Abstract
The present work studied the annual and daily aldosterone, norepinephrine and epinephrine patterns in the newt Triturus carnifex. The annual variations of the serum aldosterone were evaluated every month by radioimmunoassay, from September to July; the daily variations of this hormone were evaluated in the months of January, April, July and November. Aldosterone levels were low from September to November (lowest value 187.23 ± 19.7 pg/ml in November), increased in December, remained high until March and peaked in April (587.25 ± 58.2 pg/ml). In May, the levels decreased and remained steady until July. Considering the daily aldosterone pattern, in all the examined months, serum aldosterone levels were lowest at 5:00 am (lowest value 162.51 ± 17.4 pg/ml in November) and peaked at 5:00 pm (maximal value 2075.00 ± 213.00 pg/ml in April). As regards the annual catecholamine pattern as measured by HPLC, serum norepinephrine levels were low from September to November (lowest value 541.13 ± 39.8 pg/ml in September), increased in December, peaked in January (1457.03 ± 106.97 pg/ml) and remained high in February. In March, the levels decreased and remained steady until June, then decreased in July. Serum epinephrine levels increased from September to November, decreased in December and remained low until February. In March, the levels increased and peaked in April (721.55 ± 49.91 pg/ml), then decreased (lowest value 140.00 ± 10.0 pg/ml in June) and remained steady until July. Considering the daily catecholamines levels, in all the examined months, there were no daily variations among serum norepinephrine levels. Otherwise, serum epinephrine levels were always lowest at 5:00 am (lowest value 100.77 ± 9.9 pg/ml in January) and peaked at 11:00 pm (maximal value 3831.05 ± 211.5 pg/ml in November). The annual and daily aldosterone and catecholamine patterns appear correlated to the metabolic activity of this species.
Introduction
The adrenal gland of the newt Triturus carnifex is made up by numerous discrete bodies, separated from one another, lying on the ventral surface of the kidney, close to its medial margin. The bodies contain tightly intermingled steroidogenic and chromaffin cells, which interact with each other (Capaldo et al. Citation2004). The steroidogenic tissue mainly produces the corticosteroids aldosterone and corticosterone. There is no evidence of zonation of adrenocortical cells (Hanke Citation1978). The chromaffin tissue has only one type of chromaffin cell, producing the catecholamines, norepinephrine and epinephrine, in accordance with a functional cycle, correlated to the environmental temperature. During both the December–January and May–August periods, large quantities of norepinephrine and small quantities of epinephrine granules are present in the chromaffin cells. During the February–April and September–November periods, the production of epinephrine increases and the chromaffin cells contain almost the same quantities of norepinephrine and epinephrine granules (Laforgia & Capaldo Citation1991).
Both corticosteroids and catecholamines play a key role in the metabolism of the amphibians. Corticosterone and, to a lesser extent, aldosterone, are gluconeogenic and hyperglycaemic, but the primary role of these steroids appears to be the regulation of ion and water balance. Epinephrine is a potent stimulator of lypolysis in fat bodies and of glycogenolysis in both liver and muscle and is therefore hyperglycaemic. Hence, the adrenal gland plays a key role in the stress response, which includes the release of both corticosteroids and catecholamines. These hormones induce changes in metabolism and/or ionic regulation that work to combat physiological factors and to eliminate or to neutralize the stressful stimulus, allowing the organism to adapt to its environment, and to survive (Norris Citation2007).
Moreover, previous studies suggest an involvement of the adrenal gland in the amphibian reproductive processes. In the rough-skinned newt, Taricha granulosa, corticosterone has been implicated in the inhibition of the amphibian reproductive behavior (Moore & Miller Citation1984; Moore & Zoeller Citation1985), whereas recent studies report a positive relationship between corticosterone and reproduction (Moore & Jessop Citation2003). In T. carnifex, some studies evidence the presence of a correlation between some events of the reproductive cycle and the activity of the adrenal gland. Follicle-stimulating hormone (FSH), regulating spermatogenesis in amphibians (Galgano Citation1942, Citation1943; Mazzi & Vellano Citation1968) stimulated corticosteroid and catecholamine release from the newt adrenal gland, probably in order to produce the increase in metabolism necessary for spermatogenesis (Gay et al. Citation2008). In this species, the presence of large amounts of epinephrine in the chromaffin cells coincides, during the February–April period, with the breeding season, and during the September–November period with the beginning of both the luteinizing hormone (LH) synthesis cycle and the secondary sex characteristics cycle (Laforgia & Capaldo Citation1991).
In amphibians, both circadian and circannual variations in corticosteroid (Dupont et al. Citation1979; Licht et al. Citation1983; Pancak & Taylor Citation1983; Thurmond et al. Citation1986; Houck et al. Citation1996; Wright et al. Citation2003) release were demonstrated. In Bufo japonicus formosus, low values of plasma aldosterone levels were recorded in winter and spring, and the maximum occurred in July or August, in correlation with the increases in liver weight (Jolivet-Jaudet et al. Citation1984). In the toad Bufo japonicus, an increase in plasma epinephrine concentration during the migration to the breeding ponds was found (Wilson et al. Citation1995).
Aldosterone and the catecholamines allow amphibians to adapt to their environment. Since seasonal and daily changes in the environment occur, variations of the interrenal activity should be observed dependent on seasons or daytime. In T. carnifex, only the annual and daily pattern of corticosterone is well known (Zerani & Gobbetti Citation1993), whereas data are lacking about the variations of circulating aldosterone, norepinephrine and epinephrine during the year and the day. Therefore, our study concentrated on the annual and daily pattern of aldosterone and the two catecholamines, norepinephrine and epinephrine.
Materials and methods
Animals
Adult male specimens of Triturus carnifex (mean weight 8.0 g) were captured in the neighbourhood of Naples, Italy. After capture, the newts were kept in aquaria at seasonal temperature and photoperiod and fed minced cow liver, between 09:00 am and 10:00 am. The annual variations of the serum adrenal hormones (aldosterone, norepinephrine and epinephrine) were evaluated by using 10 newts each month, from September to July. In August, it was not possible to obtain animals because, in this period, newts hide on land. The average temperatures for the months studied were the following: 20.8°C in September, 17.0°C in October, 14.4°C in November, 9.3°C in December, 9.5°C in January, 6.2°C in February, 10.6°C in March, 13.9°C in April, 20.7°C in May, 25.3°C in June, 26.5°C in July. The natural light/dark cycles (average values) for the months studied were the following: light from 5:35 am to 6:42 pm in September; from 6:05 am to 5:51 pm in October; from 6:40 am to 5:04 pm in November; from 7:15 am to 4:41 pm in December; from 7:34 am to 4:51 pm in January; from 7:20 am to 5:26 pm in February; from 6:43 am to 6:00 pm in March; from 5:52 am to 6:34 pm in April; from 5:07 am to 7:06 pm in May; from 4:39 am to 7:35 pm in June; from 4:40 am to 7:45 pm in July. On the 15th day of each month, 2 days after capture, between 11 am and 12 pm, the newts were killed by decapitation. The daily variations of the serum adrenal hormones (aldosterone, norepinephrine and epinephrine) were evaluated in the months of January, April, July and November, corresponding to four different stages in the functional cycle of the adrenal gland. On the 15th day of each month, 2 days after capture, at four fixed intervals (5:00 am, 11:00 am, 5:00 pm, 11:00 pm), five newts for each interval were killed by decapitation. The natural light/dark cycles for the days studied were the following: light from 7:29 am to 5:11 pm in January; from 5:22 am to 6:54 pm in April; from 4:53 am to 7:37 pm in July; from 7:03 am to 4:46 pm in November. Blood was immediately collected over 3 min by heart puncture, centrifuged for 15 min at 2000 g and serum was collected and stored at –22°C until assayed. Institutional committees (Department of Health) approved the experiments, which were organized to minimize the stress and the number of animals used.
Hormone assay
Serum levels of aldosterone were determined by radioimmunoassay (RIA) as previously described (Andreoletti et al. Citation1988; Capaldo et al. Citation2006). Briefly, nonhaemolysed serum samples (80 μl) were incubated for 30 min at 37°C with known amounts of radioactive steroid (3H-aldosterone from Bio-Rad, Hercules, CA) in 0.06 M Na-phosphate buffer containing 0.01 EDTA disodium salt and 0.1% BSA pH 7.4. Samples were applied to an extraction column (Sep-Pak C18, Waters, Milford, MA) and washed with 500 μl of pure methanol. Methanol extracts were dried at 37°C under vacuum and redissolved in 1400 μl of PBS. An aliquot was taken to determine the labelled hormone recovery and on the other aliquot aldosterone was assayed by RIA. After incubation with rabbit antiserum (Biogenesis, Poole, UK) for 30 min at 37°C and for another 2 h in an ice bath, dextran-coated charcoal was used to separate free from bound steroids. After immersion for 10 min in an ice bath and centrifugation (2000 rpm), a supernatant aliquot was counted with a liquid scintillation spectrometer (Tri-Carb Packard, GMI, Albertville, MN, USA). Extraction yields ranged from 80 to 90%. Data were obtained through a standard calibration curve linearized with a log-logit method and corrected for individual extraction yield. Sensitivity was 5 pg/tube. The intra-assay coefficient of variation was 10%, and the inter-assay coefficient of variation was 12%.
Norepinephrine and epinephrine levels were determined in 150 μl serum. For catecholamine extraction, 50 μl of dihydroxybenzylamine were added as an internal standard. Ten milligrams of activated aluminium oxide (Sigma, St. Louise, MO) was used as adsorbent for catecholamines and the internal standard. After 15 min shaking and centrifugation, the supernatant was removed, and the aluminium oxide containing the adsorbed catecholamines and the internal standard was washed three times with 1 ml distilled water by shaking, centrifuging, and discarding the supernatant. After a final wash, adsorbed catecholamines and internal standard were desorbed from aluminium oxide by acidic elution. After shaking for 15 min, samples were centrifuged; the supernatant containing desorbed catecholamines and internal standard was removed and determination of catecholamines was performed using high-performance liquid chromatography (HPLC), with electrochemical detection, according to the method previously used in Triturus carnifex (Kloas & Hanke Citation1992; Capaldo et al. Citation2006). Electrochemical HPLC detection was carried out using an acid eluant; norepinephrine and epinephrine levels were calculated in comparison to the internal standard (dihydroxybenzylamine). The detection limit for NE and E was around 20 pg. The intra-assay coefficient of variation was 7%, and the inter-assay coefficient of variation was 10%.
Statistical analysis
All data were expressed as means ± standard error of mean (SEM). Statistical comparisons between two means were made by means of Duncan's multiple range test following the one-way analysis of variance (ANOVA). P < 0.05 was considered statistically significant.
Results
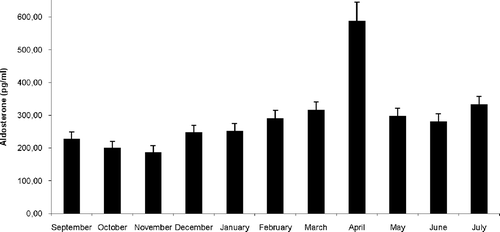
Aldosterone annual variations
Serum aldosterone levels were low from September to November; no significant variation was observed (). In December, the levels significantly increased (P < 0.05 between November and December), remained high until March and peaked in April (P < 0.001 between March and April). In May, the levels decreased (P < 0.001 between April and May) and remained steady until July. The levels of July were significantly (P < 0.05) higher than in September.
Figure 1. Annual variations of serum aldosterone levels in male Triturus carnifex. Values are means ± SE of the mean. For each group, 10 animals were used. P < 0.05 between November and December; P < 0.001 between March and April; P < 0.001 between April and May; P < 0.05 between July and September.
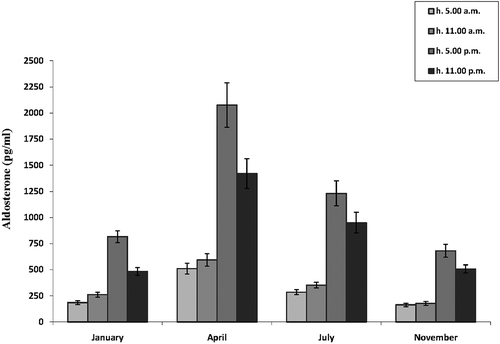
Aldosterone daily variations
In all the examined months (January, April, July, November), serum aldosterone levels were lowest at 5:00 am and remained low at 11:00 am (). Then they increased and peaked at 5:00 pm (P < 0.001 between 5:00 am and 5:00 pm levels in all the months). At 11:00 pm, the levels were significantly lower than at 5:00 pm (P < 0.001 between 5:00 pm and 11:00 pm levels in January and April; P < 0.05 between 5:00 pm and 11:00 pm levels in July and November).
Figure 2. Daily variations of serum aldosterone levels in male Triturus carnifex. Values are means ± SE of the mean. For each group, five animals were used. P < 0.001 between 5:00 am and 5:00 pm levels in all the months; P < 0.001 between 5:00 pm and 11:00 pm levels in January and April; P < 0.05 between 5:00 pm and 11:00 pm levels in July and November.
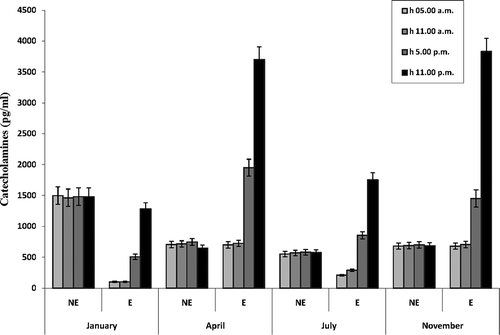
Catecholamine annual variations
Serum norepinephrine and epinephrine levels had a different pattern during the year (). Serum norepinephrine levels were low from September to November; no significant variation was observed. In December, the levels significantly increased (P < 0.05 between November and December), peaked in January (P < 0.001 between December and January) and remained high in February. In March, the levels decreased (P < 0.001 between February and March) and remained steady until June. There was a further decrease in July (P < 0.05 between June and July); no significant variation was observed between the levels of July and September.
Figure 3. Annual variations of serum catecholamines (norepinephrine and epinephrine) levels in male Triturus carnifex. Values are means ± SE of the mean. Norepinephrine: P < 0.05 between November and December; P < 0.001 between December and January; P < 0.001 between February and March; P < 0.05 between June and July. Epinephrine: P < 0.05 between September and November; P < 0.001 between November and December; P < 0.001 between February and March; P < 0.001 between April and May; P < 0.001 between July and September.
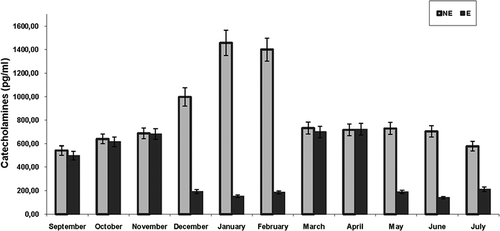
Serum epinephrine levels were high in fall, when they increased from September to November (P < 0.05 between September and November). In December, the levels decreased significantly (P < 0.001 between November and December), and remained low until February. In March, the levels increased significantly (P < 0.001 between February and March) and remained steady until April; there was a further decrease in May (P < 0.001 between April and May), and the levels remained steady until July. The levels in July were significantly lower (P < 0.001) than in September.
Finally, the comparison between the levels of the two catecholamines during the year showed that during September–November and March–April periods, the levels of the two catecholamines were similar in each month; during December–February and May–July periods, the levels of norepinephrine were significantly (P < 0.001) higher than epinephrine levels in each month.
Catecholamine daily variations
Serum norepinephrine and epinephrine levels had a different pattern during the day (). In all the examined months (January, April, July, November), there were no variations among serum norepinephrine levels at four fixed intervals (5:00 am, 11:00 am, 5:00 pm, 11:00 pm). On the contrary, in all the examined months, serum epinephrine levels were lowest at 5:00 am and remained low at 11:00 am; then, they significantly increased at 5:00 pm (P < 0.001 between 11:00 am and 5:00 pm levels) and peaked at 11:00 pm (P < 0.001 between 5:00 pm and 11:00 pm levels). The comparison between the levels of the two catecholamines during the day showed that, in January and July, at 5:00 am and 11:00 am, the levels of epinephrine were significantly (P < 0.001) lower than norepinephrine levels; then, epinephrine levels increased and, at 11:00 pm, touched (January) or went over (July) norepinephrine levels. In April and November, at 5:00 am and 11:00 am, the levels of epinephrine were like those of norepinephrine. Then, epinephrine levels increased and, at 11:00 pm, they went over norepinephrine levels.
Discussion
The results of the present research point out the existence of an annual and daily pattern of both aldosterone and catecholamines, norepinephrine and epinephrine.
The annual serum aldosterone pattern showed the lowest levels from September to November. Then, the levels rose from December up to a maximum in April, decreased in May, with values similar to those found during December–March period, and remained steady until July. Previous studies dealt with the occurrence of an annual aldosterone pattern in anuran amphibians. In Bufo japonicus formosus, low values of aldosterone levels were recorded from January to June; beginning in July, an increase was observed and the maximal level was reached in August. Then, in September, aldosterone levels decreased and remained low till December. The authors (Jolivet-Jaudet et al. Citation1984) concluded that, due to the glucocorticoid role of aldosterone in amphibians, the high levels of this hormone in July and August were related to the summer increase in the liver weight, likely due to storage of glycogen and other substances. The annual serum aldosterone pattern found in T. carnifex appears different from that observed in B. japonicus formosus. In T. carnifex, the breeding season spreads from January to May, with a peak in March–April, when the highest levels of aldosterone were found. It could be hypothesized that the high levels of this hormone could supply the newts with the energy necessary for reproduction, and/or could also be related to the newt adaptation to the aquatic environment, and therefore to the osmoregulatory role of this hormone.
The annual aldosterone pattern can be compared with the annual corticosterone pattern, found in T. carnifex (Zerani & Gobbetti Citation1993). In this species, the corticosterone levels were low from October to November, peaked in January, fell in February and remained low until March, after which they peaked in July. The low autumn levels of both aldosterone and corticosterone suggest a reduced activity of the steroidogenic tissue during this period. Beginning from December, the increase in the levels of both hormones suggests an increase in the activity of the steroidogenic tissue. However, the trend of corticosterone and aldosterone levels was different. Corticosterone peaked in January and in July (Zerani & Gobbetti Citation1993), whereas aldosterone peaked in April. Considering that, in the biosynthetic pathway, corticosterone is a precursor of aldosterone, it is as if the hormonal synthesis was shifted to corticosterone or aldosterone, according to the different periods of the year. In January and July, the biosynthetic pathway mainly produces corticosterone; in April, the biosynthetic pathway mainly produces aldosterone, probably to coincide with the peak of the breeding season.
The daily serum aldosterone pattern did not change during the year. It always showed low levels before noon, and high levels after noon. The lowest levels were at 5:00 am. Then, the levels rose up to a maximum at 5:00 pm and decreased at 11:00 pm, when they were significantly higher than the morning levels. Previous studies showed the occurrence of a daily aldosterone pattern in anuran amphibians. In Xenopus laevis, the maximal values for both aldosterone and corticosterone were found at 12:00 am, and the lowest values at 9:00 pm. Differences between both steroids were not found; moreover, these patterns were related to the feeding rhythms (Thurmond et al. Citation1986). The daily serum aldosterone pattern found in T. carnifex is different from that observed in X. laevis, and does not seem related to feeding rhythms; however, as in this species, differences between the patterns of both steroids were not found. Indeed, the daily aldosterone pattern appeared similar to that of corticosterone (Zerani & Gobbetti Citation1993); both hormones had lowest levels in the morning (5:00 am for aldosterone and 9:00 am for corticosterone) and highest levels in the afternoon (5:00 pm for both aldosterone and corticosterone). This suggests that, in the newt, there is an increased metabolic activity in the afternoon and at night.
The annual serum catecholamine pattern showed a difference between norepinephrine and epinephrine. Norepinephrine levels were low from September to November, increased in December up to a maximum in January–February, decreased in March and remained steady until June. Then, the levels decreased in July to values like those in September. The pattern of epinephrine was opposite of that of norepinephrine: epinephrine levels were high from September to November, decreased in December and remained steady until February, then increased in March and remained steady until April. The levels decreased in May and remained low until July. Above all, the examination of the catecholamine levels during the year showed that four main periods can be identified. During September–November and March–April periods, the serum levels of norepinephrine and epinephrine were almost the same; during December–February and May–July periods, the serum levels of norepinephrine were much higher than epinephrine levels. Considering that epinephrine comes from the methylation of norepinephrine, it seems that, during the December–February and May–July periods, the hormonal synthesis is shifted to norepinephrine, and only very low levels of epinephrine are present in the serum. The synthesis of epinephrine clearly increases, with values similar to those of norepinephrine, during two periods of the year: autumn (September–November) and spring (March–April). It is interesting to observe that these periods are characterized, respectively, by the beginning of both the LH synthesis cycle and the secondary sex characteristics cycle (autumn), and the breeding season (spring). Epinephrine is a potent stimulator of glycogenolysis in both liver and muscle and therefore is hyperglycaemic; moreover it mobilizes lipid stores through the release of fatty acids (Norris Citation2007). Therefore, it might be hypothesized that epinephrine synthesis could supply the newts with the energy necessary to these events of the reproductive cycle. An involvement of epinephrine in breeding was found also in another amphibian, the toad Bufo japonicus, which showed an increase in plasma epinephrine concentration during the migration to the breeding ponds (Wilson et al. Citation1995).
The present results confirm those of a previous study (Laforgia & Capaldo Citation1991), showing the existence of a functional cycle of T. carnifex chromaffin tissue, on the basis of the ultrastructure of the chromaffin cell. The only difference observed concerns the month of February that in this study showed very high levels of norepinephrine and very low levels of epinephrine, as the December–January period. It is likely that this difference could be due to differences in mean environmental temperatures.
The daily serum catecholamine pattern did not change during the year. Serum norepinephrine levels remained always steady during the day, probably due to the fact that not only adrenal chromaffin cells, but also the paraganglia and leakage from adrenergic synapses of the sympathetic nervous division may contribute to the circulating norepinephrine levels. Serum epinephrine levels always increased at 5:00 pm and peaked at 11:00 pm, also during the periods (December–February and May–July) characterized, in the morning, by high levels of norepinephrine and low levels of epinephrine. The night increase in epinephrine levels during all the year strengthens the hypothesis of an increased metabolic activity of the newt in the afternoon and in the night, and may explain why newts mate at night, when more energy is made available. Moreover, an influence of melatonin on the daily catecholamine pattern could be hypothesized, since the maximum of epinephrine levels was always found at 11:00 pm (melatonin is mainly secreted in the dark), but further studies are needed to verify this hypothesis (Norris Citation2007).
In conclusion, the present results show the existence, in T. carnifex, of an annual and daily aldosterone and catecholamine pattern, probably related to the metabolic activity of this species.
Acknowledgements
The authors thank Dr Tullio Criscuolo for assistance in the determination of serum aldosterone and catecholamine levels.
References
- Andreoletti , GE , Vellano , C , Colucci , D , Androne , C , Mazzi , V and Fasolo , A. 1988 . Anatomical organization of CRF-and AVT-like systems in the newt hypothalamus and the effects of localised lesion to the posterior hypothalamus on serum aldosterone and corticosterone . Bollettino di Zoologia , 55 : 261 – 268 .
- Capaldo , A , Gay , F , Criscuolo , T , Valiante , S , Laforgia , V and Varano , L. 2004 . Influence of acetylcholine administration on the interrenal gland of Triturus carnifex (Amphibia, Urodela) . Italian Journal of Zoology , 2 : 73 – 76 .
- Capaldo , A , Gay , F , De Falco , M , Virgilio , F , Valiante , S Laforgia , V . 2006 . The newt Triturus carnifex as a model for monitoring the ecotoxic impact of the fungicide thiophanate methyl and the adverse effects on the adrenal gland . Comparative Biochemistry and Physiology C , 143 : 86 – 93 .
- Dupont , W , Bourgeois , P , Reinberg , A and Vaillant , R. 1979 . Circannual and circadian rhythms in the concentration of corticosterone in the plasma of the edible frog (Rana esculenta L.) . Journal of Endocrinology , 80 : 117 – 125 .
- Galgano , M. 1942 . L'azione ormonale delle gonadi e dell'ipofisi sul ciclo sessuale annuale degli anfibi, in rapporto al clima . Bollettino della Società Italiana di Biologia Sperimentale , 17 : 1 – 3 .
- Galgano , M. 1943 . Tratti fondamentali del ciclo sessuale annuale negli anfibi dei nostri climi . Bollettino di Zoologia , 14 : 57 – 74 .
- Gay , F , Laforgia , V and Capaldo , A. 2008 . Human follicle-stimulating hormone modulates the adrenal gland activity of the Italian crested newt Triturus carnifex (Amphibia, Urodela) . Comparative Biochemistry and Physiology A , 151 : 126 – 132 .
- Hanke , W. 1978 . “ General, comparative and clinical endocrinology of the adrenal cortex ” . In The adrenal cortex of Amphibia , Edited by: Chester Jones , I and Henderson , IW . 419 – 495 . London : Academic Press .
- Houck , LD , Mendonça , MT , Lynch , TK and Scott , DE. 1996 . Courtship behaviour and plasma levels of androgens and corticosterone in male marbled salamanders, Ambystoma opacum (Ambystomatidae) . General and Comparative Endocrinology , 104 : 243 – 252 .
- Jolivet-Jaudet , G , Inoue , M , Takada , K and Ishii , S. 1984 . Circannual changes in plasma aldosterone levels in Bufo japonicus formosus . General and Comparative Endocrinology , 53 : 163 – 167 .
- Kloas , W and Hanke , W. 1992 . Effects of atrial natriuretic factor on corticosteroid and catecholamine secretion by the adrenals of Xenopus laevis . General and Comparative Endocrinology , 85 : 269 – 277 .
- Laforgia , V and Capaldo , A. 1991 . Annual cycle of the chromaffin cells of Triturus cristatus . Journal of Morphology , 208 : 83 – 90 .
- Licht , P , McCreery , BR , Barns , R and Pang , R. 1983 . Seasonal and stress related changes in the plasma gonadotropins, sex steroids, and corticosterone in the bullfrog, Rana catesbeiana . General and Comparative Endocrinology , 50 : 124 – 145 .
- Mazzi , V and Vellano , C. 1968 . The counterbalancing effect of follicle-stimulating hormone on the antigonadal activity of prolactin in the male newt Triturus cristatus carnifex (Laur.) . Journal of Endocrinology , 40 : 529 – 530 .
- Moore , IT and Jessop , TS. 2003 . Stress, reproduction, and adrenocortical modulation in amphibians and reptiles . Hormones and Behavior , 43 : 39 – 47 .
- Moore , FL and Miller , LJ. 1984 . Stress-induced inhibition of sexual behaviour: Corticosterone inhibits courtship behaviours of a male amphibian (Taricha granulosa) . Hormones and Behaviour , 18 : 400 – 410 .
- Moore , FL and Zoeller , RT. 1985 . Stress-induced inhibition of reproduction: Evidence of suppressed secretion of LH–RH in an amphibian . General and Comparative Endocrinology , 60 : 252 – 258 .
- Norris , DO. 2007 . “ Vertebrate endocrinology ” . In , 4th , Amsterdam : Academic Press .
- Pancak , MK and Taylor , DH. 1983 . Seasonal and daily plasma corticosterone rhythms in American toads, Bufo americanus . General and Comparative Endocrinology , 50 : 490 – 497 .
- Thurmond , W , Kloas , W and Hanke , W. 1986 . Circadian rhythm of interrenal activity in Xenopus laevis . General and Comparative Endocrinology , 61 : 260 – 271 .
- Wilson , JX , Saway , H , Kikuchi , M , Kubokawa , K and Ishii , S. 1995 . Circulating catecholamine and glucose concentration in Japanese toads (Bufo japonicus) during the breeding season . General and Comparative Endocrinology , 98 : 303 – 310 .
- Wright , ML , Guertin , CJ , Duffy , JL , Szatkowski , MC , Visconti , RF and Alves , CD. 2003 . Developmental and diel profiles of plasma corticosteroids in the bullfrog, Rana catesbeiana . Comparative Biochemistry and Physiology A , 135 : 585 – 595 .
- Zerani , M and Gobbetti , A. 1993 . Corticosterone during the annual reproductive cycle and in sexual behaviour in the crested newt, Triturus carnifex . Hormones and Behaviour , 27 : 29 – 37 .