Abstract
This paper presents the results of an application of ultrasonic telemetry on white seabream, Diplodus sargus inhabiting an artificial reef (AR) in NW Sicily (western Mediterranean Sea). The objective of the study was to investigate the movement pattern of seabreams, verify their homing behaviour and site fidelity, determine their home range and describe their use of the habitat. Four seabreams were tagged and released, and their movements were recorded with automated and manual acoustic receivers. The spatial and temporal distribution of positional data suggest that the tagged seabreams hide inside the AR during the day, staying out of their shelter at night. The nocturnal movements of the tagged fishes are suggested to be a search for food in the seagrass patches surrounding the ARs. The monitored seabreams showed clear homing behaviour and strong site fidelity. Their home range extended from 0.01 to 0.17 km and included the AR and the surrounding sandy area with seagrass patches. Home range areas increased proportionally to the distance between the refuge on AR and the foraging areas on seagrass patches. The higher activity of seabreams during the night was interpreted as a result of a trade-off between predation risk and foraging needs.
Introduction
Movement patterns and habitat use in fish are important for understanding population and community processes as well as for fisheries management and conservation purposes, i.e. to better design marine protected areas according to fish home range (Lucas & Baras Citation2000). In recent years, the need to verify and improve the efficiency of protected areas and artificial reefs has determined an increase of studies on activity pattern, habitat use and home range of several species (Ormond & Gore Citation2005). A large number of such studies focus on coral reef fishes (Zeller Citation1997; Eristhee & Oxenford Citation2001), with only a few papers dealing with Mediterranean species living in artificial reef areas. Diel movements and home range of fishes in the Mediterranean Sea have been studied only in brown meagre, Sciaena umbra (Picciulin et al. Citation2005), dusky grouper, Epinephelus marginatus (Lembo et al. Citation1999) and salema, Sarpa salpa (Jadot et al. Citation2006).
The white seabream, Diplodus sargus, is a rocky-bottom dwelling fish occurring in the Mediterranean Sea and eastern Atlantic Ocean from a few metres down to at least 50 m depth (Whitehead et al. Citation1986). It is a highly valued fish targeted by artisanal and recreational fishermen in the Mediterranean area (Harmelin-Vivien et al. Citation1995), and it has been the object of aquaculture initiatives and marine ranching experiments (D'Anna et al. Citation2004). Several different aspects of the biology and ecology of white seabream have been studied (Rosecchi Citation1987; Garcia-Rubies & Macpherson Citation1995; Harmelin-Vivien et al. Citation1995; Macpherson Citation1998; Planes et al. Citation1999; Guidetti & Sala Citation2007), and the role of white seabream as a key-stone species involved in cascade effects and other dynamic processes regulating natural systems has been highlighted. However, such a role has not been clearly stated in artificial habitats, where the white seabream is a frequent and sometimes abundant component of the fish assemblage (Relini et al. Citation2002; Guidetti et al. Citation2005).
The Gulf of Castellammare (NW Sicily, western Mediterranean Sea) hosts one of the largest artificial reef areas along the Italian coast. Research has been carried out on its benthic community, fish assemblage, food web and fishing yields (Badalamenti et al. Citation2000). Studies conducted on the benthic community living on the concrete boulders have shown the scarcity of macroalgae and a very low benthic biomass (Tumbiolo et al. Citation1997). White seabreams have been found frequently on this artificial reef during diurnal visual census of the associated fish fauna (D'Anna et al. Citation1994). Moreover, a study conducted on their feeding habits showed that they feed at night on the bare sandy bottom and on Cymodocea nodosa patches close to the artificial reef (Pepe et al. Citation1998).
No quantitative study has been made to date on the movement pattern of the white seabream in artificial reefs, primarily because of the constraints due to the structural complexity of such a heterogeneous habitat (Smith et al. Citation2000). Yet the knowledge of spatial requirements and of movement patterns of fish is considered one of the key issues related to the productivity and functioning of artificial reefs and to the efficiency of marine protected areas (MPAs) (Frazer & Lindberg Citation1990). Acoustic telemetry techniques that employ automated receivers have proved a powerful tool in the study of the behavioural ecology of marine and freshwater animals and may help to define movements inside their home range (Bridger et al. Citation2001; Taverny et al. Citation2002).
The main objective of this study is to investigate the movement pattern of white seabream in the Gulf of Castellammare artificial reef area, based on acoustic tracking of tagged individuals. This study aimed to examine their homing behaviour and site fidelity, determine their home range and describe their use of habitat.
Materials and methods
Study site
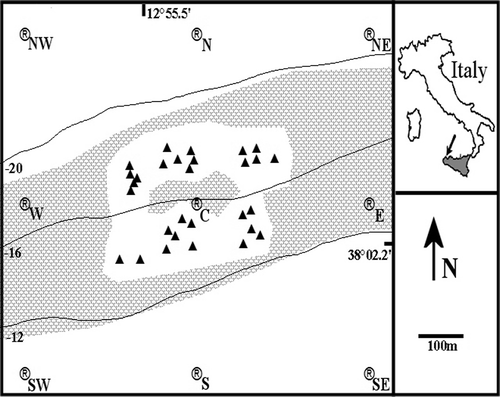
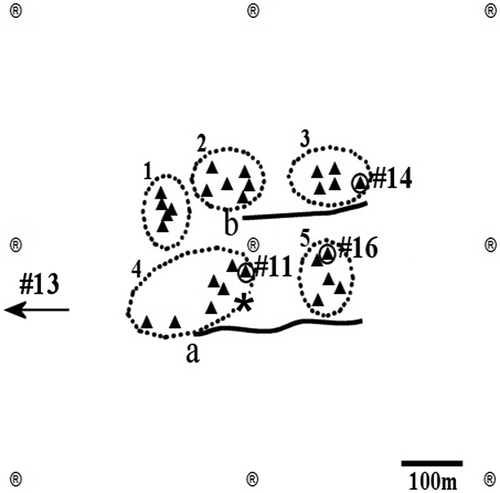
The Gulf of Castellammare is located on the NW coast of Sicily (38°03' N, 12°55' E). Two main artificial reef areas, plus several smaller isolated artificial structures, are present on the sandy bottom of the gulf between 10 and 50 m depth. The study site extended for about 1 km2 around the Alcamo Marina Artificial Reef Area (AM-ARA), located 1 km offshore between 14 and 18 m depth and included 29 artificial units distributed over an area of 0.2 km2 (). Each unit is a three-layer pyramid 6 m in height and 150 m3 in volume, made of 14 cubic concrete blocks with passing holes of various sizes (for details see Badalamenti et al. Citation2002). The units are aggregated in five reefs numbered 1 to 5 (). The seabed around AM-ARA is fine sand covered by a patchy Cymodocea nodosa meadow. For the spatial analysis of data a GIS map of AM-ARA was created based on a pre-existing side-scan sonar map (Badalamenti & D'Anna Citation1997) and on scuba dive observations.
Tagging and releasing
Twenty-five white seabreams were caught on 8 and 15 October 2004 with two longlines (labelled a and b in ) baited with holothurian flesh, set on the sandy bottom among the artificial units. The decision to use longlines was taken after interviews with local professional fishermen, in an attempt to select a method that would allow the lowest post-catch mortality in seabreams. The catch site of each fish was identified with GPS during the hauling operation and associated a posteriori with the closest reef in order to evaluate the homing behaviour. After removing hook and punching swim bladder to compensate embolism, 10 individuals survived but only 4 of them (20.5 ± 2 cm mean total length) were sufficiently healthy to be surgically implanted with a miniaturized transmitter tag (pinger mod. V8SC-1L by Vemco Ltd, length 24 mm, diameter 9 mm, weight in water 2.6 g, delay 10–30 s, frequency 69 kHz), according to the methodology suggested by Thoreau and Baras (Citation1996). Fishes were labelled after their own pinger code (##11, 13, 14 and 16). Individuals #11 and #13 were caught with longline a near reef no. 4, individuals #14 and #16 were caught with longline b between reefs nos. 3 and 5. Tagged fishes were left in a cage placed on reef no. 4 for a 15-h acclimatization period before release.
Acoustic monitoring
An array of nine submerged omnidirectional automated receivers (mod. VR2 by Vemco Ltd) was deployed in AM-ARA to continuously monitor the position of each tagged fish by means of presence/absence data. The receivers were kept in the area for 48 days from 9 October to 25 November 2004. Each receiver was moored 5 m below the surface on a thin rope vertically oriented between a hard plastic float and an anchor bar. One receiver labelled ‘C’ (centre) was deployed in the middle of AM-ARA, the others were placed 400 m apart at the vertexes of a grid centred on ‘C’ and labelled according to their geographical position, as indicated in . The total detection area covered by the receivers was 0.64 km2. Each VR2 receiver has a detection range of at least 500 m, as shown by a previous study on the performance of this telemetric system in different environmental conditions (sea current, presence of thermocline, water turbidity, etc.) in the same area (D'Anna et al. Citation2003). Detection data from VR2 receivers were used to calculate the geographic position or ‘fix’ of each fish activity centre (FAC, sensu Simpfendorfer et al. Citation2002) within 15-min time intervals. The adopted deployment design of VR2 receivers allows researchers to calculate FAC positions with a maximum error of 50 m for a fish located in an open space (Giacalone et al. Citation2005). FAC geographical coordinates were calculated using FiSAR, a custom-made software developed by Giacalone et al. (Citation2006). When the number of detections from all receivers was zero during a 15-min interval, no geographical coordinates were computed by FiSAR and that gap in the FAC time series was classified as a ‘silent fix’. The time distribution of silent fixes was investigated to distinguish between different possible fish positions: (1) randomly scattered silent fixes, where each silent fix was interpreted as a fish hiding inside an artificial unit; (2) sequences of a minimum of two silent fixes in a row, interpreted as a fish staying out of the detection area, i.e. away from AM-ARA. FAC fixes were used to estimate site fidelity and home range of each tagged fish.
FAC fixes in the data set of each fish were ‘cleaned’ following the method suggested by Skajaa et al. (Citation1998). This method, tailored to the present study and rewritten in MS Excel, analyses sets of eight successive fixes and calculates the mean coordinates (X, Y) of each set, a deviation value () and a regression of the eight X and Y values versus time. When the deviation in a set was higher than 20 and the slope of both regressions was not significantly different from zero (t-test, p < 0.005), the Excel routine replaced the set of eight fixes with the calculated mean of X and Y. The criteria chosen to clean the data allowed to correct those sets of coordinates corresponding to a wide spatial dispersion of consecutive fixes without any directional movement which should be interpreted as a positioning error due to the presence of an artificial unit (hence the non-significant regression) (Giacalone et al. Citation2005).
A fish presence index (FPI), expressed as the percent ratio between the number of calculated positional fixes (raw data) and the number of expected fixes of each fish during the whole study period, was used to estimate the occurrence of each fish inside the detection area.
After a preliminary check of FAC fixes made during the first few days of operation, we realized that the VR2 diurnal positions from all fish were always concentrated around the central receiver (labelled ‘C’ in ), probably because all tagged fish tended to stay in their refuge inside an artificial unit during daylight hours (Giacalone et al. Citation2005). To assess precisely the diurnal location of each tagged fish in the area and to verify their homing behaviour, a total of four boat trips were performed every 10 days during daylight hours using a manual receiver (mod. VR60 by Vemco Ltd). As opposed to VR2, which automatically calculates a FAC fix every 15 min, the VR60 gives the actual position of each tagged fish that occurs within its detection range while it is hand-operated. Tracking during each trip was carried out systematically in a wider area around AM-ARA (1 km away from the AR area), to be sure to detect also fish swimming a bit away from the artificial reef.
Mean distances between diurnal refuge positions (from VR60 data) and nocturnal positions (FAC fixes from VR2 data) throughout the whole study period were calculated for each fish.
Data analysis
FAC fixes were plotted onto a geo-referenced map of AM-ARA using the ArcView 3.1 GIS software, and spatial analysis was performed using the Animal Movement Analyst Extension, AMAE (Hooge et al. Citation1999). The AMAE site fidelity test was used to test the null hypothesis that the movements of each tracked fish were random. This test utilizes a Monte Carlo simulation to compare movements observed during the study with 1000 random walks incorporating the actual sequence of distances travelled by each fish during each 15-min interval. The AMAE outlier removal function was applied with the harmonic mean method to exclude 5% of fixes from the data set of each fish. The home range of each individual was determined using the 95% Kernel Utilization Distribution (KUD) in the AMAE routine. The night core area of each individual (50% KUD) was also computed for nocturnal data (from dusk to dawn). The AMAE ad-hoc value was adopted as smoothing factor for home range and core area calculation.
Results
FPI values in the monitored area ranged from 65.5% for fish #13 to 95.5% for fish #14 (). The analysis of the FAC fixes time series pointed out some individual differences in the distribution of silent fixes. In particular, fish #13 showed 0.5–6 h of absence from the VR2s detection area. Silent fixes in the data sets of fish #11, #14 and #16 were rare, randomly scattered, without any sequence of successive silent fixes, and occurred mainly in the daylight hours.
Table I. Distance between release site and diurnal refuge, mean distance between diurnal refuge and night positions, fish presence index (FPI) and home range (95% KUD) of the four tagged fish
According to VR60 diurnal manual tracking data, fish #11, #14 and #16 were located always in the same AR unit during each trip, which was interpreted as their refuge (). The distance between the release site and each refuge ranged between 59 m for fish #11 and 928 m for fish #13 (). The sequence of FAC fixes for fish #13 showed an initial straight movement from the release site to reef no. 4. This refuge was abandoned two days after the release, when this fish reached an area with quarry rocks 928 m westward of the release site (). All other fishes displayed a clear homing behaviour, leaving the release site and moving each to a different AR unit located at 50–80 m from their catch site, where they remained during daylight hours for the rest of the study period: fish #11 moved to reef no. 4, while fishes #14 and #16 moved to reef no. 3 and 5 respectively ().
The hypothesis that the observed movements were random was rejected for all individuals (AMAE site fidelity test: p = 99.9), indicating that all fishes had a high degree of site fidelity and moved consistently between the diurnal refuge and the nocturnal area in the C. nodosa meadow.
The home range of tagged fishes extended from 0.01 km2 for fish #11 to 0.17 km2 for fish #14, with a mean value of 0.11 ± 0.08 km2 () and for each fish it included the artificial reefs and parts of bare sandy bottom and of C. nodosa meadow (). Fish #13 was detected only by two or three western VR2 receivers during the whole study period: this suggests that it stayed off the western limit of the study area after the initial two-day period in reef no. 4. As a consequence, its home range based on the analysis of FAC fixes would be underestimated, also considering the low FPI value (65.5%). For this reason data on its home range were not included in and .
Figure 3. GIS maps of the home range (KUD 95%), the diurnal refuge and the night core areas (KUD 50%) of tagged white seabreams ##11, 14 and 16 over the whole study period.
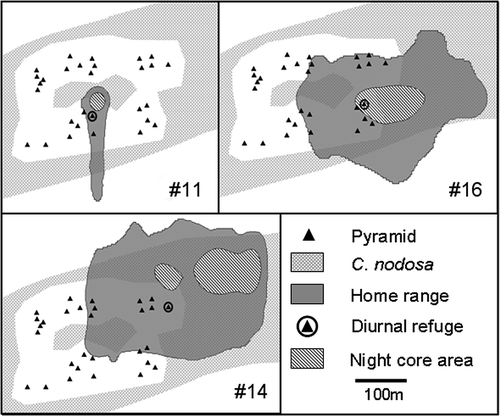
Even if home ranges partially overlapped, each 95% KUD area was centred on a different portion of AM-ARA. shows also the location of the diurnal refuge in a AR and the night core area in the C. nodosa meadow of fishes #11, #14 and #16.
Movements between diurnal positions (VR60 data) and night-time FACs inside the nocturnal core areas ranged from 45.1 ± 7.1 m for fish #11 to 181.1 ± 51.3 m for fish #14 ().
Discussion
The white seabreams tagged and released in the Gulf of Castellammare showed homing capacity and high site fidelity. Except for fish #13, all home ranges rest inside AM-ARA and are strictly linked to the artificial reef and the surrounding environment, where the seabreams find refuge and food.
We acknowledge that our findings are of limited generality due to small sample size. Sampling and handling procedures are a critical phase of any telemetry experiment. The choice and success ratio of methods for collecting individuals and implanting acoustic tags depend strongly on fish species stress-resistance, thus it is not possible to reliably estimate the percentage of collected fish that will be successfully tagged in the end. In this study only 4 out of 25 collected fish were in good enough condition to be tagged, although the best available sampling method was used. We believe anyway that the small sample size has been compensated by the very large amount of position-through-time data from the telemetry system, as documented in similar studies with few tagged specimens (Eristhee & Oxenford Citation2001; Chateau & Wantiez Citation2007). Despite the described constraints, our results demonstrate that, the application of acoustic telemetry to the monitoring of tagged white seabream in the Alcamo Marina Artificial Reef Area was successful.
The diurnal localization of fish #11, #14 and #16 and the position of their nocturnal core areas indicate diel movements between their refuge in an artificial unit and their feeding area in a C. nodosa patch (Pepe et al. Citation1998). This diel movement pattern disagrees with previous knowledge on this species in its natural rocky habitat. White seabream in the western Mediterranean has been described as a diurnally active fish, frequenting the surf zone to feed on bivalves, algae and sea urchins (Sala & Ballesteros Citation1997). Similar results were reported by Giacalone (unpublished data) for the natural rocky area nearby AM-ARA in a preliminary study on the movement of white seabream. In the Azores this species has been reported to feed between noon and dusk (Figueiredo et al. Citation2005). In contrast, as already observed by Pepe et al. (Citation1998) and D'Anna et al. (Citation2004), the white seabream living in the Gulf of Castellammare artificial reef area spend diurnal hours inside their shelter among the concrete boulders and move to the feeding area at night. Pepe et al. (Citation1998) found that leaves of C. nodosa and associated molluscs were the most frequent and abundant items in the stomach contents of white seabreams collected in AM-ARA. The differences between our data and those collected in the western Mediterranean and the Azores could be explained by the differences in habitat structure and food availability existing between natural infralittoral rocky habitats and artificial reefs. The former habitat is typically very heterogeneous and characterized by boulders of different sizes and numerous holes and crevices that provide plenty of refuges, allowing fishes to dwell safely near the bottom where they can hide rapidly from predators. More importantly, infralittoral rocky bottoms are generally widely extended and offer a continuous habitat available for feeding and hiding. In addition, natural rocks are generally covered with algae hosting an invertebrate fauna that can serve as food for fish (Figueiredo et al. Citation2005). In AM-ARA each artificial unit is isolated on the sandy bottom and the only refuges available are the holes present in the concrete boulders. Moreover, studies carried out on the benthic assemblage of AM-ARA detected poor macroalgal cover and very low invertebrate biomass (Tumbiolo et al. Citation1997; Badalamenti et al. Citation2000). In this particular habitat white seabreams seem to use the units as a shelter and the C. nodosa meadow as a foraging area.
Although the use of rocks for sheltering and of sandy bottoms for feeding is a habit shared by several reef fish species, the factors determining this behaviour have not been thoroughly investigated. In our study the movement pattern of white seabream might have been influenced by the characteristics of the artificial reef, which offers plenty of refuges in each single unit but very little food. Although the white seabream is considered a diurnal predator that relies mostly on visual cues (Eggers Citation1997), in AM-ARA a diurnal trip to the foraging area on the C. nodosa meadow could expose seabreams to a high predation risk (Hobson et al. Citation1981). Crepuscular and nocturnal activity could be a behaviour adopted by some fish to reduce risk predation (Garcia-Rubies & Zabala Citation1990; Heggenes et al. Citation1993) and to optimize foraging strategy in relation to environmental factors. The choice of tagged seabreams to move during the night is probably the result of a trade-off between predation risk and foraging needs (Gilliam Citation1987). The decision to dwell over open areas at night suggests that white seabreams in AM-ARA might add other sensory modalities to the visual localization of food as demonstrated for the cod Gadus morhua (Lokkeborg & Fernö Citation1999), for the rainbow trout Oncorhynchus mykiss and brown trout Salmo trutta (Railsback et al. Citation2005).
A clear homing behaviour was indicated by the return of released seabreams to a reef close to their respective capture site, confirming what was already known from tracking experiments with other reef fishes (Matthews et al. Citation1990; Mitamura et al. Citation2005). In addition, their ability to recognize a particular refuge after each nocturnal trip throughout the study period confirms their homing behaviour. Little is known about homing in Mediterranean fishes; such attitude has been acknowledged only for salema Sarpa salpa (Jadot et al. Citation2006) and dusky grouper Epinephelus marginatus (Lembo et al. Citation1999). The distance covered during this study by seabreams from the release site to their home reef ranged from 59 to 928 m. D. sargus is acknowledged as a territorial fish characterized by low vertical displacements but potentially wide horizontal movements. In AM-ARA this species showed a pattern of movements limited to a few hundred metres.
During the whole study period, each fish has been moving between its own refuge and the feeding area, showing a high degree of site fidelity. This behaviour is common to different reef-associated fish, especially when individuals move between selected microhabitats (Hissmann et al. Citation2000). Indeed, site fidelity can be influenced by environmental features and habitat quality. Being a reef-associated species, white seabream in AM-ARA tend to hide within the concrete blocks of the artificial reef, but the choice of one peculiar AR unit is probably dictated also by the proximity of a high-quality foraging area.
The 95% KUD of fishes #11, #14 and #16 represents a good estimate of their home range especially if referred to their FPI value (>92%). On the other hand, the 95% KUD of fish #13 underestimates its true home range because this individual seemed to stay often outside the area detected by the VR2 fixed receivers, as suggested by its low FPI value. The home range size of our tagged white seabreams varied considerably among individuals (0.01 to 0.17 km2). It is well known that several factors can influence the home range size (Kramer & Chapman Citation1999). Some authors have found a positive correlation between home range size and fish size or age (Heupel et al. Citation2004; Jones Citation2005), but due to the homogeneous length of our individuals an influence of fish size on home range size can be excluded. Other factors besides fish length may well affect home range, such as food limitation (Hansen & Closs Citation2005), movement rate (Popple & Hunte Citation2005) and habitat topography (Zeller Citation1997; Eristhee & Oxenford Citation2001; Topping et al. Citation2005). The high variability among the home ranges observed in this study could be explained by individual movement patterns and by bottom topography. Fish are expected to discriminate between suitable and unsuitable microhabitats. The homing behaviour and high site fidelity of our tagged seabreams seem to rely on their skill in recognizing their own refuge among 29 units and one or more defined C. nodosa patch in a wide seagrass meadow. Actually, tagged seabreams were able to keep their own diurnal refuge and nocturnal core area throughout the whole study period with consistent diel movement patterns. In this sense, our seabreams follow a typical reef refuging and off-reef foraging pattern, and their home range depends on the AM-ARA microhabitat mosaic. The spatial arrangement of the distinct microhabitats is likely to affect the distances travelled by each individual between its diurnal refuge and nocturnal foraging area. This fact would explain the difference among the sizes of their home range. Actually the 95% KUD areas of fishes #11, #16 and #14 is proportional to their respective mean refuge-to-night positions distance (). This finding is in agreement with previous studies on different reef-associated fish (i.e. Serranidae and Labridae), which demonstrated a strong relationship between the home range size and the position of the microhabitat visited by each single fish (Zeller Citation1997; Eristhee & Oxenford Citation2001) or the rate of fish movement (Popple & Hunte Citation2005; Topping et al. Citation2005).
Conclusion
The technology employed in this study proved to be an efficient tool for assessing position-through-time of tagged white seabream from simple presence/absence data. The effectiveness of the telemetric system allowed us to examine for the first time the movement pattern of this reef-associated species in an artificial reef area. The knowledge gained on site fidelity, habitat use and homing behaviour of white seabream has shed light on the efficiency and functioning of an artificial reef system.
The study of the diel movement pattern contributed to clarify the role of artificial substrates in providing shelter to white seabream and the function of C. nodosa as a feeding ground. The topographic aspects of the study area and the different use that seabreams make of habitats suitable for refuge and feeding influence the home range size and the movement pattern of fish.
Based on our results, the artificial reef can provide seabream with suitable refuges. This finding can be used to enhance future design of artificial reefs and to integrate artificial structures and natural environments.
Acknowledgements
We thank Giuseppe A. Trunfio and Tomas Vega Fernandez for a precious help in data analysis and proof review, Paolo La Scala from Palermo University and the IAMC-CNR staff for their support during field works.
References
- Badalamenti , F , Chemello , R , D'Anna , G , Henriquez Ramos , P and Riggio , S . 2002 . Are artificial reefs comparable to neighbouring natural rocky areas? A mollusc case study in the Gulf of Castellammare (NW Sicily) . ICES Journal of Marine Science , 59 ( Supplement ) : S127 – S131 .
- Badalamenti , F and D'Anna , G . 1997 . Sperimentazione di un modulo pilota di maricoltura integrata in un'ipotesi di gestione della fascia costiera nel Golfo di Castellammare (Sicilia occidentale): ruolo delle strutture artificiali nella rete trofica e nel reclutamento di forme giovanili per la maricoltura. Ministero della Marina Mercantile, III piano triennale della Pesca e dell'Acquacoltura 91
- Badalamenti , F , D'Anna , G and Riggio , S . 2000 . “ Artificial reefs in the Gulf of Castellammare (North-West Sicily): A case study ” . In Artificial reefs in European seas , Edited by: Jensen , AC , Collins , KJ and Lockwood , APM . 75 – 96 . London : Kluwer Academic Publishers .
- Bridger , CJ , Booth , RS , Mc Kinley , RS and Scruton , DA . 2001 . Site fidelity and dispersal patterns of domestic triploid steelhead trout (Oncorhynchus mykiss Walbaum) released to the wild . ICES Journal of Marine Science , 58 : 510 – 516 .
- Chateau , O and Wantiez , L . 2007 . Site fidelity and activity patterns of a humphead wrasse, Cheilinus undulatus (Labridae), as determined by acoustic telemetry . Environmental Biology of Fishes , 80 : 503 – 508 .
- D'Anna , G , Badalamenti , F , Giacalone , VM and Lembo , G . 2003 . Progettazione ed implementazione di un sistema di rilevamento telemetrico per lo studio degli spostamenti di specie ittiche in un'area marina protetta con barriere artificiali nel Golfo di Castellammare (Sicilia NO). Ministero delle Politiche Agricole e Forestali, V Piano Triennale della Pesca e dell'Acquicoltura , Rapporto finale Progetto 5C 121, dicembre 2003: 71 .
- D'Anna , G , Badalamenti , F , Gristina , M and Pipitone , C . 1994 . Influence of artificial reefs on coastal fish communities of the Gulf of Castellammare (N/W Sicily) . Bulletin of Marine Science , 55 : 418 – 433 .
- D'Anna , G , Giacalone , VM , Badalamenti , F and Pipitone , C . 2004 . Releasing of hatchery-reared juveniles of the white seabream Diplodus sargus (L., 1758) in the Gulf of Castellammare artificial reef area (NW Sicily) . Aquaculture , 233 : 251 – 268 .
- Eggers , DM . 1997 . Factors in interpreting data obtained by diel sampling of fish stomachs . Journal of the Fisheries Research Board of Canada , 34 : 290 – 294 .
- Eristhee , N and Oxenford , HA . 2001 . Home range size and use of space by Bermuda chub Kyphosus sectatrix (L.) in two marine reserves in the Soufrière Marine Management Area, St Lucia, West Indies . Journal of Fish Biology , 59 : 129 – 151 .
- Figueiredo , M , Morato , T , Barreiros , JP , Afonso , P and Santos , RS . 2005 . Feeding ecology of the white seabream, Diplodus sargus, and the ballan wrasse, Labrus bergylta, in the Azores . Fishery Research , 75 : 107 – 119 .
- Frazer , TK and Lindberg , WJ . 1990 . Refuge spacing similarly affects reef-associated species from three phyla . Bulletin of Marine Science , 55 : 388 – 400 .
- Garcia-Rubies , A and Macpherson , E . 1995 . Substrate use and temporal pattern of recruiment in juvenile fishes of the Mediterranean littoral . Marine Biology , 124 : 35 – 42 .
- Garcia-Rubies , A and Zabala , M . 1990 . Effects of total fishing prohibition on the rocky fish assemblages of Medes Islands Marine Reserve (NW Mediterranean) . Scientia Marina , 54 : 317 – 328 .
- Giacalone , VM , D'Anna , G , Garofalo , G , Collins , K and Badalamenti , F . 2005 . “ Estimation of positioning error from an array of automated omnidirectional receivers in an artificial reef area ” . In Aquatic telemetry: advances and applications , Edited by: Spedicato , MT , Lembo , G and Marmulla , G . 245 – 253 . Rome : FAO – Coispa .
- Giacalone , VM , Garofalo , G , D'Anna , G , Badalamenti , F and Pipitone , C . 2006 . Fi.S.A.R.: A data-managing and processing software for automated telemetry systems . Marine Technology Society Journal , 40 : 47 – 50 .
- Gilliam , JF . 1987 . Habitat selection under predation hazard: Test of a model with foraging minnows . Ecology , 68 : 1856 – 1862 .
- Guidetti , P , Bussotti , S and Boero , F . 2005 . Evaluating the effects of protection on fish predators and sea urchins in shallow artificial rocky habitats: A case study in the northern Adriatic Sea . Marine Environment Research , 59 : 333 – 348 .
- Guidetti , P and Sala , E . 2007 . Community-wide effects of marine reserves in the Mediterranean Sea . Marine Ecology Progress Series , 335 : 43 – 56 .
- Hansen , EA and Closs , GP . 2005 . Diel activity and home range size in relation to food supply in a drift-feeding stream fish . Behavioural Ecology , 16 : 640 – 648 .
- Harmelin-Vivien , ML , Harmelin , JG and Leboulleux , V . 1995 . Microhabitat requirements for settlement of juvenile sparid fishes Mediterranean rocky shores . Hydrobiologia , 300/301 : 309 – 320 .
- Heggenes , J , Krog , O , Londos , O and Dokk , KK . 1993 . Homeostatic behavioural responses in a changing environment: brown trout (Salmo trutta) become nocturnal during winter . Journal of Animal Ecology , 62 : 295 – 308 .
- Heupel , MR , Simpfendorfer , CA and Hueter , RE . 2004 . Estimation of shark home ranges using passive monitoring system . Environmental Biology of Fishes , 71 : 135 – 142 .
- Hissmann , K , Fricke , H and Schauer , J . 2000 . Patterns of time and space utilisation in coelacanths (Latimeria columnae), determined by ultrasonic telemetry . Marine Biology , 136 : 943 – 952 .
- Hobson , ES , McFarland , WN and Chess , JR . 1981 . Crepuscular and nocturnal activities of Californian nearshore fishes, with consideration of their scotopic visual pigments and the photic environment . Fishery Bulletin , 79 : 1 – 30 .
- Hooge , PN , Eichenlaub , WM and Solomon , EK . 1999 . The Animal Movement Program , Fairbanks : USGS, Alaska Biological Science Center .
- Jadot , C , Donnay , A , Acolas , ML , Cornet , Y and Begout Anras , ML . 2006 . Activity patterns, home-range size, and habitat utilization of Sarpa salpa (Teleostei: Sparidae) in the Mediterranean Sea . ICES Journal of Marine Science , 63 : 128 – 139 .
- Jones , KMM . 2005 . Home range areas and activity centres in six species of Caribbean wrasses (Labridae) . Journal of Fish Biology , 66 : 150 – 166 .
- Kramer , DL and Chapman , MR . 1999 . Implications of fish home range size and relocation for marine reserve function . Environmental Biology of Fishes , 5 : 65 – 79 .
- Lembo , G , Fleming , IA , Okland , F , Carbonara , P and Spedicato , MT . 1999 . Site fidelity of the dusky grouper Epinephelus marginatus (Lowe, 1834) studied by acoustic telemetry . Marine Life , 9 : 37 – 43 .
- Lokkeborg , S and Fernö , A . 1999 . Diel activity pattern and food search behaviour in cod, Gadus morhua . Environmental Biology of Fishes , 54 : 345 – 353 .
- Lucas , MC and Baras , E . 2000 . Methods for studying spatial behaviour of freshwater fishes in the natural environment . Fish and Fisheries , 1 : 283 – 316 .
- Macpherson , E . 1998 . Ontogenetic shifts in habitat use and aggregation in juvenile sparid fishes . Journal of Experimental Marine Biology and Ecology , 220 : 127 – 150 .
- Matthews , KR , Quinn , TP and Miller , BS . 1990 . Use of ultrasonic transmitters to track demersal rockfish movements on shallow rocky reefs . American Fisheries Society Symposium , 7 : 375 – 379 .
- Mitamura , H , Arai , N , Sakamoto , W , Mitsunaga , Y , Tanaka , H , Mukai , Y , Nakamura , K , Sasaki , M and Yoneda , Y . 2005 . Role of olfaction and vision in homing behaviour of black rockfish Sebastes inermis . Journal of Experimental Marine Biology and Ecology , 322 : 123 – 134 .
- Ormond , RFG and Gore , MA . 2005 . “ No-take zones: Does behaviour matter? ” . In Aquatic telemetry: Advances and applications , Edited by: Spedicato , MT , Lembo , G. and Marmulla , G . 71 – 90 . Rome : FAO – Coispa .
- Pepe , P , Badalamenti , F and D'Anna , G . 1998 . Abitudini alimentari di Diplodus sargus nell'area delle strutture artificiali di Alcamo Marina (Golfo di Castellammare, Sicilia Nord-Occidentale) . Biologia Marina Mediterranea , 5 : 367 – 370 .
- Picciulin , M , Umani , M , Costantini , M , Spoto , M and Ferrero , EA . 2005 . “ Preliminary results from an exploratory translocation study in the Natural Marine Reserve of Miramare (Trieste, Italy) ” . In Aquatic telemetry: Advances and applications , Edited by: Spedicato , MT , Lembo , G and Marmulla , G . 203 – 210 . Rome : FAO – Coispa .
- Planes , S , Macpherson , E , Biagi , F , Garcia-Rubies , A , Harmelin , J , Harmelin-Vivien , M , Jouvenel , J-Y , Tunesi , L , Vigliola , L and Galzin , R . 1999 . Spatio-temporal variability in growth of juvenile sparid fishes fron the Mediterranean littoral zone . Journal of Marine Biology Association UK , 79 : 137 – 143 .
- Popple , ID and Hunte , W . 2005 . Movement patterns of Cephalopholis cruentata in a marine reserve in St Lucia, W.I., obtained from ultrasonic telemetry . Journal of Fish Biology , 67 : 981 – 992 .
- Railsback , SF , Harvey , BC , Hayse , JW and LaGory , KE . 2005 . Tests of theory for diel variation in salmonid feeding activity and habitat use . Ecology , 86 : 947 – 959 .
- Relini , G , Relini , M , Torchia , G and Palandri , G . 2002 . Ten years of censuses of fish fauna on the Loano artificial reef . ICES Journal of Marine Science , 59 : 132 – 137 .
- Rosecchi , E . 1987 . L'alimentation de Diplodus annularis, Diplodus sargus, Diplodus vulgaris et Sparus aurata (Pisces, Sparidae) dans le Golfe du Lion et les lagunes littorales . Revue des Travaux de l'Institut des Pêches maritime , 49 : 125 – 141 .
- Sala , E and Ballesteros , E . 1997 . Partitioning of space and food resources by three fish of the genus Diplodus (Sparidae) in a Mediterranean rocky infralittoral ecosystem . Marine Ecology Progress Series , 152 : 273 – 283 .
- Simpfendorfer , CA , Heupel , MR and Hueter , RE . 2002 . Estimation of short-term centers of activity from an array of omnidirectional hydrophones and its use in studying animal movements . Canadian Journal of Fish Aquatic Science , 59 : 23 – 32 .
- Skajaa , K , Fernö , A , Løkkeborg , S and Haugland , EK . 1998 . Basic movement pattern and chemo-oriented search towards baited pots in edible crab (Cancer pagurus L.) . Hydrobiologia , 371–372 : 143 – 153 .
- Smith , IP , Collins , KJ and Jensen , AC . 2000 . Digital electromagnetic telemetry system for studying behaviour of decapod crustaceans . Journal of Experimental Marine Biology and Ecology , 247 : 209 – 222 .
- Taverny , C , Lepage , M , Piefort , S , Dumont , P and Rochard , E . 2002 . Habitat selection by juvenile European sturgeon Acipenser sturio in the Gironde estuary (France) . Journal of Applied Ichthyology , 18 : 536 – 541 .
- Thoreau , X and Baras , E . Anaesthesia and surgery procedures for implanting telemetry transmitters into the body cavity of tilapia Oreochromis aureus . Underwater Biotelemetry. Proceedings of the First Conference and Workshop on First Telemetry in Europe . Edited by: Baras , E and Philippart , JC . pp. 13 – 22 . Belgium : University of Liege .
- Topping , DT , Lowe , CG and Caselle , JE . 2005 . Home range and habitat utilization of adult California sheephead, Semicossyphus pulcher (labridae), in a temperate no-take marine reserve . Marine Biology , 147 : 301 – 311 .
- Tumbiolo , ML , Badalamenti , F and D'Anna , G . Preliminary assessment of zoobenthic biomass living on an artificial reef in the Gulf of Castellammare (NW Sicily) . The responses of marine organisms to their environments. Proceedings of the 30th European Marine Biology Symposium . Southampton, United Kingdom. Edited by: Hawkins , LE , Hutchinson , S , Jensen , AC , Sheader , M and Williams , JA . pp. 353 – 359 . Southampton, , UK : University of Southampton .
- Whitehead , PJP , Bauchot , ML , Hureau , JC , Nielsen , J and Tortonese , E . 1986 . Fishes of the North-Eastern Atlantic and the Mediterranean , Vol. II , Paris : Unesco .
- Zeller , DC . 1997 . Home range and activity patterns of the coral trout Plectropomus leopardus (Serranidae) . Marine Ecology Progress Series , 154 : 65 – 77 .