dormouse Eliomys Wagner, 1840 (Gliridae, Mammalia). 1 – Karyotype divergence . Italian Journal of Zoology , 55 : 35 – 45 .
- Filippucci , MG , Rodinò , E , Nevo , E and Capanna , E. 1988b . Evolutionary genetics and systematics of the garden dormouse Eliomys Wagner, 1840. 2 – Allozyme diversity and differentiation of chromosomal races . Italian Journal of Zoology , 55 : 47 – 54 .
- Fritz , U , Fattizzo , T , Guicking , D , Tripepi , S , Pennisi , MG Lenk , P . 2005 . A new cryptic species of pond turtle from southern Italy, the hottest spot in the range of the genus Emys (Reptilia, Testudines, Emydidae) . Zoologica Scripta , 34 : 351 – 371 .
- Godena , G , D'Alonzo , F and Cristaldi , M. 1978 . Corrélations entre caryotypes et biotypes chez le lérot (Eliomys quercinus) et les autres rongeurs de l'île Lipari . Mammalia , 42 : 382 – 384 .
- Graphodatsky , A and Fokin , I. 1993 . Comparative cytogenetics of Gliridae . Zoolicheskii Zhurnal , 72 : 194 – 213 .
- Hewitt , GM. 1999 . Post-glacial recolonization of European biota . Biological Journal of the Linnean Society , 68 : 87 – 112 .
- Imai , HT , Satta , Y and Takahata , N. 2001 . Integrative study on chromosome evolution of mammals, ants and wasps based on the minimum interaction theory . Journal of Theoretical Biology , 210 : 475 – 497 .
- Irwin , DM , Kocher , TD and Wilson , AC. 1991 . Evolution of the cytochrome b gene of mammals . Journal of Molecular Evolution , 32 : 28 – 144 .
- Kimura , M. 1980 . A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences . Journal of Molecular Evolution , 16 : 111 – 120 .
- Kotsakis , A. 1991 . Late Pleistocene fossil microvertebrates of Grotta Breuil (Monte Circeo, Central Italy) . Quaternaria Nova , 1 : 325 – 332 .
- Lenk , P , Fritz , U , Joger , U and Wink , M. 1999 . Mitochondrial phylogeography of the European pond turtle, Emys orbicularis (Linnaeus, 1758) . Molecular Ecology , 8 : 1911 – 1922 .
- Lichter , P , Boyle , A , Wienberg , J , Arnold , N , Popp , S , Cremer , T and Ward , DC. 1992 . In situ hybridization to human metaphase chromosomes using DIG- or biotin-labeled DNA probes and detection with fluorochrome conjugates. In: Nonradioactive in situ hybridization (Application manual) . Boehringer Mannheim, Biochemica, Mannheim , : 25 – 27 .
- McKenna , MC and Bell , SK . 1997 . Classification of mammals – above the species level , XII – 631 . New York : Columbia University Press .
- Michaux , JR , Hardy , OJ , Justy , F , Fournier , P , Kranz , A Cabria , M . 2005 . Conservation genetics and population history of the threatened European mink Mustela lutreola, with an emphasis on the west European population . Molecular Ecology , 14 : 2373 – 2388 .
- Moreno , S. 2002 . Lirón careto Eliomys quercinus (Linnaeus, 1766) . Galemys , 14 : 1 – 16 .
- Murariu , D , Lungeanu , A , Gavrila , L and Stepan , C. 1985 . Preliminary data concerning the study of the karyotype of Eliomys quercinus (Linnaeus, 1766) (Mammalia, Gliridae) . Travaux du Museum d'Histoire naturelle “Grigore Antipa” , 27 : 325 – 327 .
- Ramalhinho , MG and Libois , R. 2001 . The karyotype of the Formentera island garden dormouse, Eliomys quercinus ophiusae . Belgian Journal of Zoology , 131 : 83 – 85 .
- Ruiz-Herrera , A , Nergadze , SG , Santagostino , M and Giulotto , E. 2008 . Telomeric repeats far from the ends: Mechanisms of origin and role in evolution . Cytogenetic and Genome Research , 122 : 219 – 228 .
- Sánchez , A , Burgos , M , Jimenez , R , Chamorro , S and Diaz de la Guardia , R . 1991 . Evolution of Eliomys karyotypes in western Mediterranean regions . Cytobios , 66 : 25 – 33 .
- Sánchez , A , Burgos , M , Jimenez , R and Diaz de la Guardia , R . 1989 . Quantitative analysis of silver staining of the nucleolar organizing region in Eliomys quercinus . Genome , 32 : 978 – 982 .
- Santucci , F , Nascetti , G and Bullini , L. 1996 . Hybrid zones between two genetically differentiated forms of the pond frog Rana lessonae in southern Italy . Journal of Evolutionary Biology , 9 : 429 – 450 .
- Sarà , M and Casamento , G. 1994 . Distribution and ecology of dormice (Myoxidae) in Sicilia . A preliminary account. Hystrix , 6 : 161 – 168 .
- Stankiewicz , P and Lupski , JR. 2002 . Genome architecture, rearrangements and genomic disorders . Trends in Genetics , 18 : 74 – 82 .
- Sumner , AT. 1972 . A simple technique for demonstrating centromeric heterochromatin . Experimental Cell Research , 75 : 304 – 306 .
- Sumner , AT , Evans , HJ and Buckland , RA. 1971 . New technique for distinguishing between human chromosomes . Nature New Biology , 232 : 31 – 32 .
- Tamura , K , Dudley , J , Nei , M and Kumar , S. 2007 . MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0 . Molecular Biology and Evolution , 24 : 1596 – 1599 .
- Volobouev , VT , Aniskin , VM , Lecompte , E and Ducroz , JF. 2002 . Patterns of karyotype evolution in complexes of sibling species within three genera of African murid rodents inferred from the comparison of cytogenetic and molecular data . Cytogenetic and Genome Research , 96 : 261 – 275 .
- Volobouev , VT , Hoffmann , A , Sicard , B and Granjon , L. 2001 . Polymorphism and polytypy for pericentric inversions in 38-chromosome Mastomys (Rodentia, Murinae) and possible taxonomic implications . Cytogenetic and Genome Research , 92 : 237 – 242 .
- Vujošević , M , Filippucci , MG , Blagojević , J and Kryštufek , B. 1993 . Evolutionary genetics and systematics of the garden dormouse, Eliomys Wagner, 1840. 4. Karyotype and allozyme analyses of E. quercinus dalmaticus from Yugoslavia . Italian Journal of Zoology , 60 : 47 – 51 .
- Zima , J , Macholan , M and Filippucci , MG. 1994 . “ Proceedings of II Conference on dormice (Rodentia, Myoxidae). Hystrix 6: 1–340 ” . In Chromosomal variation and systematics of myoxids Edited by: Filippucci , MG . 63 – 76 .
- Zurita , F , Jiménez , R , Diaz de la Guardia , RS and Burgos , M. 1999 . The relative rDNA content of a NOR determines its level of expression and its probability of becoming active . A sequential silver staining and in-situ hybridization study. Chromosome Research , 7 : 563 – 570 .